Adverse environmental conditions due to climate change, combined with declining soil fertility, threaten food security. Modern agriculture is facing a pressing situation where novel strategies must be developed for sustainable food production and security. Biostimulants, conceptually defined as non-nutrient substances or microorganisms with the ability to promote plant growth and health, represent the potential to provide sustainable and economically favorable solutions that could introduce novel approaches to improve agricultural practices and crop productivity. Current knowledge and phenotypic observations suggest that biostimulants potentially function in regulating and modifying physiological processes in plants to promote growth, alleviate stresses, and improve quality and yield.
- abiotic stresses
- biostimulants
- food security
- metabolomics
- growth-promoting rhizobacteria (PGPR)
- plant defenses
1. Introduction
Plants are continuously exposed to biotic (e.g., pest and pathogen attacks) and abiotic stresses (e.g., drought, extreme temperatures and salinity), which lead to frequent adjustment and remodeling of the plant defense machinery, as well as involving reconfiguration of the plant metabolism [1][2]. Evolutionarily, plants have developed a multilayered and dynamic defense system that renders them as adept as animals in responding effectively to ever-changing environments [3]. Furthermore, plant defense strategies can be enhanced and sensitized using various approaches such as biological and chemical priming. Defense priming is a phenomenon whereby the plant immune system and abiotic defenses are preconditioned, resulting in faster, stronger and effective defense and resistance mechanisms against subsequent biotic and abiotic stresses [4][5][6]. This immune-stimulation of plants, which is postulated to be an adaptive and low-cost defensive measure, is a result of interactions of plants with beneficial microbes, chemical compounds, insect herbivores or environmental cues [7][8].
There is emerging evidence of biostimulants as plant priming agents, as demonstrated by the observed effectiveness of these formulations in promoting and sensitizing plant defenses and resistance against different environmental stresses [9]. In the last decade, the field of plant biostimulants has been steadily growing in the agricultural industry and has positioned itself as one of the key emerging strategies for enhancing crop production and resilience to the changing climate. Plant biostimulants have received considerable attention lately, and are increasingly being integrated into agriculture and production systems as plant growth and yield regulators/promoters as well as pre-stress conditioners [10]. However, the limited fundamental research into the modes of action of many biostimulant products is among the knowledge gaps that require scientific attention. Elucidation of the biological basis of biostimulant function, and a broad mechanism of action at a cellular and molecular levels, is a prerequisite for the development of a scientifically-based biostimulant industry, leading to an effective exploration and application of formulations in agriculture [9][10]. Few emerging studies have shown the impeccable capabilities and potential of metabolomics approaches to reveal the mechanistic insights describing biostimulant-plant interactions [11][12]. Furthermore, these studies have proven that metabolomics studies can provide key fundamental knowledge and understanding required to explore novel biostimulant-based strategies for improved crop health and resilience in a changing climate.
Metabolomics is classically defined as a comprehensive and holistic measurement of the entire complement of small molecular weight molecules, namely metabolites (≤1500 Da in size), within a biological system [13]. The metabolome, being the chemical space and language of metabolism, carries imprints of genetic and environmental factors, and is expectedly more sensitive to perturbations in both metabolic fluxes and enzyme activity than either the transcriptome or proteome [14][15]. Hence, the global quantitative measurements of the metabolome provide an exploration of small cellular worlds, revealing hidden patterns and (predictively) reflecting functional signatures of the biochemical landscape and cellular physiology of the system under consideration [13][16][17].
2. Basic and Overlapping Framework of Plant Immunity and Defense against Biotic and Abiotic Stresses
In their natural habitats, plants coexist with highly dynamic microbial communities, some of which are harmful to plant health. Furthermore, the environmental constraints such as drought, salt and extreme temperatures negatively affect plant growth and development, both at the cellular and organismal levels [18][19]. Thus, for optimal growth and development, plants must have a protective immune and defense system that properly integrates both microbial signals and abiotic factors at both local and systemic levels. Studies have revealed that, evolutionarily, plants have developed constitutive, active, inducible and tightly regulated immune and defense systems that mediate interactions with heterogeneous environments comprising both biotic and abiotic stresses. The result of these dynamic and complex interactions is a determining factor for plant survival and fitness [20][21]. Several models have been proposed for describing plant immune responses to biotic stresses, with a common denominator that the innate immune system is based on the surveillance and perception of non-self, damaged-self and altered-self, broadly termed "danger" signals [22][23]. Generally, the first line of defense in plant response to biotic stresses involves preformed physical and chemical barriers such as cutin and waxes (cuticles), cell walls, antimicrobial enzymes and secondary metabolites to prevent or attenuate invasion of various pathogens [23][24]. In a case of a successful pathogen entry or plant alteration and/or damage, the second line of defense is launched, triggered by the recognition of danger signals through structurally diverse transmembrane or intracellular pattern recognition receptors (PRRs). This recognition of danger signals activates several levels of induced defense responses through a complex network of signal transduction and amplification, both locally at the site of infection and systemically in distant tissues [22][25].
