Organic cation transporters (OCT) 1, 2 and 3 and novel organic cation transporters (OCTN) 1 and 2 of the solute carrier 22 (SLC22) family are involved in the cellular transport of endogenous compounds such as neurotransmitters, l-carnitine and ergothioneine. OCT/Ns have also been implicated in the transport of xenobiotics across various biological barriers, for example biguanides and histamine receptor antagonists. In addition, several drugs used in the treatment of respiratory disorders are cations at physiological pH and potential substrates of OCT/Ns. OCT/Ns may also be associated with the development of chronic lung diseases such as allergic asthma and chronic obstructive pulmonary disease (COPD) and, thus, are possible new drug targets.
- pulmonary drug delivery
- SLC22A1–5
- lung epithelium
- drug uptake
- β2-agonists
- chronic lung diseases
- anticholinergics
1. Introduction
Organic cation transmembrane transporters belonging to the solute carrier family 22 (i.e., SLC22A1–A5) are increasingly recognised as "impactors" of drug disposition in the respiratory tract [1][2][3]. SLC22A1–A5 transporters can be further divided according to the driving force of cation transport into membrane-potential-sensitive organic cation transporters (OCTs) or Na+ and pH-dependent novel organic cation transporters (OCTNs) [4][5]. The human OCT subclass consists of OCT1 (SLC22A1), OCT2 (SLC22A2) and OCT3 (SLC22A3), whereas the OCTN subclass includes OCTN1 (SLC22A4) and OCTN2 (SLC22A5). According to the human protein atlas, hOCT1 is a 61 kDa protein exhibiting a broad tissue distribution with high expression levels in the liver [6][7]. hOCT2, with a less ubiquitous expression pattern than hOCT1 and hOCT3, is most strongly expressed in the kidneys with an approximate molecular size of 63 kDa [6][8]. The tissue expression pattern of OCT3 (61 kDa) is very broad, with high levels being observed in the liver, skeletal muscle, placenta and heart [8][9]. OCTN1 and OCTN2 also exhibit broad tissue distribution, and both are approximately 62 kDa proteins [8][9]. OCT/N transporters participate in the cellular transport of a broad spectrum of endogenous and exogenous organic cations and zwitterions such as neurotransmitters and xenobiotics. Thus, they are involved in clinical drug–transporter interactions and at the same time, they perform important physiological functions [9][10]. OCT1, for example mediates thiamine uptake, modulates hepatic glucose and lipid metabolism and thereby plays a role in hepatic steatosis [11][12]. In the brain, OCT2 and OCT3 are involved in the regulation of a variety of normal central nervous system functions related to mood as well as salt-intake behaviour and osmoregulation [13][14][15]. Detailed information on OCTs' expression and function in organs (except for the lung) was recently reviewed and can be found in this Special Issue of International Journal of Molecular Sciences [16]. With a focus on the lung, reviews by Salomon et al. [2] and Nickel et al. [3] have provided comprehensive overviews of pulmonary OCT/Ns' expression and function to the reader. Generally, OCTs in airway epithelial and smooth muscle cells accept physiological substrates such as dopamine, histamine, serotonin and acetylcholine. OCTN1 and OCTN2 mediate the uptake of ergothioneine (ESH) and l-carnitine, respectively.
2. Expression and Subcellular Localisation of OCT/Ns in Lung-Derived Cell Lines, Pulmonary Cell Cultures and Lung Tissues in Health and Disease
The expression of OCT/Ns in the lung has been studied in several cell lines of human respiratory epithelial origin (e.g., A549, NCl-H441, BEAS-2B, Calu-3 and 16HBE14o-), in primary airway epithelial cells and in lung tissues on the mRNA and protein level [17][18][19][20][21][22][23], and has been reviewed comprehensively in our pervious publication [3]. There is some consensus that OCT1, OCT3, OCTN1 and OCTN2 are found ubiquitously throughout the lung epithelium. OCT2 expression is more controversial. The transporter was absent in many of lung-derived cell lines [17][18][23][24][25][26], except for NCl-H441 [20][27]. In the case of primary cultures of human tracheal, bronchial and alveolar epithelial cells and in human whole-lung tissue, a lot of conflicting data have been published [17][19][21][23][25][28][29].
