Terpenes, shikimates, polyketides, acetogenins, peptides, alkaloids, and many uncharacterized structures, extracted and purified from marine resources, showed various pharmacological activities as antioxidant, antibacterial, anticancer, antiviral, anti-inflammatory, antidiabetic, antihypertensive, and anticoagulant.
1. Introduction
PD is the most common neurodegenerative disorder, affecting 1% of the population over 65 [115]. Clinically, most patients present with a motor disorder and suffer from bradykinesia, resting tremor, rigidity, and postural instability. Other manifestations include behavioral, cognitive, and autonomic disturbances. As previously mentioned, PD is a multifactorial disease. The main causes beyond aging are oxidative stress, mitochondrial dysfunction, and the consequent loss of dopaminergic neurons; for this reason, the candidate molecules to be used as a possible protective therapy in PD should have antioxidant and anti-apoptotic activities; affecting the PI3K/Akt pathway, as well as downstream signaling.
2. Fucoidan
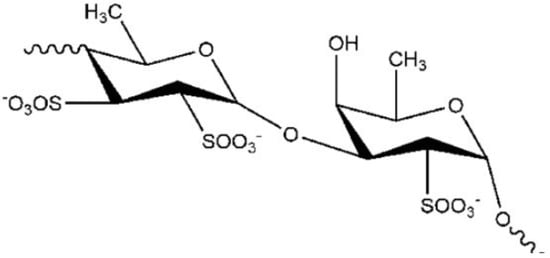
One molecule showing these properties, is the fucoidan [
53], a polysaccharide () extracted from the brown algae
Saccharina japonica (sugar content, 48%, fucose content 28%, sulfate content 29%).
Figure 1. Chemical structure of fucoidan.
Notably, fucoidan exhibited a protective effect in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) C57BL/6 mice. In the study by Luo and colleagues [
116], fucoidan treatment significantly improved the motor impairment in a MPTP mice model of PD. It was also able to counteract the depletion of striatal DA and reduced tyrosine hydroxylases-positive neurons in the
substantia nigra pars compacta [
116]. In an in vitro model of PD, using the 1-methyl-4-phenylpyridinium-induced MN9D dopaminergic cell line, fucoidan pre-treatment preserved cell morphology, increased mitochondrial activity, and reduced 1-methyl-4-phenylpyridinium-induced lactate dehydrogenase release; however, in line with other marine compounds, the pharmacological mechanisms underlying this protective effect is still unknown. Jhamandas et al. demonstrated that fucoidan’s neuroprotection depends on its antioxidant effect observed in rat cholinergic neurons of the basal forebrain treated with Aβ by blocking the generation of ROS. Luo et al. [
116], confirmed that the neuroprotective mechanism of fucoidan might depend on its antioxidant activity even if at low concentration showed no significant effects on the other parameters. These data indicated that the change in antioxidant status contributes to the protective effect of fucoidan from MPTP-induced loss of dopaminergic neurons. Still, it is not the only mechanism exerted by this compound. Indeed, other mechanisms involved may concern the anti-inflammatory action of fucoidan.
3. Xyloketal B
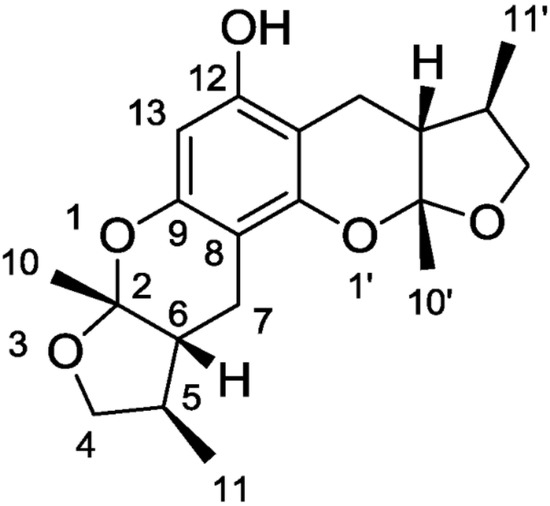
A study by Nakamura et al., 2006, showed that the modulation of elements in the inflammatory pathway could regulate neurons’ state. A molecule that exhibits anti-oxidant, anti-inflammatory, and anti-apoptotic activities is the Xyloketal B () obtained by mangrove fungus of the South China Sea coast.