The first layer of these inducible defenses is put in motion through the perception of microbe/pathogen-associated molecular patterns (M/PAMPs) or damage-associated molecular patterns (DAMPs) and termed M/PAMP-triggered immunity (M/PTI). The latter involves a series of complex cellular and molecular reprogramming events that are translated into distinct biochemical and physiological phenomena, such as activation of a signaling network involving phytohormones and other signaling molecules, stomatal closure to stop pathogen penetration, production of reactive oxygen species (ROS) and nitric oxide (NO), callose deposition and the de novo biosynthesis of antimicrobial metabolites due to reconfiguring of cellular metabolism [21][26]. However, due to the coevolution of plant-pathogen interactions, specialized pathogens have developed means to suppress M/PTI, through a repertoire of effector molecules that are translocated into the plant cell where they can alter cellular metabolism and homeostasis to promote disease. This state is known as effector-triggered susceptibility (ETS). On the other hand, plants have evolved mechanisms to perceive these effectors through intracellular receptors known as resistance (R) proteins. The recognition of these effectors activates the second layer of defense known as effector-triggered immunity (ETI). One of the descriptive markers of ETI events is the hypersensitive response (HR), which is an induced localized programmed cell death, to limit the spread of pathogen infection [21][23][26].
Studies have shown that there is an interplay (or overlap) between M/PTI and ETI, which is explained by the convergence between signaling and downstream biochemical events induced by both layers of defenses. This highly regulated coordination of M/PTI- and ETI-related defenses defines the basal defensive metabolism and subsequent physiological state of the plant against pathogen attack [22][27]. Furthermore, locally induced defense responses can trigger a defensive state in distant parts of the plant, a phenomenon known as systemic acquired resistance (SAR). The latter confers long-lasting protection against a range of pathogens in the systemic healthy tissue of plants undergoing a localized pathogen infection. Another form of an induced resistance mechanism is induced systemic resistance (ISR). The latter results from the interactions of plants and beneficial microbes, such as plant growth-promoting rhizobacteria and fungi [28][29]. Elaborated details of cellular and molecular events underlying these layers and models of plant immune responses to biotic stresses are beyond the scope of this review but can be found in the literature cited herein.
Plants are not only exposed to harmful microorganisms but also to harsh environmental conditions such as drought, salinity, extreme temperatures and nutrient deficiency, which adversely affect plant growth and productivity [30][31]. Some of the general defense responses include a highly-regulated cascade of signal transduction events, activation of defense-related genes, the accumulation of ROS and antioxidant mechanisms, production of defense-related metabolites and subsequent physiological and morphological changes [32]. Furthermore, one of the primary events in the plant responses to abiotic stresses involves a highly regulated and coordinated web of plant hormones such as abscisic acid (ABA), auxins (indole acetic acid, IAA), ethylene (ET), cytokinins (CK), gibberellins (GA), salicylic acid (SA), jasmonic acid (JA) and brassinosteroids (BRs). These phytohormones often act as primary signaling molecules that trigger complex signaling cascades, subsequently leading to stimulation of the expression of stress-related genes and induction of physiological and morphological changes, which eventually lead to abiotic stress tolerance or resistance (Figure 1) [33][34][35].
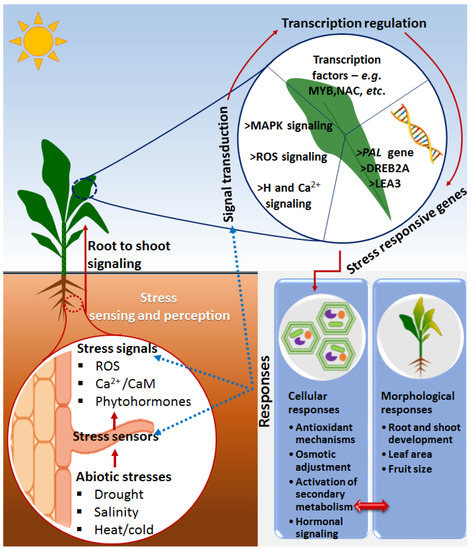
Figure 1. A descriptive model showing some general molecular events in a plant responding to abiotic stresses. Upon the perception of stress signals via evolutionarily developed rapid-sensing mechanisms, the systematic signal transduction/pathways such as ROS, Ca2+ and phytohormone signals are activated, leading to regulation of stress-responsive genes as facilitated by transcription factors. The stress-related genes are (de)activated to orchestrate the induction of stress adaptation/resistance cellular mechanisms such as antioxidant mechanisms, osmotic adjustment and secondary metabolism adjustment, as well as morphological responses such as root and shoot development, leaf area and fruit size. The blue dotted lines indicate the influence of the stress responses to sensors, signals and signal transductions. Abbreviations: Ca2+ = calcium ion, Ca2+/CaM = calcium calmodulin, ROS = reactive oxygen species, MAP kinases = mitogen-activated protein kinase, H = hormone, MYB = myeloblastosis, NAC = non-amyloid-β component, PAL = phenylalanine ammonia-lyase, DREB2A = dehydration-responsive element-binding protein 2A, LEA3 = late embryogenesis abundant 3.
