he Coronavirus disease (COVID-19) pandemic has resulted in challenges to cancer management, exacerbated by limited clinical resources and caution in preventing COVID-19 transmission between patients and healthcare professionals. The neglect of breast cancer (in particular, triple-negative breast cancer (TNBC)) patients during the outbreak could negatively impact their overall survival, as delays in treatment and consultations provide vital time for tumor progression and metastasis. Herein, we review the shifting clinical management of TNBCs during the COVID-19 outbreak. The suggested treatment recommendations can hopefully minimize virus exposure without sacrificing patient care during times when healthcare systems are overburdened. Further, we review published RNA-seq data to assess the theoretical infectability of metastatic TNBCs to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection.
1. Introduction
The treatment of patients with various cancers during the COVID-19 pandemic presents a unique set of challenges. Cancer patients or those with a history of cancer face a disproportionately higher risk of SARS-CoV-2 infection and a poor prognosis relative to COVID-19 patients without cancer [
11,
12,
13,
14]. However, many of these studies have been limited by low numbers of patients and a lack of diversity in geography, age, sex, race/ethnicity and cancer treatment information. The disparity in SARS-CoV-2 infections among cancer patients relative to non-cancer patients may be explained by evidence that cancer patients are immunocompromised while receiving anti-cancer treatments (i.e., surgery, chemotherapy, targeted therapies) or supporting medications (i.e., steroids) and experience the inherently immunosuppressive nature of their cancer [
11,
12,
15]. Furthermore, cancer patients are often older than 60 years of age with one or more co-morbidities and are in frequent contact with healthcare services and facilities during and after cancer treatment, increasing their risk of SARS-CoV-2 infection and COVID-19 mortality [
15]. Clinicians are now presented with the difficult task of healthcare restructuring that concurrently favors the reduction of COVID-19 transmission and the delivery of high-quality care for cancer patients. The high degree of heterogeneity among cancers and the broad spectrum of cancer progression profiles and variation in subtype-specific clinical implications mean that prioritizations for treatment must be made.
These studies have highlighted cancer patients as an important at-risk group for adverse clinical prognosis following SARS-CoV-2 infection. While more data on cancer patients with COVID-19 are required, a retooling of patient cancer treatment that reduces the risk of SARS-CoV-2 infection without sacrificing potentially life-saving cancer treatments is favorable. An abundance of literature has been published focusing on the clinical outlook of cancer patients with COVID-19. However, less information exists on specific cancers and clinically relevant cancer subtypes. Thus, more research will be necessary to fully understand the effects of COVID-19 on these patients and to inform cancer type-specific treatment guidelines, as is the case for TNBC.
2. TNBC Management during COVID-19
For the effective prioritization of treatment for breast cancer patients not suspected to have COVID-19 during the pandemic, Dietz and colleagues have suggested the categorization of breast cancer patients into three priority levels (A, B & C) [
16]. They also provide treatment considerations and recommendations specific to the COVID-19 pandemic for each level. Priority A patients are considered to have an immediately life-threatening or symptomatic condition requiring urgent clinical intervention, while priority B patients have conditions that require treatment prior to the conclusion of the pandemic. The treatment of priority C patients can be postponed pending the end of the pandemic. Most breast cancer patients will fit into the priority B category, which is further subdivided into groups according to priority (B1–B3, where B1 patients are the highest priority). Given the aggressiveness of their disease, patients with TNBC are often among the highest priority for treatment. In accordance, TNBC patients are category B1 along with patients with HER2+ breast cancers.
