l-Asparaginase (ASNase, EC 3.5.1.1) is a tetrameric aminohydrolase enzyme that catalyses the hydrolysis of the amino acid L-Asparagine into ammonia and L-aspartic acid. ASNase is present in different organisms such as bacteria, fungi, plant tissues and algae. ASNase is used in the pharmaceutical field as an anticancer drug for the treatment of acute lymphoblastic leukemia (ALL) and other malignant diseases such as Hodgkin’s disease. In the food sector, ASNase is used to prevent the formation of acrylamide, a toxic compound formed when starch-rich foods are cooked at temperatures above 100 °C. ASNase can also be used as a biosensor for the detection of L-asparagine levels.
- l-asparaginase
- confinement strategies
- nanomaterials
- therapeutic agents
- acrylamide mitigation
- biosensors
1. Introduction
ASNase can be produced by a wide variety of natural sources, namely microorganisms (bacteria, yeast, filamentous fungi, algae), plants and vertebrates. Microorganisms, such as Aspergillus tamarii , Aerobacter spp., Bacillus spp., Photobacterium spp., Serratia spp., Xanthomonas spp., Pseudomonas aeruginosa, Proteus vulgaris, Streptomyces griseus and Vibrio succinogenes are preferred sources for ASNase production [1]. In 1967, two ASNase isozymes with different properties were discovered in Escherichia coli, namely type I and type II [2]. Type I ASNase is a homodimeric cytosolic constitutive enzyme, while type II ASNase, normally assuming a homotetrameric configuration, is located in the enzyme periplasm, being secreted only when exposed to low nitrogen concentrations. Even though both isozymes show enzymatic activity for L-asparagine and L-glutamine, the main difference between them is the specificity for L-asparagine [3]. Type II is known to have anti-tumour activity due to the higher specific affinity for L-asparagine [4].
The L-asparagine hydrolysis by ASNase occurs in two main steps (Figure 1). The first step involves the enzyme nucleophilic residue activation by NH2, a powerful base, and the subsequent attack on the L-asparagine amide carbon atom, generating the beta-acyl-enzyme intermediate; the second one comprises the nucleophile activation by a water molecule, attacking the ester carbon successively, providing L-aspartic acid and liberating ammonia [5].
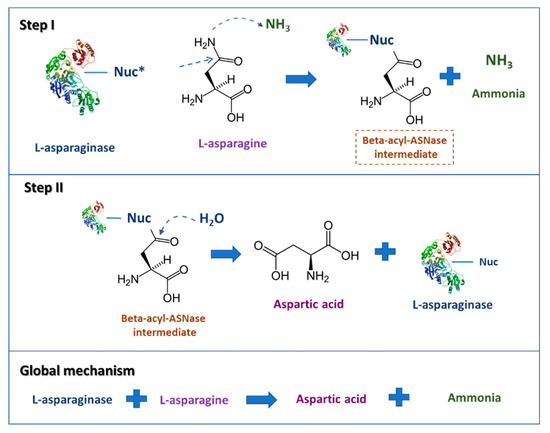
Figure 1. Scheme describing the L-asparaginase reaction mechanisms. * Nuc: nucleophilic residue (adapted from Hill et al. [6]).
In the pharmaceutical sector, type II ASNase has been applied in the treatment of lymphoproliferative disorders and lymphomas, namely ALL, T-cell lymphomas, subtypes of myeloid leukaemias and NK tumours [7]. Furthermore, due to its glutaminase activity, ovarian carcinomas and further solid tumours have been projected as ASNase additional targets [7]. In fact, in vitro ASNase sensitivity was exhibited for soft tissue sarcoma [8], β-catenin mutated hepatocellular carcinoma [8], hepatocellular carcinoma with low asparagine synthetase expression [9] and gastric adenocarcinoma [10]. ASNase can deplete L-asparagine, an essential amino acid to tumour cells. More specifically, healthy cells synthesise L-asparagine through transaminase enzyme, which converts oxaloacetate into an intermediate aspartate that subsequently transfers an amino group from glutamate to oxaloacetate, generating α-ketoglutarate and aspartate. They are then transformed into asparagine through asparagine synthase or glutamine-dependent asparagine synthetase via an ATP-dependent reaction, which takes advantage of the amido-N of L-glutamine in order to form the amido group of asparagine.
