G Protein-Coupled Receptor Kinases (GRK) are known to phosphorylate G Protein Coupled Receptors GPCRs on the plasma membrane to turn off their intracellular signaling [
24,
25]. Therefore, GRKs exert a critical role in both physiological states and pathological conditions that are mainly associated with alterations of the adrenergic signaling. Besides this classical effect, GRKs are also known to inactivate non-GPCR receptors, including tyrosine kinase receptors, by phosphorylative events [
24]. Moreover, they can interact with and regulate the activity of several cytosolic substrates [
26,
27,
28]. Therefore, GRKs are involved in the regulation of key processes within the cell and are, consequently, potential therapeutic targets [
26,
29]. Among GRKs, GRK2 is known to regulate cell metabolism [
29,
30,
31]. GRK2 shuttles among the different subcellular compartments following the energetic requests of the cell [
32,
33]. In particular, we showed that GRK2 localizes in mitochondria and regulates critical mitochondrial processes, such as ATP production and mitochondrial biogenesis [
32]. The overexpression of the kinase favors such processes preventing ATP loss after hypoxia/reperfusion [
32] and mitochondrial damage in response to acute exposure to ionizing radiation [
34]. Despite energy metabolism, the suggestion of GRK2 involvement in cell metabolism comes from the demonstration that insulin induces an up-regulation of the kinase expression levels and the association between GRK2 and IRS1 [
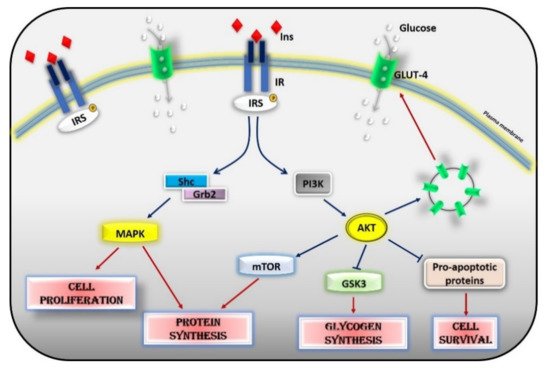
35]. GRK2 phosphorylates IRS1 in serine/threonine and inhibits the activation of the downstream signaling. In particular, GRK2 inhibits the phosphorylation of IRS1 in Tyr612, blocking the insulin signaling, and induces its phosphorylation in Ser307, promoting IRS degradation [
35,
36]. These results are in line with the reciprocal relationship between these two sites of IRS phosphorylation that is described in previous reports [
37,
38]. Chronic stimulation of beta-adrenergic receptors (
βAR) is involved in the pathogenesis of insulin resistance. In cells overexpressing the
βAR, GRK2 accumulates in the plasma membrane and inhibits IRS1 activation and glucose uptake in response to insulin. The selective inhibition of the kinase potentiates insulin signaling both in vitro and in vivo suggesting that GRK2 mediates adrenergic insulin resistance while its inhibition increases insulin sensitivity [
35]. These observations point to GRK2 as a potential target, also considering that conditions associated with insulin resistance (diabetes, hypertension, or chronic activation of β adrenergic receptor), are all characterized by elevated kinase levels. Indeed, GRK2 expression is increased in key tissues in different experimental models of insulin resistance, and its downregulation ameliorates the alterations of glucose homeostasis and insulin signaling in response to different insults [
39]. Recently, the role of GRK2 in myeloid cells has also been investigated. Vila-Bedmar and colleagues suggest that the downregulation of GRK2 in these cells reduces the pro-inflammatory features of macrophages and prevents high fat diet-induced metabolic alterations [
40]. GRK2 expression levels, as well as its subcellular localization and activity, are finely regulated. Several molecules are involved in regulating different steps of kinase transcription, mRNA regulation and translation, protein maturation, localization, activation, and degradation, as extensively described in the review from Penela and colleagues [
41]. Here, we will discuss the recent findings on the metabolic role of GRK2 in insulin resistance-related conditions, such as diabetes, hypertension, and heart failure.