Some of the common physiological responses to major abiotic stresses include a reduction in both transpiration and photosynthesis rates, a decrease in stomatal conductance and in leaf water content and a reduced relative growth rate [36][37]. For instance, responding to drought stress, molecular and cellular events deployed by the plant are translated into a reconfiguration of plant cellular metabolism, similar to defense responses to biotic stress. The latter leads to physiological modulation for plant survival. Some of the reported defense-related morpho-physiological changes due to drought stress are root swelling to promote nutrient uptake, leaf stomatal closure and leaf rolling to prevent water loss via transpiration, decreased chlorophyll content, leaf abscission and decrease in leaf area contributing to the reallocation of nutrients stored in older and diseased leaves to new leaves or the shoot [32][38].
Growth arrest is also one of the primary effects of drought stress. The inhibition of shoot growth is known to minimize the metabolic demands under water stress, thus increasing the biosynthesis and assimilation of metabolites such as osmolytes. The latter, also known as osmoprotectants, are low molecular weight, soluble compounds that play fundamental roles in osmotic adjustment, consequently conferring protection against cell drying-out. Osmoprotectants efficiently maintain osmotic balance, and stabilize membranes and macromolecules under water stress conditions. These include betaines, amino acids, polyols and non-reducing sugars (e.g., glycine betaine, proline and inositol) [39]. Root growth arrest maintains the function of root meristem and promotes rapid growth once the stress has been alleviated [40]. The inhibition of lateral root growth was also observed as an adaptive mechanism that promotes primary root elongation to reach the water from lower layers in the soil [41]. The other key cellular event that is observed under both biotic and abiotic stresses is the perturbations in the highly-regulated ROS homeostasis, causing an over-accumulation of ROS such as singlet oxygen (1O2), superoxide anion radical (O2•−), hydroxyl radical (•OH) and hydrogen peroxide (H2O2). The accumulation of these species results from the imbalance between the ROS production and the effectiveness of ROS scavenging systems, subsequently causing an oxidative burst and adversely affecting the plant metabolism by damaging cellular components such as DNA, proteins and lipids [42][43][44]. ROS generation can be detrimental as well as advantageous; it can also serve as a biological marker during abiotic stress conditions and can induce stress-signaling pathways to inhibit further damages [43][45]. To effectively manage and alleviate the oxidative stress, plants utilize ROS scavenging systems comprising (i) the enzymatic antioxidant system and (ii) a non-enzymatic antioxidant mechanism also known as the "low molecular weight" antioxidant system [45][46].
The enzymatic antioxidant system is made up of interrelated antioxidant enzymes including superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX), peroxidase (POX) and glutathione reduction (GR) [47][48]. On the other hand, the non-enzymatic antioxidant machinery is made-up of low molecular weight compounds such as phenolic compounds, amino acids, carotenoids, glutathione (GSH), ascorbic acid and α-tocopherol, which serve important roles in the detoxification of ROS. Apart from their antioxidant properties, these low molecular weight compounds can also function as osmoprotectants under abiotic stress, particularly under drought stress [49][50][51]. Furthermore, the antioxidant machinery (non-enzymatic and enzymatic) functions synergistically for stress alleviation or to enhance the plant tolerance to abiotic stress conditions (Figure 2) [46][52]. For instance, the ascorbate-glutathione cycle (also known as the Halliwell-Asada pathway) functions alongside enzymes such as CAT, GR and APX in the high capacity redox-homeostatic H2O2-scavenging pathways [53]. In this cycle, APX catalyzes the reduction of H2O2 to water, utilizing ascorbate (ASC). This reaction then yields monodehydroascorbate (MDHA), which is fated to dismutate to ASC and dehydroascorbate (DHA) or be reduced to NADP+ via monodehydroascorbate reductase (MDHAR). Furthermore, dehydroascorbate reductase (DHAR) reduces DHA to ASC using GSH as the reducing substrate. This reaction yields oxidized glutathione (GSSG), which is then converted back to the reduced state (GSH) by NADPH via the action of GR. ASC and GSH take part in the cyclic transfer of reducing equivalents without being consumed in the reactions, thus allowing the scavenging of H2O2, with NADPH as the reducing equivalent donor (Figure 2) [54].