The regional expression and subcellular localisation of OCT/Ns in the airways remains, to some extent, elusive. Immunohistochemistry (IHC) studies carried out in human bronchi revealed positive OCT1 and OCT2 staining in the apical membrane of ciliated epithelial cells, intracellular OCT1 staining in ciliated epithelial cells, and OCT3 staining in the basolateral membrane of intermediate cells and the entire plasma membrane of basal cells [28]. OCT3 transcript and protein expression were confirmed in primary human bronchial and vascular smooth muscle cells [30]. Positive OCTN1 and OCTN2 stainings were observed on the apical and the lateral membranes of human primary bronchial epithelial cells [31]. OCTN1 showed the strongest expression of all OCT/Ns in bronchi but was found to be expressed at a lower degree in peripheral lung tissue [32]. Data are still scarce on cell-type-specific expression of OCT/Ns, and hence it is rather difficult to identify the physiological function of each transporter protein. We highlighted this issue in another review in 2016 [3], however, the field has not advanced significantly since then.
A limited number of studies have been published connecting OCT/N expression to lung diseases. Genome-wide association studies (GWAS) have identified SLC22A5 variants being linked to primary systemic carnitine deficiency and asthma [33][34]. Furthermore, due to its potential role in histamine release and clearance in the airways, an association between SLC22A3 gene polymorphisms and the severity of asthma has been proposed [35][36]. No differences in mRNA expression levels of OCT1, OCT3, OCTN1 and OCTN2 between ex-smokers with a severe stage of COPD and healthy subjects were reported in a study by Berg and colleagues [32]. Subsequently, the same group's IHC analysis confirmed OCTN1 and OCTN2 expression in the epithelial cells of the bronchi, bronchioles and alveolar type II epithelial cells as well as in alveolar macrophages. However, no differences between COPD and healthy subjects were visible in IHC [31]. It should be noted that neither was immunoblot analysis carried out nor were IHC signal intensities measured in order to quantify potential differences in protein expression between the two groups. In another study, Calu-3 cells were grown for 21 days under air-interfaced culture (AIC) conditions and exposed to pro-inflammatory lipopolysaccharide (LPS) or house dust mite extract (HDM) to simulate asthmatic-like conditions at the epithelium in vitro [37]. The LPS challenge significantly upregulated the expression of OCT1, OCT3, OCTN1 and OCTN2 on mRNA and protein levels. HDM had similar effects on OCT1, OCT3 and OCTN2 mRNA and protein expression. However, when Calu-3 cells grown for the same duration and under similar conditions were exposed to LPS for shorter periods of time, other researchers did not observe any change in mRNA levels of OCT1, OCT3 [24] or OCTN2 [38]. Stimulation with tumour necrosis factor-α (TNF-α) and the Th2-cytokine, IL-4 also did not result in any change in OCT1, OCT3 [24] and OCTN2 mRNA levels [38]. Regretfully, in neither study, were mRNA expression data supported by protein expression analysis. In contrast, when alveolar A549 cells were exposed to similar LPS concentrations, mRNA and protein levels of OCTN1 and OCTN2 were downregulated, which was accompanied by a significant reduction in the uptake of the model substrate 4-[4-(dimethylamino)-styryl]-N-methylpyridinium (ASP+) [39]. Likewise, challenging A549 cells with cigarette smoke extract (CSE), LPS or both caused a significant reduction in OCTN1 and OCTN2 mRNA expression levels [40]. It has been demonstrated that A549 and BEAS-2B cells respond distinctly, in terms of cytokine release, to LPS stimulation [41]. The latter may suggest different pathways involved in alveolar and bronchial epithelial cells upon LPS challenge and, together with different LPS exposure times used in the above-mentioned studies, may explain the discrepancies in OCT/N expression regulation observed in response to LPS stimulation in Calu-3 and A549 cells. Overall, some promising first results suggest that there indeed could be differences in OCT/N expression (and function) in healthy vs. diseased lungs, which needs to be further investigated using appropriate in vitro and experimental animal models as well as lung tissue specimens from patients with respiratory diseases.
OCT/N expression and subcellular localisation in the lungs need to be conclusively studied. For example, cell surface protein biotinylation should be performed to obtain clear-cut subcellular localisation data for OCT/Ns. However, it is essential to use high-quality antibodies, which might have to be generated first. Proteomics profiling will help not only to quantify expression levels of OCT/Ns but also to look into the association between molecular pathways of OCT/Ns in the lung, in health and disease [42]. Lastly, single-cell analysis approaches will allow to sort cell populations of the lung and to determine the expression of OCT/Ns in individual cells (i.e., epithelial vs. immune cells). In this context, first evidence was given that alveolar macrophages also express OCTN1 and OCTN2 [31].