In the food industry, ASNase can prevent the acrylamide formation, a carcinogenic compound produced during the heat of food processed products [11]. Thus, the pre-treatment of starchy foods with ASNase, before heating, converts L-asparagine to aspartic acid, preventing the acrylamide formation by the Maillard reaction between L-asparagine and carbonyl compounds at high temperatures [11]. In 2003, Zyzak et al. [12] reported the ASNase application for acrylamide reduction in a potato matrix. This observation led to the inclusion of monographs on ASNase from Aspergillus oryzae and Aspergillus niger in World Health Organization (WHO) food additives series in 2008 (59th series) [13] and 2009 (60th series) [14], respectively. However, as the enzyme action could be affected by food composition, the ideal ASNase to be used in the food industry must be stable throughout the food processing and proteolysis and, once consumed, it should not cause allergic or toxic reactions.
The manufacture of ASNase-based biosensors to detect and/or quantify L-asparagine levels is also considered a promising technology in both clinical and food industries[15]. These biosensors’ mechanism of action is due to the ASNase activity. Ammonium ions generated during the asparagine hydrolysis lead to a pH variation and subsequent change of colour and absorption wavelength [16].
2. Commercial ASNase
Currently, there are several type II ASNase commercially available, produced industrially for medical applications (detailed in Table 1): (i) native ASNase from E. coli (Elspar® from Ovation Pharmaceuticals, Illinois, IL, USA [17]; Leukanase® from Sanofi-aventis, New South Wales, Australia; Kidrolase® from EUSA Pharma, SAS, Lyon, France [18], etc.); (ii) PEGylated ASNase from recombinant E. coli, pegaspargase (Oncaspar® from Enzon Pharmaceuticals, Florida, FL, USA) [19]; (iii) native ASNase, but as a recombinant form, being produced in E. coli and E. chrysanthemi as host cells (Spectrila® from Medac Gesellschaft, Wedel, Germany [20] and Erwinase® (from Erwinia chrysanthemi) from EUSA Pharma, SAS, Lyon, France [21], respectively).
Elspar® was the first ASNase to be available on the market and to be approved (1978) by the U.S. Food and Drug Administration (FDA) for use as a component of a multi-agent chemotherapeutic regimen for the treatment of patients with acute lymphoblastic leukaemia (ALL). In 1994, Oncaspar® received the same approval by FDA, but only for patients with hypersensitivity to native Elspar®. Only in 2006 it was approved as part of the first-line therapy for any ALL patient [22]. In November 2011, FDA approved Erwinase®, indicating its use as a component of a multi-agent chemotherapeutic regimen for ALL patients treatment who have developed hypersensitivity to either Elspar® or Oncaspar® [23]. Finally, in January 2016, the European Commission granted a marketing authorisation valid throughout the European Union for Spectrila® (from E. coli). However, all these ASNase products are associated with several noteworthy toxicities and should be used with care because of the possibility of severe reactions, including anaphylaxis and sudden death [24].
Commercially approved ASNases to be used in food industries (detailed in Table 1) comprise the fungal ones from A. oryzae (Acrylaway® from Novozymes A/S, Bagsvaerd, Denmark) and A. niger (PreventASeTM from DSM, Heerlen, The Netherlands) [25]. The US government attributed the status of “generally recognised as safe” (GRAS) to both ASNases, and in 2007-2008 they received a favourable evaluation as a food additive by the Joint FAO/WHO Expert Committee [26].
Table 1. Commercial ASNase for therapeutic and food applications.
|
ASNase Application |
ASNase Form |
Microorganism |
ASNase Commercial Name |
ASNase Manufacturer |
|
Therapeutic/Pharmaceutical |
Native ASNase |
E. coli |
Elspar® |
Ovation Pharmaceuticals |
|
Leukanase® |
Sanofi-aventis |
|||
|
Kidrolase® |
EUSA Pharma |
|||
|
PEGylated ASNase |
E. coli |
Oncaspar® |
Enzon Pharmaceuticals |
|
|
Native recombinant ASNase |
E. coli |
Spectrila® |
Medac Gesellschaft |
|
|
E. chrysanthemi |
Erwinase® |
EUSA Pharma |
||
|
Food Industry |
Native ASNase |
A. oryzae |
Acrylaway® |
Novozymes A/S |
|
A. niger |
PreventASeTM |
DSM |
3. Confined ASNase
The use of ASNase in its free form is challenging due to its unstable nature and limitation to a single use. Thus, the improvement of ASNase enzymatic and therapeutic properties has been achieved by introducing chemical modifications and physical integration within several supports. These techniques, if properly designed, can improve the stability of the enzymes and allow their reuse, also contributing to the reduction of operation costs [27]. Due to enzymes protection (enhanced activity and stability [28]) and expanded catalytic half-life [29], confined ASNase can find improved applications in the previous cited areas [30]. Nevertheless, as the enzymes confinement on support materials could result in several enzyme modifications, the changes in the enzyme structure and activity should be thoroughly studied and evaluated according to the target application [31]. Therefore, the choice of the support material and the confinement procedure are aspects of maximum importance. A high number of ASNase confinement possibilities have been recently developed, which may be grouped into three main approaches: (i) physical adsorption; (ii) covalent attachment; (iii) entrapment.