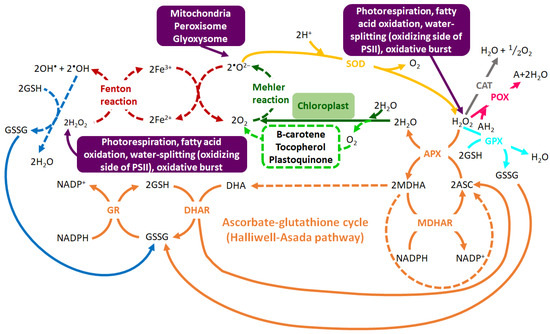
Figure 2. The intricate web of the highly-regulated oxidant-antioxidant system. Hydroxyl radical (•OH) and superoxide anion radical (•O2−) are generated via the Fenton reaction (in red). •O2− is produced by Mehler reaction (in dark green) and singlet oxygen (1O2) from water molecules (in neon green). The initial step in the ROS scavenging is the conversion of •O2− into hydrogen peroxide (H2O2) by superoxide dismutase SOD (in yellow), followed by the detoxification of H2O2 by GPX (in turquoise), CAT (in grey), APX (in orange) and POX (in pink). H2O2 is also scavenged through the ascorbate-glutathione cycle (in orange) which uses ASC and GSH as the cyclic transfer of reducing equivalents and NADPH as reducing power. The removal of •OH by GSH (in blue) forms GSSG which is regenerated to GSH via the GR-mediated reaction. The enzymatic pathways are depicted by bold lines and non-enzymatic pathways are indicated by dotted lines. Abbreviations: GPX = glutathione peroxidase, APX = ascorbate peroxidase, GR = glutathione reductase, ASC = ascorbate, MDHAR = monodehydroascorbate reductase, MDHA = monodehydroascorbate, AH2 = oxidizable substrate, DHA = dehydroascorbate, DHAR = dehydroascorbate reductase, POX = non-specific peroxidase, GSH = reduced glutathione, GSSG = oxidized glutathione. Adapted from [54].
Recent studies have begun to reveal molecular intersections and biochemical networks between biotic and abiotic stress responses, as well as overlapping regulatory mechanisms in combined stress responses [20][55][56]. A detailed account of plant responses to combined abiotic and biotic stresses is beyond the scope of this review, but the reader is referred to the literature cited herein for more details. However, this observation (i.e., combination of stresses) points to the reality of plants in their natural environments, where the plants interact with both abiotic and biotic stresses, individually or simultaneously, and in the presence of microbial communities (composed of bacteria, fungi and oomycetes) that inhabit the host plant, soil and surroundings, often in symbiotic relationships [57][58]. Some of these beneficial microorganisms found in the rhizosphere have been shown to induce systemic defense and tolerance mechanisms or precondition the plant protective responses against a range of environmental stresses.
2.1. Priming against Abiotic Stresses
Plant priming is a natural phenomenon, described as potentiating the protective and defensive responsiveness of plants upon the perception of some signals from the environment. Studies have demonstrated that plant priming—the preconditioning of plant defense mechanisms by beneficial microbes or agrochemicals—results in a faster, stronger and effective defense and resistance response to environmental stresses [59][60]. Mechanistically, priming can be described as a systematic multistage process consisting of three main stages, namely: (i) the priming phase, (ii) the post-challenge primed state and (iii) the transgenerational primed state. Some of the essential metabolic events observed during the priming phase include the biosynthesis or increase in the levels of amino acids, hormone conjugates and sugars, and these metabolic changes are collectively referred to as the "priming fingerprint" in the priming phenomenology [4][15]. These molecular and cellular changes are stored in the form of "metabolic memory", "molecular memory", "stress memory" or "primed memory"’, which can be described as "sensitization" or "immunization" strategies of plant defense systems. Upon a subsequent (or secondary) challenge—the post-challenge primed state—the "primed" plant effectively launches rapid and stronger defense responses [61][62]. The metabolic reprogramming observed during this phase mainly involves changes in secondary metabolite levels—i.e., defense-related compounds such as phenylpropanoids, terpenoids, volatiles, glucosinolates and tryptophan-related metabolites [4][63].