Figure 2. Chemical structure of xyloketal B.
Lin et al. [
117] isolated a class of compounds in 2001 (xyloketals A-G); among these compounds, xyloketal B exhibited a scavenger effect for free oxygen radicals [
118] and prevented neuronal cell damage. The first to examine the protective effect of xyloketal B was Chen with his research group [
119]; in particular, for testing this molecule, they used human umbilical vein endothelial cells (HUVECs) and mimicked endothelial injury with oxidized low-density lipoprotein (oxLDL). The stress with oxLDL cause cell morphological changes and decreased cell viability in this in vitro model, while the xyloketal B treatment significantly reverted these effects. This research group studied oxLDL and xyloketal B’s effect on NADPH oxidase activity (an enzyme with a key role in ROS production) to understand this protective mechanism. Interestingly, oxLDL increased the NADPH oxidase activity [
120]. At the same time, the pre-treatment with xyloketal B was able to significantly lower both oxLDL-induced superoxide anion production and the mRNA expression of NADPH oxidase subunits gp91phox and p47phox [
119]. These findings indicate that xyloketal B counteract ROS production via inhibiting NADPH oxidase activity and decreasing its subunits’ mRNA expression. In 2009, Zhao et al. [
118] studied the neuroprotective potential of xyloketal B on an in vitro model of PC12 cell line exposed to oxygen and glucose deprivation. Initially, they tested the effect of xyloketal B pre-treatment after oxygen and glucose deprivation on cell viability, and after that, they evaluated the protective effect on mitochondria with MitoSOX dosage. The MTT assay reported significantly reduced stress with oxygen and glucose deprivation the number of viable cells, while the pre-treatment with xyloketal B counteracted this effect. For mitochondrial activity, disturbance in cellular respiration due to the glucose deficiency during ischemia causes nicotinamide adenine dinucleotide(H) accumulation in the mitochondria and ROS overproduction, which further leads to mitochondrial damage indicated by the reduction in mitochondrial membrane potential and cytochrome c release. In this study [
118], mitochondrial ROS production resulted strongly enhanced upon oxygen and glucose deprivation by MitoSOX assay, while upon xyloketal B showed the MitoSOX signal intensity reduced, thus the mitochondria may represent a potential target in the anti-apoptotic effect of xyloketal B in neuronal cells.
4. Seaweeds
Another source of marine compounds with antioxidant activity is seaweeds, which have been the target of numerous studies (extensively reviewed in [
121]), standing out as major producers of bioactive molecules with high antioxidant ability. Another research group tested the effects of different seaweed extracts (
S. muticum.,
S. polyschides.,
P. pavonica) in SH-SY5Y cells stressed with high concentration (1M) of 6-OHDA for 24 h (cell viability reduced more than 45%). The presence of the different extracts substantially increased the cell viability, counteracting DA’s neurotoxicity. These results suggested that the extracts exerted an antiapoptotic mechanism, as observed by the mitochondrial membrane potential analyzed by JC-1 assay parallel with the inhibition of caspase-3 activity. This protective effect may be due to the antioxidant capacity of the seaweeds tested; in particular, regarding the treatments with
Ascophyllum nodosum,
S. muticum, and
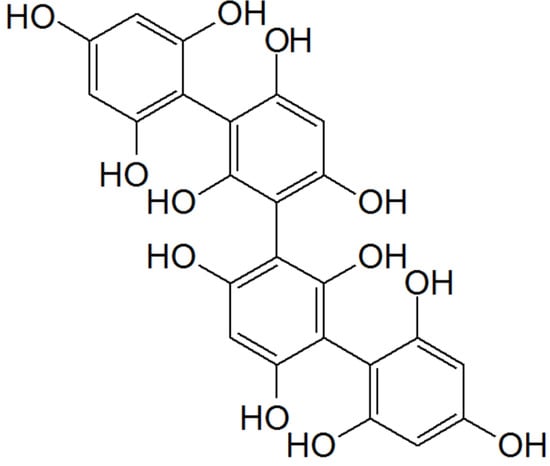
S. polyschides, this positive effect may be due to phlorotannins (), exclusively found in brown seaweeds, which are characterized by high antioxidant activities.