3. OCT/Ns (SLC22A1–A5) Transporter Function in Lung Physiology and Pathophysiology
Despite being involved in the transport of essential endogenous substrates (Figure 1), little information is available about the role of OCT/Ns in physiological functions and under pathological conditions in the lung. Uncovering (patho)physiological functions of OCT/Ns in the lung is a key step in developing new drug therapies for respiratory disorders [10][43]. Molecular investigations are necessary to further identify and validate potential endogenous substrates of OCT/Ns. Transport studies of substrate candidates are traditionally performed utilising radioactive isotopes or liquid chromatography–mass spectrometry. Potentially, a novel assay that detects shifts in thermostability of the transporter protein in the presence of specific substrates may be employed to detect transporter–substrate interactions of lung OCT/Ns [44]. The main advantage of this assay is that it allows large-scale screening to identify candidate substrates from libraries of unlabelled compounds. However, the assay cannot discriminate between substrates, inhibitors or competitors and requires purified proteins. Novel approaches such as transporter tandems might also help to assess the influence of gene polymorphisms on OCT/Ns functional activity and to determine their (patho)physiological consequences in the lung [45].
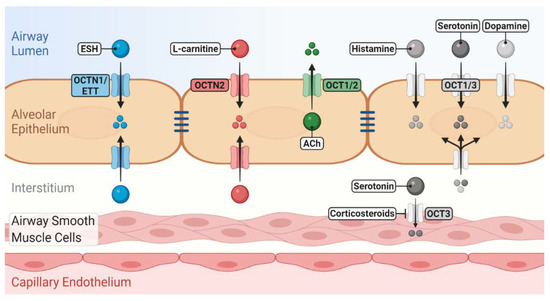
Figure 1. Role of novel/organic cation transporters (OCT/Ns) in pulmonary disposition of endogenous substrates. OCTN1/ergothioneine (ESH) transporter (ETT) mediates the uptake of ESH, which possesses antioxidant and anti-inflammatory properties. OCTN2 mediates l-carnitine uptake, which is essential for cellular energy production. OCTs may participate in the uptake/release of a number of neurotransmitters into/from airway epithelial and smooth muscle cells. Many of these induce bronchoconstriction, regulate mucus secretion and clearance and are linked to the pathophysiology of asthma. The localisation of OCT/Ns, whether apical or basolateral in airway epithelium, is still elusive.
4. Pharmacological Aspects of OCT/N Transporters in the Lung
4.1. Interaction of OCT/N Transporters with Inhaled Drugs
Inhaled bronchodilator drugs must pass through the airway epithelial barrier to reach their target receptors in the underlying airway smooth muscle cells. Many of these drugs belonging to the muscarinic receptor antagonists and β2-agonists are cations and are positively charged at physiological pH-values of the lung lining fluid. Thus, OCT/Ns may play a potential role in their pulmonary disposition, pharmacokinetics, safety and efficacy profile. The involvement of OCT/N transporters in interactions with inhaled drugs has been intensively discussed in previous reviews [2][3].
The role of OCT/Ns in the pulmonary disposition of inhaled drugs is an ongoing research topic; however, data are mainly limited to in vitro studies. Despite being essential for initial screenings of inhaled drugs' interactions with membrane transporters, in vitro studies are insufficient to confirm the clinical significances of such interactions, and a number of challenges and disadvantages are associated with them. First, an ideal in vitro lung epithelial model for drug disposition studies still does not exist. Second, the different cell composition of proximal and distal lung epithelium may result in different OCT/N expression and activity profiles [17][46]. Third, determination of inhaled drug concentrations in the epithelial lining fluid following drug inhalation is extremely difficult because of the complex anatomical nature of the lung [25][47]. Thus, the concentrations of inhaled drugs applied in in vitro studies to assess their interaction with OCT/N transporters may be clinically irrelevant. The concentration of drugs used in in vitro studies is of particular importance because it influences the ratio of passive membrane diffusion and transporter-mediated uptake [9]. Generally, when high concentrations are used, passive membrane permeation predominates resulting in an underestimation of the transporter impact [9]. In vitro data may, therefore, not predict the in vivo airway-to-blood absorption process accurately and should be carefully considered. Validation can be achieved by ex vivo, in vivo and clinical studies [10]. Recent reviews have covered preclinical models (in vitro, ex vivo and in vivo) that are currently implemented in pulmonary drug delivery studies [48][49]. Moreover, new experimental in silico models such as Mimetikos Preludium™ (Emmace Consulting) and SimCyp Simulator™ (Certara) can be used to estimate the regional absorption of inhaled drugs [50][51]. It is necessary to point out that such in silico models may fail to precisely describe the pulmonary pharmacokinetics of drugs because the validity of the simulation depends on the quality (and quantity) of data used to inform the model. These data, however, are scarce and were generated under different experimental conditions. Moreover, none of these models has been specifically designed for use in pulmonary drug delivery. Nonetheless, in silico models might prove useful to assess the impact of OCT/Ns in pulmonary disposition of inhaled drugs, once sufficient and reliable in vitro and in vivo data have been generated to inform them.