Emerging studies have demonstrated that various priming agents/stimuli can be applied to sensitize the plant defense system against abiotic stresses. For instance, parental drought priming has been shown to enhance drought tolerance in wheat offspring via increased accumulation of proline and glycine betaine [64]. Relatedly, silicon (Si)-based biostimulants have been shown to prime wheat seedlings against salt stress by reducing the accumulation of sodium ions, thus improving salt stress tolerance [65]. In another study by Zhang [66], drought priming was also shown to potentiate the defense response against heat stress in tall fescue (Festuca arundinacea) via lipidome reconfiguration. Furthermore, Shehu et al. [67] reviewed the priming effects of β-aminobutyric acid (BABA) against abiotic stresses such as salt stress, drought, nutrient stress and heavy metal stress; some of the general physiological, biochemical and molecular alterations potentiated by BABA included improved stomatal regulation and photosynthesis, water use efficiency, cell membrane remodeling, enhanced ROS detoxification and stress-related gene expression. The molecular and cellular events underlying the priming phenomenon may differ and/or overlap depending on the priming stimuli and the secondary stress [68]. Despite the exponentially increasing efforts to elucidate the metabolic changes that define the priming events in time and space, there is still much to uncover in order to understand this potentiation of plant protective defenses. The comprehensive molecular and biochemical networks involved in the establishment of priming stages are still far from being fully characterized. Regardless of the shortcomings and limitations as stated above, defense priming is undoubtedly one of the key adaptive strategies employed by nature. Thus, this preconditioning of plant defense systems is considered a prospective and complementary alternative means that offers new avenues for plant resistance against environmental stresses.
3. Biostimulants as Agronomic Tools to Promote Plant Growth and Counteract Abiotic Stresses
In the last decade, there has been exponentially-growing attention to plant biostimulants as a potential solution to mitigate the negative impacts of the changing climate on agriculture, and becoming one of the pillars of a new agricultural revolution for sustainable food production. Even though the concept of biostimulants surfaced in 1933, only in more recent years has attention and studies from different fields emerged to define, describe and understand plant biostimulants, as well as their modes of action [10][69]. Historically, the definition of "biostimulants" has evolved and been reformulated over the years, although it has often been poorly described due to a lack of a scientifically-based theoretical foundation for the conceptualization and characterization of these materials that show potential in improving plant health and development. The recent review by Yakhin et al. [10] provides a detailed account and a chronological evolution of the concept of the term biostimulant. Currently, a biostimulant is conceptually defined as any substance or microorganism that is not a nutrient, pesticide or any of the soil improvers, but has the ability to promote the health and growth of a plant through the induction of natural biological processes [10][70][71].
4. Omics Sciences to Study Plant Biology in Abiotic Stress Conditions
The application of omics approaches such as genomics, transcriptomics, proteomics and metabolomics (Figure 3) have proven to be instrumental in the investigation and identification of the key multilayered biochemical events and mechanisms underpinning the effects of biostimulant formulations on plants’ physiology. For instance, a previous transcriptomics study reported that the use of a novel technology, next generation sequencing (NGS), allowed the monitoring of the impact of biostimulants on the transcriptome of plants, thus revealing the molecular mechanisms of action by which the biostimulant enhanced growth promotion in corn and soybean. The mechanisms of action elucidated in corn included the upregulation of nitrogen and phosphate assimilation and metabolism, maltose biosynthesis, sugar transport and phloem loading and hormone (cytokinin) metabolism, whereas in soybean, growth enhancing metabolic processes that were upregulated included nitrogen metabolism, sulfate reduction, metal and ion transport and amino acid biosynthesis [72].
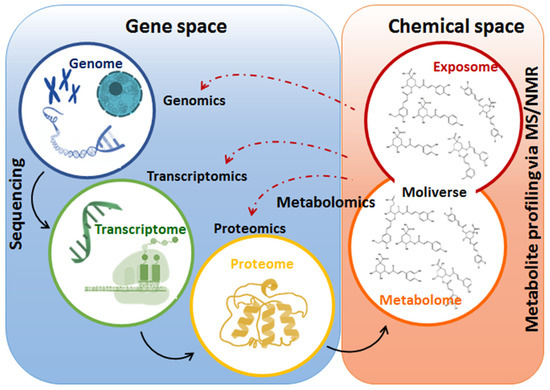
Figure 3. Summary of the omics disciplines comprising systems biology. The figure illustrates the flow of biological information from gene to metabolome, highlighting the interconnection of gene and chemical spaces of a biological system. In a stressful event, gene expression is altered, and chemical spaces are reprogrammed to maintain cellular and molecular life-defining equilibria. At the metabolome level, changes in the levels of primary and secondary metabolites play an important role in the readjustment of cellular metabolism towards adaptation to various environmental conditions.