Even though in recent years numerous works about ASNase confinement have been published, in which the enhanced biochemical and pharmacological features of ASNase are reported, more work is still needed to fulfil the requirements of regulatory agencies and reach the biopharmaceutical industry. Within these recent reports, the ASNase entrapment displayed the most promising results for an intravenous application and in vivo safety. Although the ASNase entrapment into (nano)materials has been reported in the literature, there are no related commercial solutions currently in the market. Since the majority of ASNases are thermolabile and active in a narrow pH range, ASNase confinement for food applications has been recently investigated to improve the enzymatic stability and activity over a broad range of temperature and pH, in order to lower the processing time and costs. In recent times, confined ASNase also started to emerge in the biosensing technology, opening new possibilities up at the industrial level, namely in therapeutic/pharmaceutical and food industries due to its potential to monitor asparagine levels in blood serum samples of ALL and lymphosarcoma patients and to detect asparagine in food samples. The need for a thermostable ASNase to improve its catalytic performance reinforces the need for further research on the use of confined ASNase.
Acknowledgments
This entry is adapted from the peer-reviewed paper 10.3390/molecules25245827
References
- Neelam Verma; Kuldeep Kumar; Gurnoor Kaur; Sneh Anand; L-Asparaginase: A Promising Chemotherapeutic Agent. Critical Reviews in Biotechnology 2007, 27, 45-62, 10.1080/07388550601173926.
- H. A. Campbell; L. T. Mashburn; E. A. Boyse; L. J. Old; Two l-asparaginases from Escherichia coli B. Their separation, purification, and antitumor activity. Biochemistry 1967, 6, 721-730, 10.1021/bi00855a011.
- J. H. Schwartz; J. Y. Reeves; J. D. Broome; Two L-asparaginases from E. coli and their action against tumors. Proceedings of the National Academy of Sciences 1966, 56, 1516-1519, 10.1073/pnas.56.5.1516.
- Mi-Kyung Yun; Amanda Nourse; Stephen W. White; Charles O. Rock; Richard J. Heath; Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli l-asparaginase. Journal of Molecular Biology 2007, 369, 794-811, 10.1016/j.jmb.2007.03.061.
- Ganeshan Shakambari; Balasubramaniem AshokKumar; Perumal Varalakshmi; L-asparaginase – A promising biocatalyst for industrial and clinical applications. Biocatalysis and Agricultural Biotechnology 2019, 17, 213-224, 10.1016/j.bcab.2018.11.018.
- Joseph M. Hill; Joseph Roberts; Ellen Loeb; Amanullah Khan; Ayten MacLellan; Robert W. Hill; l-Asparaginase therapy for leukemia and other malignant neoplasms. Remission in human leukemia. JAMA 1967, 202, 882-888, 10.1001/jama.1967.03130220070012.
- Daniele Covini; Saverio Tardito; Ovidio Bussolati; Laurent R. Chiarelli; Maria V. Pasquetto; Rita Digilio; Giovanna Valentini; Claudia Scotti; Expanding Targets for a Metabolic Therapy of Cancer: L-Asparaginase. Recent Patents on Anti-Cancer Drug Discovery 2012, 7, 4-13, 10.2174/157489212798358001.
- Saverio Tardito; J. Uggeri; C. Bozzetti; M. G. Bianchi; B. M. Rotoli; R. Franchi-Gazzola; G. C. Gazzola; R. Gatti; Ovidio Bussolati; The inhibition of glutamine synthetase sensitizes human sarcoma cells to l-asparaginase. Cancer Chemotherapy and Pharmacology 2007, 60, 751-758, 10.1007/s00280-007-0421-z.
- B Zhang; L-W Dong; Y-X Tan; J Zhang; Y-F Pan; C Yang; M-H Li; Z-W Ding; L-J Liu; T-Y Jiang; et al. Asparagine synthetase is an independent predictor of surgical survival and a potential therapeutic target in hepatocellular carcinoma. British Journal of Cancer 2013, 109, 14-23, 10.1038/bjc.2013.293.
- Claudia Scotti; Patrizia Sommi; Maria Valentina Pasquetto; Donata Cappelletti; S. Stivala; Paola Mignosi; Monica Savio; Laurent Roberto Chiarelli; Giovanna Valentini; Victor M. Bolanos-Garcia; et al. Correction: Cell-Cycle Inhibition by Helicobacter pylori L-Asparaginase. PLoS ONE 2012, 7, 13892, 10.1371/annotation/c6c16a43-aadd-4cd3-a916-e935534dab38.