4.2. OCTN2 as a Target to Enhance Pulmonary Drug Delivery
Conjugation of drugs or nanodrug delivery systems with a specific transporter's substrate to promote drug transfer across biological barriers has emerged as a strategy to improve drug delivery [52][53]. OCTN2-targeted nanodrug delivery systems have been successfully used to enhance the oral bioavailability of nanoparticles [54]. As far as the pulmonary drug delivery is concerned, Mo and colleagues synthesised a carnitine ester prodrug of prednisolone (i.e., prednisolone succinate-l-carnitine (PDSC)). The uptake of the prodrugs into BEAS-2B cells was enhanced and could be inhibited by l-carnitine, indicating it was an OCTN2-mediated process. The prodrug displayed improved duration of action with the free prednisolone being slowly released inside the cells resulting in longer suppression of LPS-induced release of IL-6 by BEAS-2B cells in vitro [55]. In a follow-up study, the asthmatic guinea pig model was treated with the prodrug and showed less severe vascular pathologies, restricted asthma induced airway thickenings and lower inflammatory cell count in bronchoalveolar fluid when compared to animals treated with unconjugated prednisolone [56]. However, a link to alterations in OCTN2 expression levels was not investigated in this model.
OCTN2 may be a potential target to enhance the pulmonary delivery of inhaled drugs to achieve a better therapeutic outcome. As mentioned before, l-carnitine itself has antioxidant properties and may confer additional beneficial effects when conjugated with inhaled drugs used for the treatment of respiratory disorders such as asthma and COPD (Figure 2).
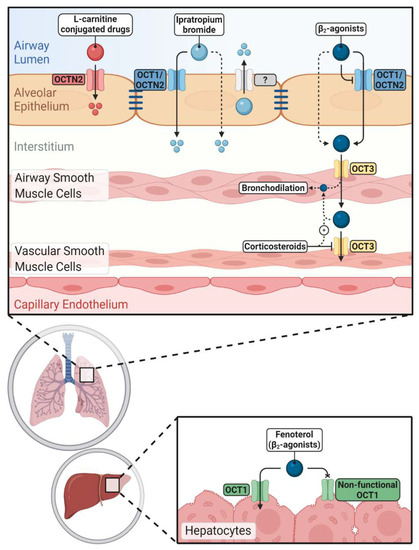
Figure 2. Impacts of novel/organic cation transporters (OCT/Ns) on pulmonary and systemic disposition of inhaled drugs. Inhaled cationic β2-agonists' epithelial transport is mediated either by OCT/Ns (mainly OCT1 and OCTN2) or passive diffusion. Corticosteroids can increase the airway retention of β2-agonists by inhibiting OCT3 in vascular smooth muscle cells. Hepatic clearance of fenoterol is reduced in carriers of non-functional OCT1 alleles. Ipratropium bromide uptake via airway epithelium is more complex and OCT/Ns, unidentified efflux transporters and passive diffusion are participating. OCTN2 mediates the uptake of l-carnitine-conjugated prodrugs and nanoparticles into pulmonary epithelial cells improving pulmonary delivery of the parent drugs.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21239168
References
- Bosquillon, C. Drug transporters in the lung—Do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 2010, 99, 2240–2255, doi:10.1002/jps.21995.
- Salomon, J.J.; Ehrhardt, C. Organic cation transporters in the blood–air barrier: Expression and implications for pulmonary drug delivery. Ther. Deliv. 2012, 3, 735–747, doi:10.4155/tde.12.51.
- Nickel, S.; Clerkin, C.G.; Selo, M.A.; Ehrhardt, C. Transport mechanisms at the pulmonary mucosa: Implications for drug delivery. Expert Opin. Drug Deliv. 2016, 13, 667–690, doi:10.1517/17425247.2016.1140144.