As exemplified above, transcriptomics studies can contribute to the ongoing scientific efforts to provide fundamental research insights that describe the mechanisms and modes of action of biostimulants. However, these studies present challenges such as the fact that an increase in the levels of mRNA does not always correlate with protein levels and not all the translated proteins are enzymatically active. Furthermore, the outcome of the transcriptome and proteome profiling can be limited by the identification of mRNA and proteins which depends on organism-specific genome information. Thus, due to these limitations, changes in the transcriptome or proteome level do not necessarily correspond to the alteration in biochemical phenotypes and may not accurately reflect the biochemical status of the plant in response to abiotic stresses and biostimulants [73]. Thus, an integration of upper omics levels in the systems biology, with metabolomics studies (Figure 3), can provide a holistic and comprehensive understanding of the molecular mechanisms of actions mediated by biostimulants to mitigate abiotic stresses.
This entry is adapted from the peer-reviewed paper 10.3390/metabo10120505
References
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, doi:10.1038/ncomms1926.
- Stael, S.; Rocha, A.G.; Robinson, A.J.; Kmiecik, P.; Vothknecht, U.C.; Teige, M. Arabidopsis calcium-binding mitochondrial carrier proteins as potential facilitators of mitochondrial ATP-import and plastid SAM-import. FEBS Lett. 2011, 585, 3935–3940, doi:10.1016/j.febslet.2011.10.039.
- Balmer, A.; Pastor, V.; Glauser, G.; Mauch-Mani, B. Tricarboxylates induce defense priming against bacteria in Arab. Thaliana. Front. Plant Sci. 2018, 9, 1–15, doi:10.3389/fpls.2018.01221.
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The “prime-ome”: Towards a holistic approach to priming. Trends Plant Sci. 2015, 20, 443–452, doi:10.1016/j.tplants.2015.04.002.
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 1–4, doi:10.3389/fpls.2014.00642.
- Westman, S.M.; Kloth, K.J.; Hanson, J.; Ohlsson, A.B.; Albrectsen, B.R. Defence priming in Arabidopsis—A Meta-Analysis. Sci. Rep. 2019, 9, 1–13, doi:10.1038/s41598-019-49811-9.
- Martinez-medina, A.; Flors, V.; Heil, M.; Mauch-mani, B.; Pieterse, C.M.J. Recognizing plant defense priming. Trends Microbiol. 2016, 21, 818–822.
- Tugizimana, F.; Djami-tchatchou, A.T.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic analysis of defense-related reprogramming in Sorghum bicolor in response to Colletotrichum sublineolum infection reveals a functional metabolic web of phenylpropanoid and flavonoid pathways. Front. Plant Sci. 2019, 9, 1–20, doi:10.3389/fpls.2018.01840.
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.B.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants enhance growth and drought tolerance in Arabidopsis thaliana and exhibit chemical priming action. Ann. Appl. Biol. 2019, 174, 153–165, doi:10.1111/aab.12482.
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 1–32, doi:10.3389/fpls.2016.02049.
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 1–18, doi:10.3389/fpls.2019.00493.
- Wu, L.; Gao, X.; Xia, F.; Joshi, J.; Borza, T.; Wang-Pruski, G. Biostimulant and fungicidal effects of phosphite assessed by GC-TOF-MS analysis of potato leaf metabolome. Physiol. Mol. Plant Pathol. 2019, 106, 49–56, doi:10.1016/j.pmpp.2018.12.001.
- Tugizimana, F.; Piater, L.; Dubery, I. Plant metabolomics: A new frontier in phytochemical analysis. S. Afr. J. Sci. 2013, 109, 1–11, doi:10.1590/ sajs.2013/20120005.
- Rosato, A.; Tenori, L.; Cascante, M.; De Atauri Carulla, P.R.; Martins dos Santos, V.A.P.; Saccenti, E. From correlation to causation: Analysis of metabolomics data using systems biology approaches. Metabolomics 2018, 14, 1–20, doi:10.1007/s11306-018-1335-y.
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Labuschagne, N.; Dubery, I.A. Unravelling the metabolic reconfiguration of the post-challenge primed state in Sorghum bicolor responding to Colletotrichum sublineolum infection. Metabolites 2019, 9, 1–25, doi:10.3390/metabo9100194.
- Beisken, S.; Eiden, M.; Salek, R.M. Getting the right answers: Understanding metabolomics challenges. Expert Rev. Mol. Diagn. 2015, 15, 97–109, doi:10.1586/14737159.2015.974562.
- Szecowka, M.; Heise, R.; Tohge, T.; Nunes-Nesi, A.; Vosloh, D.; Huege, J.; Feil, R.; Lunn, J.; Nikoloski, Z.; Stitt, M.; et al. Metabolic fluxes in an illuminated Arabidopsis rosette. Plant Cell 2013, 25, 694–714, doi:10.1105/tpc.112.106989.