Figure 3. Chemical structure of tetrafucol A: a phlorotannin found in the brown algae Ascophyllum nodosum.
One promising seaweed with neuroprotective potential is the
Codium Tomentosum that showed antioxidative and antigenotoxic capacity [
122]. Valentao et al. [
123] tested its ability to scavenge the reactive oxygen and nitrogen species and characterized this seaweed’s chemical composition collected from the Atlantic Ocean by high-pressure liquid chromatography analysis. This species showed different organic acid, such as oxalic acid, aconitic acid, ketoglutaric acid, pyruvic acid, malic acid, malonic and fumaric acids, and a great variety of volatile compounds, such as phenolic compounds, secondary metabolites with multiple biological activities, as the plant defense mechanism under different environmental stress conditions.
5. Astaxanthin
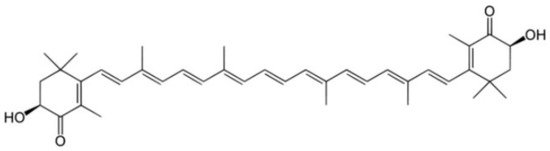
Another promising class of molecules with therapeutic potential versus PD are carotenoids. Most carotenoids are tetraterpenoid compounds consisting of eight isoprene units and are responsible for the red, orange, and yellow colors of archaea and fungi, algae, plants. In the marine environment, these molecules are obtained by macroalgae, bacteria, and unicellular phytoplankton and play numerous and important functions, including protect chlorophyll via absorbing light energy and scavenging free radicals of oxygen [124]. Animals cannot synthesize carotenoids de novo and need to ingest carotenoids via supplementation or diet. Aquatic animals swallow carotenoids from foods, such as algae and other animals, and convert their structure via metabolic reactions leading to structural diversity. Carotenoids have been intensely studied for their role in human health, not only as natural antioxidants but also as pharmaceuticals because they generally promote health, as in cancer prevention, by boosting immune function and cognitive performance antiaging effects and anti-inflammatory agents. Despite these important pharmacological activities, carotenoid use has some limitations: for example, easy degradation, low shelf life, low solubility in water, and low bioavailability. Most carotenoids are tetraterpenoid compounds consisted of a sequence of eight isoprene units. One important carotenoid from marine organisms is AXT (introduced in previous chapters). AXT () is a xanthophyll carotenoid produced primarily by the marine algae Haematococcus Pluvialis, and it has been widely studied in a broad range of clinical applications, including cardiovascular diseases, metabolic syndrome, gastric ulcers, and cancer, which share a common feature, including inflammation and/or oxidative stress [125].
Figure 4. Chemical structure of AXT.
AXT is already approved as a dietary supplement and commercially available, showing no significant adverse effects. Interestingly, emerging evidence suggested that biological activities for AXT’s may counteract the pathophysiology features of PD, revealing a promising therapeutic potential in the prevention or onset of symptoms in PD patients. In the study of Castelli et al. [115], the researchers evaluated AXT’s effect, in an in vitro model of SH-SY5Y stressed with H2O2. Notably, AXT showed a cytoprotective effect, preventing or modulating the severity of neuronal death following oxidative stress injury. The authors suggested that the mechanism of action involves the modulation of neuroprotective markers, including neurotrophic pathways, i.e., BDNF and antioxidant enzymes (i.e., manganese superoxide dismutase, catalase). In another study, Grimming and collaborators [126] evaluated AXT’s effect on an in vivo model of PD. The researchers explored the capacity of a dietary pre-treatment of AXT (mice consumed an AXT-enriched diet formulated to deliver a dose of 3 mg/kg/day) in protecting against neuronal damage caused by the neurotoxin MPTP. These results indicated that the AXT-enriched diet, attenuated the loss of dopaminergic neurons induced by MPTP. The protective effects were related to a decrease in inflammation and oxidative stress. In agreement, in another investigation, the administration of AXT could block some inflammatory sequelae of the lipopolysaccharide injections and inhibit NF-κB translocation to the nucleus, thereby down-regulating tumor necrosis factor-α expression. Furthermore, AXT intake was associated with a more favorable ratio of GSH, reduced GSH to oxidized GSH in the plasma.
This entry is adapted from the peer-reviewed paper 10.3390/md19010024