- Engelhart, D.; Granados, J.C.; Shi, D.; Saier, M.H.; Baker, M.E.; Abagyan, R.; Nigam, S.K.; Saier, M.H. Systems Biology Analysis Reveals Eight SLC22 Transporter Subgroups, Including OATs, OCTs, and OCTNs. Int. J. Mol. Sci. 2020, 21, 1791, doi:10.3390/ijms21051791.
- Selo, M.A., Al-Alak, H.H.; Ehrhardt, C. Lung transporters and absorption mechanisms in the lungs. In Inhalation Aerosols: Physical and Biological Basis for Therapy, 3rd ed., Hickey, A.J., Mansour, H.M., Eds.; CRC press: New York, NY, USA, 2019; Volume 1, pp. 57–70.
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific Organic Cation Transporters: Structure, Function, Physiological Roles, and Biopharmaceutical Implications. Pharm. Res. 2007, 24, 1227–1251, doi:10.1007/s11095-007-9254-z.
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250, doi:10.1038/nbt1210-1248.
- The Human Protein Atlas 19 November 2020. Available online: https://www.proteinatlas.org/ (accessed on 23 November 2020).
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319, doi:10.1124/pr.118.015578.
- The International Transporter Consortium; International Transporter Consortium; Giacomini, K.M.; Huang, S.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236, doi:10.1038/nrd3028.
- Chen, L.; Yee, S.W.; Giacomini, K.M. OCT1 in hepatic steatosis and thiamine disposition. Cell Cycle 2015, 14, 283–284, doi:10.1080/15384101.2015.1006532.
- Chen, L.; Shu, Y.; Liang, X.; Chen, E.C.; Yee, S.W.; Zur, A.A.; Li, S.; Xu, L.; Keshari, K.R.; Lin, M.J.; et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc. Natl. Acad. Sci. USA 2014, 111, 9983–9988, doi:10.1073/pnas.1314939111.
- Bacq, A.; Balasse, L.; Biala, G.; Guiard, B.P.; Gardier, A.; Schinkel, A.; Louis, F.; Vialou, V.; Martres, M.-P.; Chevarin, C.; et al. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol. Psychiatry 2012, 17, 926–939, doi:10.1038/mp.2011.87.
- Couroussé, T.; Gautron, S. Role of organic cation transporters (OCTs) in the brain. Pharmacol. Ther. 2015, 146, 94–103, doi:10.1016/j.pharmthera.2014.09.008.
- Vialou, V.V.; Amphoux, A.; Zwart, R.; Giros, B.; Gautron, S. Organic Cation Transporter 3 (Slc22a3) Is Implicated in Salt-Intake Regulation. J. Neurosci. 2004, 24, 2846–2851, doi:10.1523/JNEUROSCI.5147-03.2004.
- Samodelov, S.L.; Kullak-Ublick, G.A.; Gai, Z.; Visentin, M. Organic Cation Transporters in Human Physiology, Pharmacology, and Toxicology. Int. J. Mol. Sci. 2020, 21, 7890, doi:10.3390/ijms21217890.
- Endter, S.; Francombe, D.; Gumbleton, M.; Ehrhardt, C. RT?PCR analysis of ABC, SLC and SLCO drug transporters in human lung epithelial cell models. J. Pharm. Pharmacol. 2009, 61, 583–591, doi:10.1211/jpp/61.05.0006.
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional characterization of the organic cation transporters (OCTs) in human airway pulmonary epithelial cells. Biochim. Biophys. Acta 2015, 1848, 1563–1572, doi:10.1016/j.bbamem.2015.04.001.
- Horvath, G.; Schmid, N.; Fragoso, M.A.; Schmid, A.; Conner, G.E.; Salathe, M.; Wanner, A. Epithelial Organic Cation Transporters Ensure pH-Dependent Drug Absorption in the Airway. Am. J. Respir. Cell Mol. Biol. 2007, 36, 53–60, doi:10.1165/rcmb.2006-0230oc.
- Sakamoto, A.; Matsumaru, T.; Yamamura, N.; Suzuki, S.; Uchida, Y.; Tachikawa, M.; Terasaki, T. Drug Transporter Protein Quantification of Immortalized Human Lung Cell Lines Derived from Tracheobronchial Epithelial Cells (Calu-3 and BEAS2-B), Bronchiolar–Alveolar Cells (NCI-H292 and NCI-H441), and Alveolar Type II-like Cells (A549) by Liquid Chromatography–Tandem Mass Spectrometry. J. Pharm. Sci. 2015, 104, 3029–3038, doi:10.1002/jps.24381.