- Rodríguez-Calzada, T.; Qian, M.; Strid, Å.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 134, 94–102, doi:10.1016/j.plaphy.2018.06.025.
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324, doi:10.1016/j.cell.2016.08.029.
- Nobori, T.; Tsuda, K. The plant immune system in heterogeneous environments. Curr. Opin. Plant Biol. 2019, 50, 58–66, doi:10.1016/j.pbi.2019.02.003.
- Ramirez-Prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M.; Hirt, H. Plant immunity: From signaling to epigenetic control of defense. Trends Plant. Sci. 2018, 23, 833–844, doi:10.1016/j.tplants.2018.06.004.
- Gust, A.A.; Pruitt, R.; Nürnberger, T. Sensing danger: Key to activating plant immunity. Trends Plant. Sci. 2017, 22, 779–791, doi:10.1016/j.tplants.2017.07.005.
- Sanabria, N.M.; Huang, J.C.; Dubery, I.A. Self/nonself perception in plants in innate immunity and defense. Self/Nonself—Immune Recognit. Signal. 2010, 1, 40–54, doi:10.4161/self.1.1.10442.
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051, doi:10.1093/aob/mcv054.
- Ádám, A.L.; Nagy, Z.; Kátay, G.; Mergenthaler, E.; Viczián, O. Signals of systemic immunity in plants: Progress and open questions. Int. J. Mol. Sci. 2018, 19, 1–21, doi:10.3390/ijms19041146.
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329, doi:10.1038/nature05286.
- Yu, X.; Feng, B.; He, P.; Shan, L. From chaos to harmony: Responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol. 2017, 55, 109–137, doi:10.1146/annurev-phyto-080516-035649.
- Conrath, U. Systemic acquired resistance. Plant. Signal. Behav. 2006, 1, 179–184, doi:10.1016/0091-3057(88)90118-9.
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant. Physiol. 2012, 158, 844–853, doi:10.1104/pp.111.187468.
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant. Biol. 2011, 11, 1–14, doi:10.1186/1471-2229-11-163.
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant. Sci. 2017, 8, 1–16, doi:10.3389/fpls.2017.01147.
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant. Biol. 2003, 30, 239–264, doi:10.1071/FP02076.
- Azevedo, R.A.; Gratão, P.L.; Monteiro, C.C.; Carvalho, R.F. What is new in the research on cadmium-induced stress in plants? Food Energy Secur. 2012, 1, 133–140, doi:10.1002/fes3.10.
- Redondo-Gómez, S. Abiotic and biotic stress tolerance in plants. In Molecular Stress Physiology of Plants; Shanker, A., Venkateswarlu, B., Eds.; London, UK, IntechOpen: 2013; pp. 1–20, ISBN 9788132208075.
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant. Sci. 2016, 7, 1–26, doi:10.3389/fpls.2016.00571.
- Saud, S.; Li, X.; Chen, Y.; Zhang, L.; Fahad, S.; Hussain, S.; Sadiq, A.; Chen, Y. Silicon application increases drought tolerance of Kentucky bluegrass by improving plant water relations and morphophysiological functions. Sci. World J. 2014, 2014, 1–10, doi:10.1155/2014/368694.
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and physiological plant responses to drought stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 1–8, doi:10.1155/2016/4165750.
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 1–12, doi:10.1038/s41598-019-40362-7.
- Nahar, K.; Hasanuzzaman, M.; Fujita, M. Roles of osmolytes in plant adaptation to drought and salinity. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Eds.; Springer: Chennai, India, 2015; pp. 1–170, ISBN 9788132226161.
- Hsiao, T.C.; Xu, L.K. Sensitivity of with of roots versus leaves to water stress: Biophysical analysis and relation to water. J. Exp. Bot. 2000, 51, 1595–1616, doi:10.1093/jexbot/51.350.1595.
- Xiong, L.; Wang, R.G.; Mao, G.; Koczan, J.M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant. Physiol. 2006, 142, 1065–1074, doi:10.1104/pp.106.084632.
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240, doi:10.1093/jxb/ert375.
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13, doi:10.3389/fenvs.2014.00053.
- García-Martí, M.; Piñero, M.C.; García-Sanchez, F.; Mestre, T.C.; López-Delacalle, M.; Martínez, V.; Rivero, R.M. Amelioration of the oxidative stress generated by simple or combined abiotic stress through the K+ and Ca2+ supplementation in tomato plants. Antioxidants 2019, 8, 81, doi:10.3390/antiox8040081.