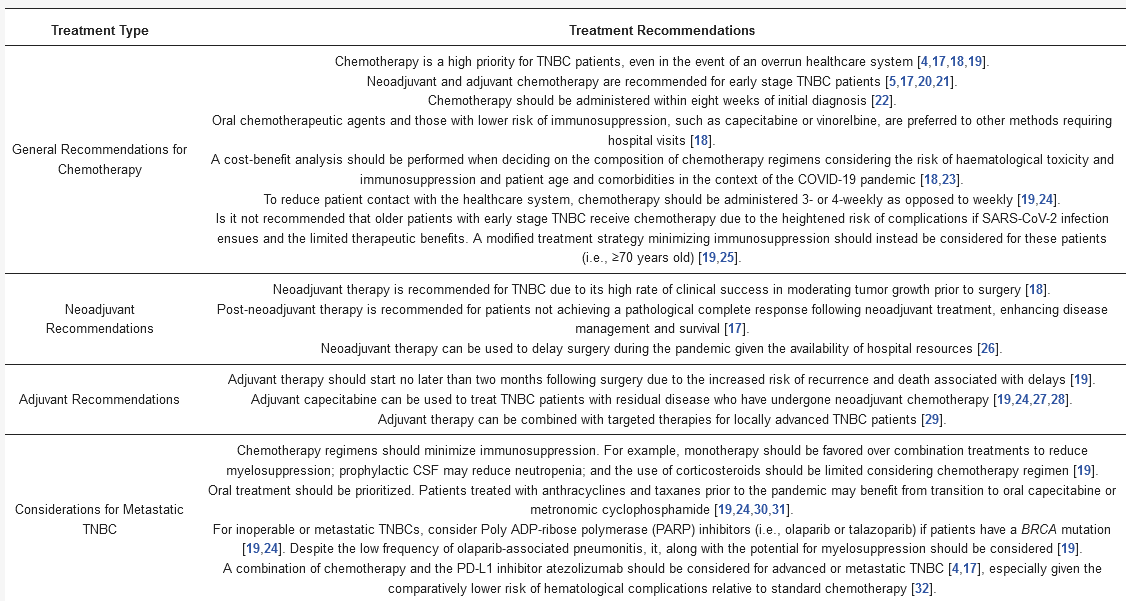
To explore changes in TNBC clinical strategies induced by the COVID-19 pandemic, we conducted a literature search to provide a detailed summary of suggested TNBC treatment strategies in response to COVID-19. We queried NCBI PubMed with the keywords “breast cancer” and “COVID-19” which returned 118 articles. From these, we eliminated articles with titles lacking our search terms. For these papers, we were solely focused on retrieving information pertaining to TNBC. We summarize our findings in the “Traditional TNBC therapies” section and emphasize chemotherapy-related recommendations in .
Table 1. Chemotherapy and targeted therapy recommendations for TNBC patients during COVID-19.
nt, rather than in an adjuvant setting, is still debated. Evidence suggests that TNBC is resistant to radiation when used alone; however, radiotherapy with poly ADP-ribose polymerase (PARP) inhibitors may be effective due to their synergistic DNA damage-enhancing effects [
47].
3. Targeted TNBC Therapies
A recent report by the national health service (NHS) classified cancer patients receiving treatments including targeted anti-PD-1/PD-L1 and PARP inhibitors are at an elevated risk of SARS-CoV-2 infection [
48]. Hence, the administration of such targeted therapies, which occurs in combination with traditional therapies in TNBC patients, presents a unique set of COVID-19- related challenges that pertains to this subtype.
Anti-PD-L1 therapy atezolizumab (ATZ) plus paclitaxel is an approved therapy for patients with PD-L1 positive metastatic TNBC [
32]. Fortunately, immune checkpoint inhibitors such as ATZ do not exert immunosuppressive effects and thus should not augment the risk of TNBC of SARS-CoV-2 infection due to greater immunosuppression [
49,
50]. Strikingly, immune checkpoint inhibitors may even boost pathogen-specific immune responses, and cases of viral or bacterial infections following treatment with ICIs are limited [
51,
52]. However, there are other side effects of PD-1/PD-L1 inhibition which may exacerbate the risk of SARS-CoV-2 infection and adverse outcomes [
53]. For example, cytokine release syndrome is a systemic inflammatory disease characterized by the release of circulating inflammatory cytokines such as interleukin-6 (IL-6) and interferon gamma [
54]. Conversely, it has been suggested that this cytokine storm may be beneficial for patients with TNBC who are receiving anti-PD-1/PD-L1 therapy and chemotherapy [
55]. While the cytokine storm is associated with acute respiratory distress in COVID-19 patients, it is suggested that TNBC patients with isolated lung metastases receiving combination immunotherapy may benefit from the COVID-19-associated cytokine storm in the lungs which may also act on metastatic lung nodules [
55,
56].
Many TNBCs exhibit a deficiency in homologous recombination and the associated repair of double strand DNA breaks, which makes them sensitive to PARP inhibitors. Intriguingly, there is evidence to support the use of PARP inhibitors to hamper SARS-CoV-2 infection and associated severe COVID-19 disease [
57]. Specifically, PARP inhibition may function against COVID-19 by limiting macrophage overactivation and associated cytokine storm along with protection against cell death. PARP inhibition reduces the levels of inflammatory cytokines associated with SARS-CoV-2-mediated cytokine storms. Thus, TNBC patients with concomitant COVID-19 receiving PARP inhibitors may experience better outcomes.
4. Specific Implications for Metastatic TNBC during the COVID-19 Pandemic
Within five years of their diagnosis, women with TNBC are more likely to experience metastasis relative to women with other breast cancer subtypes [
58]. Patients with TNBCs experience metastasis the bone, lungs, liver and brain. Therefore, in the context of management of TNBC during the COVID-19 pandemic, there are likely different factors to be considered if the TNBC is local and surgically removed versus a patient with metastatic disease.