- Sakamoto, A.; Matsumaru, T.; Yamamura, N.; Uchida, Y.; Tachikawa, M.; Ohtsuki, S.; Terasaki, T. Quantitative expression of human drug transporter proteins in lung tissues: Analysis of regional, gender, and interindividual differences by liquid chromatography–tandem mass spectrometry. J. Pharm. Sci. 2013, 102, 3395–3406, doi:10.1002/jps.23606.
- Salomon, J.J.; Gausterer, J.C.; Selo, M.A.; Hosoya, K.-I.; Huwer, H.; Schneider-Daum, N.; Lehr, C.-M.; Ehrhardt, C. OCTN2-Mediated Acetyl-l-Carnitine Transport in Human Pulmonary Epithelial Cells In Vitro. J. Pharm. Sci. 2019, 11, 396, doi:10.3390/pharmaceutics11080396.
- Courcot, E.; Leclerc, J.; Lafitte, J.-J.; Mensier, E.; Jaillard, S.; Gosset, P.; Shirali, P.; Pottier, N.; Broly, F.; Lo-Guidice, J.-M. Xenobiotic Metabolism and Disposition in Human Lung Cell Models: Comparison with In Vivo Expression Profiles. Drug Metab. Dispos. 2012, 40, 1953–1965, doi:10.1124/dmd.112.046896.
- Barilli, A.; Visigalli, R.; Ferrari, F.; Di Lascia, M.; Riccardi, B.; Puccini, P.; Dall’Asta, V.; Rotoli, B.M. Organic Cation Transporters (OCTs) in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. Biomedicines 2020, 8, 127, doi:10.3390/biomedicines8050127.
- Mukherjee, M.; Pritchard, D.; Bosquillon, C. Evaluation of air-interfaced Calu-3 cell layers for investigation of inhaled drug interactions with organic cation transporters in vitro. Int. J. Pharm. 2012, 426, 7–14, doi:10.1016/j.ijpharm.2011.12.036.
- Mukherjee, M.; Latif, M.; Pritchard, D.; Bosquillon, C. In-cell Western™ detection of organic cation transporters in bronchial epithelial cell layers cultured at an air–liquid interface on Transwell ® inserts. J. Pharmacol. Toxicol. Methods 2013, 68, 184–189, doi:10.1016/j.vascn.2013.05.007.
- Salomon, J.J.; Muchitsch, V.E.; Gausterer, J.C.; Schwagerus, E.; Huwer, H.; Daum, N.; Lehr, C.-M.; Ehrhardt, C. The Cell Line NCl-H441 Is a Usefulin VitroModel for Transport Studies of Human Distal Lung Epithelial Barrier. Mol. Pharm. 2014, 11, 995–1006, doi:10.1021/mp4006535.
- Lips, K.S.; Volk, C.; Schmitt, B.M.; Pfeil, U.; Arndt, P.; Miska, D.; Ermert, L.; Kummer, W.; Koepsell, H. Polyspecific Cation Transporters Mediate Luminal Release of Acetylcholine from Bronchial Epithelium. Am. J. Respir. Cell Mol. Biol. 2005, 33, 79–88, doi:10.1165/rcmb.2004-0363oc.
- Fallon, J.K.; Houvig, N.; Booth-Genthe, C.L.; Smith, P.C. Quantification of membrane transporter proteins in human lung and immortalized cell lines using targeted quantitative proteomic analysis by isotope dilution nanoLC–MS/MS. J. Pharm. Biomed. Anal. 2018, 154, 150–157, doi:10.1016/j.jpba.2018.02.044.
- Horvath, G.; Mendes, E.S.; Schmid, N.; Schmid, A.; Conner, G.E.; Salathe, M.; Wanner, A. The effect of corticosteroids on the disposal of long-acting β2-agonists by airway smooth muscle cells. J. Allergy Clin. Immunol. 2007, 120, 1103–1109, doi:10.1016/j.jaci.2007.08.034.