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant. J. 2017, 90, 856–867, doi:10.1111/tpj.13299.
- Tuteja, N.; Gill, S.S.; Tuteja, R. Plant responses to abiotic stresses: Shedding light on salt, drought, cold and heavy metal stress. In Omics and plant abiotic stress tolerance; Tuteja, N., Gill, S.S., Tuteja, R., Eds.; Bentham Science Publishers: Sharjah, UAE, 2011; pp. 39–64, ISBN 9781608053841.
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant. Sci. 2018, 871, 1–18, doi:10.3389/fpls.2018.01771.
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177, doi:10.1016/j.envexpbot.2017.05.002.
- Arbona, V.; Flors, V.; Jacas, J.; García-Agustín, P.; Gómez-Cadenas, A. Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, a salt-sensitive citrus rootstock, to different levels of salinity. Plant. Cell Physiol. 2003, 44, 388–394, doi:10.1093/pcp/pcg059.
- De Pinto, M.C.; De Gara, L. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J. Exp. Bot. 2004, 55, 2559–2569, doi:10.1093/jxb/erh253.
- Kharusi, L. Al; Yahyai, R. Al; Yaish, M.W. Antioxidant response to salinity in salt-tolerant and salt-susceptible cultivars of date palm. Agriculture 2019, 9, 1–17, doi:10.3390/agriculture9010008.
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830, doi:10.1242/jeb.01730.
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant. Physiol. 2011, 155, 2–18, doi:10.1104/pp.110.167569.
- da Silva, E.C.; de Albuquerque, M.B.; de Azevedo Neto, A.D.; da Silva Junior, C.D. Drought and its consequences to plants – From individual to ecosystem. In Responses of Organisms to Water Stress; Akıncı, S., Ed.; London, UK, IntechOpen: 2013; pp. 18–47, doi:10.5772/53833.
- Saijo, Y.; Loo, E.P. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2019, 225, 87–104, doi:10.1111/nph.15989.
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43, doi:10.1111/nph.12797.
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360, doi:10.1016/j.soilbio.2019.05.011.
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant. Sci. 2018, 23, 25–41, doi:10.1016/j.tplants.2017.09.003.
- Conrath, U. Molecular aspects of defence priming. Trends Plant. Sci. 2011, 16, 524–531, doi:10.1016/j.tplants.2011.06.004.
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant. Sci. 2018, 9, 1–17, doi:10.3389/fpls.2018.00112.
- Kanjariya, K.G.; Parihar, A. Prime-ome: A molecular approach towards defence mechanisms. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3606–3610, doi:10.20546/ijcmas.2017.608.433.
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant. Biol. 2017, 68, 485–512, doi:10.1146/annurev-arplant-042916-041132.
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, doi:10.1146/annurev-phyto-080614-120132.
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T. Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front. Plant Sci. 2018, 9, 1–13, doi:10.3389/fpls.2018.00261.
- Azeem, M.; Iqbal, N.; Kausar, S.; Javed, M.T.; Akram, M.S.; Sajid, M.A. Efficacy of silicon priming and fertigation to modulate seedling’s vigor and ion homeostasis of wheat (Triticum aestivum L.) under saline environment. Environ. Sci. Pollut. Res. 2015, 22, 14367–14371, doi:10.1007/s11356-015-4983-8.
- Zhang, X. Lipidomic reprogramming associated with drought stress priming—Enhanced heat tolerance in tall fescue (Festuca arundinacea). Plant. Cell Environ. 2019, 42, 947–958, doi:10.1111/pce.13405.
- Shehu, A.A.; Alsamadany, H.; Alzahrani, Y. β -Aminobutyric acid ( BABA ) priming and abiotic stresses : A review. Int. J. Biosci. 2019, 14, 450–459.
- Tugizimana, F.; Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. Metabolomics in plant priming research: The way forward? Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19061759.
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant. Sci. 2018, 9, 1–17, doi:10.3389/fpls.2018.01473.
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14, doi:10.1016/j.scienta.2015.09.021.
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant. Sci. 2019, 10, 1–8, doi:10.3389/fpls.2019.00494.
- Briglia, N.; Petrozza, A.; Hoeberichts, F.A.; Verhoef, N.; Povero, G. Investigating the impact of biostimulants on the row crops corn and soybean using high-efficiency phenotyping and next generation sequencing. Agronomy 2019, 9, 1–15, doi:10.3390/agronomy9110761.
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in plant stress physiology. In Advances in Biochemical Engineering/Biotechnology; Scheper, T., Ed.; Cham, Switzerland, Springer International Publishing: 2018; Volume 164, pp. 187–236, doi:10.1007/10_2017_55.