- Fei Xu; Maria Jose Oruna-Concha; J. Stephen Elmore; The use of asparaginase to reduce acrylamide levels in cooked food. Food Chemistry 2016, 210, 163-171, 10.1016/j.foodchem.2016.04.105.
- David V. Zyzak; Robert A. Sanders; Marko Stojanovic; Daniel H. Tallmadge; B. Loye Eberhart; Deborah K. Ewald; David C. Gruber; Thomas R. Morsch; Melissa A. Strothers; George P. Rizzi; et al. Acrylamide formation mechanism in heated foods. Journal of Agricultural and Food Chemistry 2003, 51, 4782-4787, 10.1021/jf034180i.
- Safety Evaluation of Certain Food Additives and Contaminants, Who Food Additive Series; World Health Organization: Geneva, Switzerland, 2008.
- Safety Evaluation of Certain Food Additives and Contaminants, Who Food Additive Series; World Health Organization: Geneva, Switzerland, 2009.
- Tahira Batool; Essam A. Makky; Muna Jalal; Mashitah M. Yusoff; A comprehensive review on l-asparaginase and its applications. Applied Biochemistry and Biotechnology 2015, 178, 900-923, 10.1007/s12010-015-1917-3.
- Kuldeep Kumar; Mandeep Kataria; Neelam Verma; Plant asparaginase-based asparagine biosensor for leukemia. Artificial Cells, Nanomedicine, and Biotechnology 2012, 41, 184-188, 10.3109/10731199.2012.716062.
- Elspar® (Asparaginase); Merck & Co., Inc.: Riverside, PA, USA, 2000.
- Subhash Chand; Richi V. Mahajan; Jai Prakash Prasad; Debendra K. Sahoo; Kanti Nandan Mihooliya; Mahesh S. Dhar; Girish Sharma; A comprehensive review on microbial l ‐asparaginase: Bioprocessing, characterization, and industrial applications. Biotechnology and Applied Biochemistry 2020, 67, 619-647, 10.1002/bab.1888.
- Assessment Report Oncaspar; European Medicines Agency: London, UK, 2016.
- Assessment Report Spectrila; European Medicines Agency: London, UK, 2015.
- Public Assessment Report Crisantaspase; Medicines Evaluation Board: Utrecht, The Netherlands, 2015.
- Patricia Anne Dinndorf; Joseph Gootenberg; Martin H. Cohen; Patricia Keegan; Richard Pazdur; FDA drug approval summary: Pegaspargase (Oncaspar®) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). The Oncologist 2007, 12, 991-998, 10.1634/theoncologist.12-8-991.
- T.A. Costa-Silva; I.M. Costa; H.P. Biasoto; G.M. Lima; C. Silva; Adalberto Pessoa; Gisele Monteiro; Critical overview of the main features and techniques used for the evaluation of the clinical applicability of L-asparaginase as a biopharmaceutical to treat blood cancer. Blood Reviews 2020, 43, 100651, 10.1016/j.blre.2020.100651.
- BC Cancer Drug Manual. Available online: http://www.cdha.nshealth.ca/nova-scotia-cancer-care-program-25 (accessed on 25 January 2021).
- Krishnakumar T; Visvanathan R; Acrylamide in food products: A review. Journal of Food Processing & Technology 2014, 5, 344, .
- JECFA. Compendium of Food Additive Specifications; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007.
- Joanna Bodakowska-Boczniewicz; Zbigniew Garncarek; Immobilization of naringinase from Aspergillus niger on a magnetic polysaccharide carrier. Molecules 2020, 25, 2731, 10.3390/molecules25122731.
- Claudia Bernal; Karen Rodríguez; Ronny Martínez; Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnology Advances 2018, 36, 1470-1480, 10.1016/j.biotechadv.2018.06.002.
- Valeria E. Bosio; Germán A. Islan; Yanina N. Martínez; Nelson Durán; Guillermo Raul Castro; Nanodevices for the immobilization of therapeutic enzymes. Critical Reviews in Biotechnology 2015, 36, 447–464, 10.3109/07388551.2014.990414.
- Kushagri Singh; Abha Mishra; Deepankar Sharma; Kavita Singh; Nanotechnology in enzyme immobilization: An overview on enzyme immobilization with nanoparticle matrix. Current Nanoscience 2019, 15, 234-241, 10.2174/1573413714666181008144144.
- Uglas S. Clark; Can immobilization be exploited to modify enzyme activity?. Trends in Biotechnology 1994, 12, 439-443, 10.1016/0167-7799(94)90018-3.