- Berg, T.; Hegelund-Myrbäck, T.; Öckinger, J.; Zhou, X.; Brännström, M.; Hagemann-Jensen, M.; Werkstrom, V.; Seidegård, J.; Grunewald, J.; Nord, M.; et al. Expression of MATE1, P-gp, OCTN1 and OCTN2, in epithelial and immune cells in the lung of COPD and healthy individuals. Respir. Res. 2018, 19, 68, doi:10.1186/s12931-018-0760-9.
- Berg, T.; Myrbäck, T.H.; Olsson, M.; Seidegård, J.; Werkström, V.; Zhou, X.; Grunewald, J.; Gustavsson, L.; Nord, M. Gene expression analysis of membrane transporters and drug‐metabolizing enzymes in the lung of healthy and COPD subjects. Pharmacol. Res. Perspect. 2014, 2, e00054, doi:10.1002/prp2.54.
- Shrine, N.; Portelli, M.A.; John, C.; Artigas, M.S.; Bennett, N.; Hall, R.; Lewis, J.; Henry, A.P.; Billington, C.K.; Ahmad, A.; et al. Moderate-to-severe asthma in individuals of European ancestry: A genome-wide association study. Lancet Respir. Med. 2019, 7, 20–34, doi:10.1016/s2213-2600(18)30389-8.
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; Von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O. A Large-Scale, Consortium-Based Genomewide Association Study of Asthma. N. Engl. J. Med. 2010, 363, 1211–1221, doi:10.1056/nejmoa0906312.
- Yamauchi, K.; Shikanai, T.; Nakamura, Y.; Kobayashi, H.; Ogasawara, M.; Maeyama, K. Roles of Histamine in the Pathogenesis of Bronchial Asthma and Reevaluation of the Clinical Usefulness of Antihistamines. Yakugaku Zasshi 2011, 131, 185–191, doi:10.1248/yakushi.131.185.
- Yamauchi, K.; Ogasawara, M. The Role of Histamine in the Pathophysiology of Asthma and the Clinical Efficacy of Antihistamines in Asthma Therapy. Int. J. Mol. Sci. 2019, 20, 1733, doi:10.3390/ijms20071733.
- Mukherjee, M.; Cingolani, E.; Pritchard, D.; Bosquillon, C. Enhanced expression of Organic Cation Transporters in bronchial epithelial cell layers following insults associated with asthma – Impact on salbutamol transport. Eur. J. Pharm. Sci. 2017, 106, 62–70, doi:10.1016/j.ejps.2017.05.052.
- Rotoli, B.M.; Visigalli, R.; Barilli, A.; Ferrari, F.; Bianchi, M.G.; Di Lascia, M.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional analysis of OCTN2 and ATB0,+ in normal human airway epithelial cells. PLoS ONE 2020, 15, e0228568, doi:10.1371/journal.pone.0228568.
- Li, D.; Qi, C.; Zhou, J.; Wen, Z.; Zhu, X.; Xia, H.; Song, J. LPS-induced inflammation delays the transportation of ASP+ due to down-regulation of OCTN1/2 in alveolar epithelial cells. J. Drug Target. 2019, 28, 437–447, doi:10.1080/1061186x.2019.1678169.
- Qi, C.; Zhou, J.; Wang, Z.; Fang, X.; Li, D.; Jin, Y.; Song, J. Cigarette smoke extract combined with lipopolysaccharide reduces OCTN1/2 expression in human alveolar epithelial cells in vitro and rat lung in vivo under inflammatory conditions. Int. Immunopharmacol. 2020, 87, 106812, doi:10.1016/j.intimp.2020.106812.
- Schulz, C.; Farkas, L.; Wolf, K.; Krätzel, K.; Eissner, G.; Pfeifer, M. Differences in LPS-Induced Activation of Bronchial Epithelial Cells (BEAS-2B) and Type II-Like Pneumocytes (A-549). Scand. J. Immunol. 2002, 56, 294–302, doi:10.1046/j.1365-3083.2002.01137.x.
- Al‐Majdoub, Z.M.; Al Feteisi, H.; Achour, B.; Warwood, S.; Neuhoff, S.; Rostami-Hodjegan, A.; Barber, J. Proteomic Quantification of Human Blood–Brain Barrier SLC and ABC Transporters in Healthy Individuals and Dementia Patients. Mol. Pharm. 2019, 16, 1220–1233, doi:10.1021/acs.molpharmaceut.8b01189.
- Liang, Y.; Ligong, C.; Chen, L. The physiological role of drug transporters. Protein Cell 2015, 6, 334–350, doi:10.1007/s13238-015-0148-2.