To our knowledge, the mortality risk of lung metastasis in TNBC patients has not yet been assessed in the context of COVID-19. However, a recent study found that both lung cancer patients or patients with lung metastasis and COVID-19 were at a heightened risk of death compared to non-metastatic cancer patients with COVID-19 [
13]. Based on this finding, TNBC patients with lung metastasis likely have elevated mortality risk, although the specific implications have yet to be determined.
Additionally, the elevated risk of lung metastasis in TNBC complicates the diagnosis from respiratory-related symptoms in this patient cohort. In a case study reported by Chen and Li, the presence of a cough in an advanced gynecological cancer patient was initially perceived as a sign of lung spread [
59]. Follow-up analyses determined that the patient was positive for COVID-19, attributing the source of the respiratory-associated symptoms to the virus rather than lung metastasis [
59]. Other reports state that pleural metastasis can mimic COVID-19 symptoms, further complicating the source of suspicious symptoms [
60]. COVID-19 testing in cancer patients at multiple points in their care is thus critical for accurate disease tracking and management.
The implications of SARS-CoV-2 infection for patients with metastatic TNBCs are multifaceted and complex. SARS-CoV-2 infection in an environment where lung function is already reduced may significantly worsen the risk of COVID-19 mortality. COVID-19 patients requiring hospitalization often require ventilation and experience adverse outcomes, which is associated with viral load. Importantly, elevated viral load has been associated with advanced age, comorbidities and recent chemotherapy, all of which are associated with cancer. Further, intubation and mortality following hospitalization were highest in patients with the greatest viral loads [
61]. The SARS-CoV-2 viral load is also associated with substantially increased IL-6 levels, indicative of cytokine storm and poor outcomes [
62].
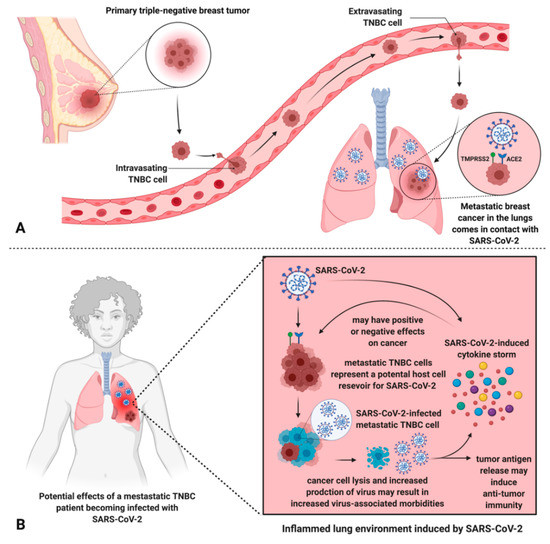
Metastasized TNBC cells may represent a cell population with enhanced susceptibility to viral infection and replication. In line with this, during the multi-step process whereby normal cells become cancerous, among other “hallmark” acquisitions contributing to their oncogenic phenotype, cells become capable of evasion of host immune-mediated recognition and destruction [
63]. Specifically, cancer cells acquire defective anti-viral defenses, including pathways mediated by interferons, increasing their susceptibility to viral infection [
7]. Thus, assuming adequate cellular expression of ACE2 and TMPRSS2, patients with metastatic TNBC represent a patient population with potential enhanced vulnerability to SARS-CoV-2 infection and poor outcomes due to widespread viral infection facilitated by cancer metastasis.
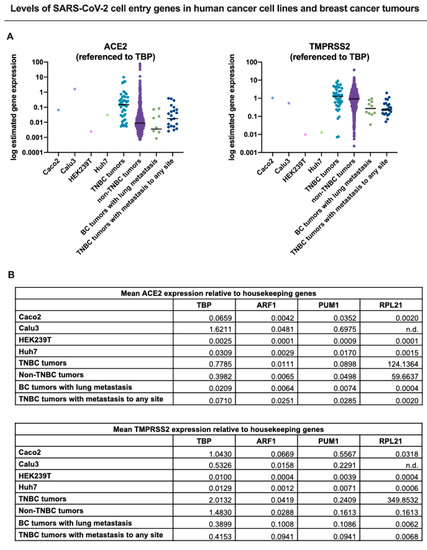
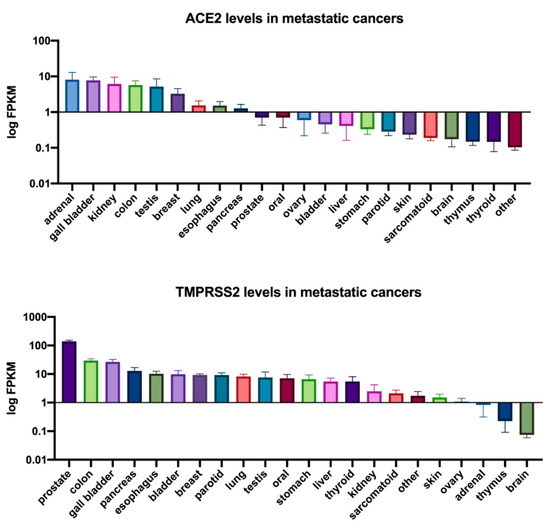
To determine whether TNBCs are possibly susceptible to SARS-CoV-2 infection, we interrogated published RNA-seq datasets to analyze the expression of both ACE2 and TMPRSS2 in TNBC patient tumors (primary tumors and metastatic) in comparison to cell lines known to be highly infectable by SARS-CoV-2 relative to a panel of known housekeeping/reference genes () [
64]. These analyses showed that TNBC patient tumors and metastasized breast cancers exhibited expression of both ACE2 and TMPRSS2 at least within the range of the infectable cell lines, suggesting that TNBC cells that come in contact with SARS-CoV-2 may be susceptible to infection. Consistent with these findings, analysis of ACE2 and TMPRSS2 expression across a panel of metastatic tumors also showed that both genes are expressed in metastatic breast tumors (). This further illustrates the potential for SARS-CoV-2 infection of metastatic breast cancer cells which may lead to heightened viral load and associated morbidities () [
61,
62]. Alternatively, it is also important to mention possible effects that SARS-CoV-2 infection may have on cancer cells present in the lung, due to the enhanced susceptibility of cancer cells to viral infection (). Future case reports and studies may reveal that metastatic cancer patients that were infected with SARS-CoV-2 experience discernable improvements in the tumor burden.
Figure 1. ACE2 and TMPRSS2 expression in breast cancer is comparable to cell lines permissive to SARS-CoV-2 infection. To compare expression of ACE2 and TMPRSS2 across datasets, we normalized the data to four different reference genes and depicted these as dot plots in (
A) to one reference gene (
TBP) and summarized in table format in (
B) for all four reference genes (
TBP, ARF1,
PUM1, RPL21). TPM normalized gene expression for ACE2 and TMPRSS2 in four human cell lines permissive to COVID-19 infection and replication: Caco-2 (colorectal adenocarcinoma cells), Calu-3 (lung cancer cells), HEK239T (embryonic kidney 293 cells), Huh7 (hepatoma cells), were calculated from raw RNA-seq counts obtained from NCBI’s Gene Expression Omnibus (
GEO) Datasets database (GSE140066, GSE147507, GSE137556, GSE129277, respectively). Only the control/non-treated samples from each dataset were included in the above analysis. RNA Seq V2 RSEM normalized gene expression for TNBC and non-TNBC tumours was obtained through the Breast Invasive Carcinoma (TCGA, Cell 2015) dataset accessed through cBioPortal [
65,
66]. All metastatic tumor data (breast cancer (BC) tumors with lung metastasis, metastatic TNBC tumors) were acquired through The Metastatic Breast Cancer Project (Provisional, February 2020) retrieved using cBioPortal. The results for the metastatic dataset were normalized by RNA Seq V2 RSEM.). The results presented in were derived in part from data generated by The Cancer Genome Atlas (TCGA) research network.
https://www.cancer.gov/tcga.
Figure 2. ACE2 and TMPRSS2 expression in metastatic cancers. ACE2 and TMPRSS2 FPKM expression values for metastatic cancers were obtained from the MET500 dataset [
67] accessed through xenabrowser.net. The mean expression of ACE2 or TMPRSS2 for each tumor site is reported with the standard error of the mean (SEM). The results presented in were derived in part from data generated by The Cancer Genome Atlas (TCGA) research network.
https://www.cancer.gov/tcga
Figure 3. Metastatic TNBC cells in the lungs may be infected by SARS-CoV-2, leading to unknown effects on the patient’s cancer and potential amplification of viral infection/morbidities. (A) TNBC metastasized to the lungs theoretically permits the interaction between SARS-CoV-2 and TNBC cells via ACE2 and TMPRSS2, exploited by SARS-CoV-2 for host cell entry. (B) An inflamed lung environment induced by SARS-CoV-2 results in a cytokine storm which leads to unknown effects on metastatic cells but may increase virus-associated morbidities. Further, metastatic breast cancer cells may represent an additional host cell SARS-CoV-2 reservoir, leading to increased viral load and virus-associated morbidities, and unknown consequences on the progression of the cancer. Tumor cell lysis can also liberate tumor antigens, inducing anti-tumor immune responses.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13020296