- Majd, H.; King, M.S.; Palmer, S.M.; Smith, A.C.; Elbourne, L.D.; Paulsen, I.T.; Sharples, D.; Henderson, P.J.; Kunji, E.R. Screening of candidate substrates and coupling ions of transporters by thermostability shift assays. eLife 2018, 7, doi:10.7554/elife.38821.
- Tschirka, J.; Bach, M.; Kisis, I.; Lemmen, J.; Gnoth, M.J.; Gründemann, D. Transporter tandems—Precise tools for normalizing active transporter in the plasma membrane. Biochem. J. 2020, 477, 4191–4206, doi:10.1042/bcj20200666.
- Salomon, J.J.; Endter, S.; Tachon, G.; Falson, F.; Buckley, S.T.; Ehrhardt, C. Transport of the fluorescent organic cation 4-(4-(dimethylamino)styryl)-N-methylpyridinium iodide (ASP+) in human respiratory epithelial cells. Eur. J. Pharm. Biopharm. 2012, 81, 351–359, doi:10.1016/j.ejpb.2012.03.001.
- Selo, M.A.; Delmas, A.-S.; Springer, L.; Zoufal, V.; Sake, J.A.; Clerkin, C.G.; Huwer, H.; Schneider-Daum, N.; Lehr, C.-M.; Nickel, S.; et al. Tobacco Smoke and Inhaled Drugs Alter Expression and Activity of Multidrug Resistance-Associated Protein-1 (MRP1) in Human Distal Lung Epithelial Cells in vitro. Front. Bioeng. Biotechnol. 2020, 8, 1030, doi:10.3389/fbioe.2020.01030.
- Sakagami, M. In vitro, ex vivo and in vivo methods of lung absorption for inhaled drugs. Adv. Drug Deliv. Rev. 2020, doi:10.1016/j.addr.2020.07.025.
- Bosquillon, C.; Madlova, M.; Patel, N.; Clear, N.; Forbes, B. A Comparison of Drug Transport in Pulmonary Absorption Models: Isolated Perfused rat Lungs, Respiratory Epithelial Cell Lines and Primary Cell Culture. Pharm. Res. 2017, 34, 2532–2540, doi:10.1007/s11095-017-2251-y.
- Kolli, A.R.; Kuczaj, A.K.; Martin, F.; Hayes, A.W.; Peitsch, M.C.; Hoeng, J. Bridging inhaled aerosol dosimetry to physiologically based pharmacokinetic modeling for toxicological assessment: Nicotine delivery systems and beyond. Crit. Rev. Toxicol. 2019, 49, 725–741, doi:10.1080/10408444.2019.1692780.
- Bäckman, P.; Arora, S.; Couet, W.; Forbes, B.; De Kruijf, W.; Paudel, A. Advances in experimental and mechanistic computational models to understand pulmonary exposure to inhaled drugs. Eur. J. Pharm. Sci. 2018, 113, 41–52, doi:10.1016/j.ejps.2017.10.030.
- Su, H.; Wang, Y.; Liu, S.; Wang, Y.; Liu, Q.; Liu, G.; Chen, Q. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm. Sin. B 2019, 9, 49–58, doi:10.1016/j.apsb.2018.10.005.
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27, doi:10.3389/fphar.2018.00027.
- Kou, L.; Yao, Q.; Sun, M.; Wu, C.; Wang, J.; Luo, Q.; Wang, G.; Du, Y.; Fu, Q.; He, Z.; et al. Cotransporting Ion is a Trigger for Cellular Endocytosis of Transporter-Targeting Nanoparticles: A Case Study of High-Efficiency SLC22A5 (OCTN2)-Mediated Carnitine-Conjugated Nanoparticles for Oral Delivery of Therapeutic Drugs. Adv. Health Mater. 2017, 6, doi:10.1002/adhm.201700165.
- Mo, J.; Shi, S.; Zhang, Q.; Gong, T.; Sun, X.; Zhang, Z.-R. Synthesis, Transport and Mechanism of a Type I Prodrug: L-Carnitine Ester of Prednisolone. Mol. Pharm. 2011, 8, 1629–1640, doi:10.1021/mp100412z.
- Mo, J.; Lim, L.Y.; Zhang, Z.-R. l-Carnitine ester of prednisolone: Pharmacokinetic and pharmacodynamic evaluation of a type I prodrug. Int. J. Pharm. 2014, 475, 123–129, doi:10.1016/j.ijpharm.2014.08.049.
