Metastasis is the process of dissemination of a tumor, whereby cells from the primary site dislodge and find their way to other tissues where secondary tumors establish. Metastasis is the primary cause of death related to cancer. This process warrants changes in original tumoral cells and their microenvironment to establish a metastatic niche. Traditionally, cancer therapy has focused on metastasis prevention by systematic treatments or direct surgical re-sectioning. However, metastasis can still occur. More recently, new therapies direct their attention to targeting cancer stem cells. As they propose, these cells could be the orchestrators of the metastatic niche.
- Metastasis
1. Introduction
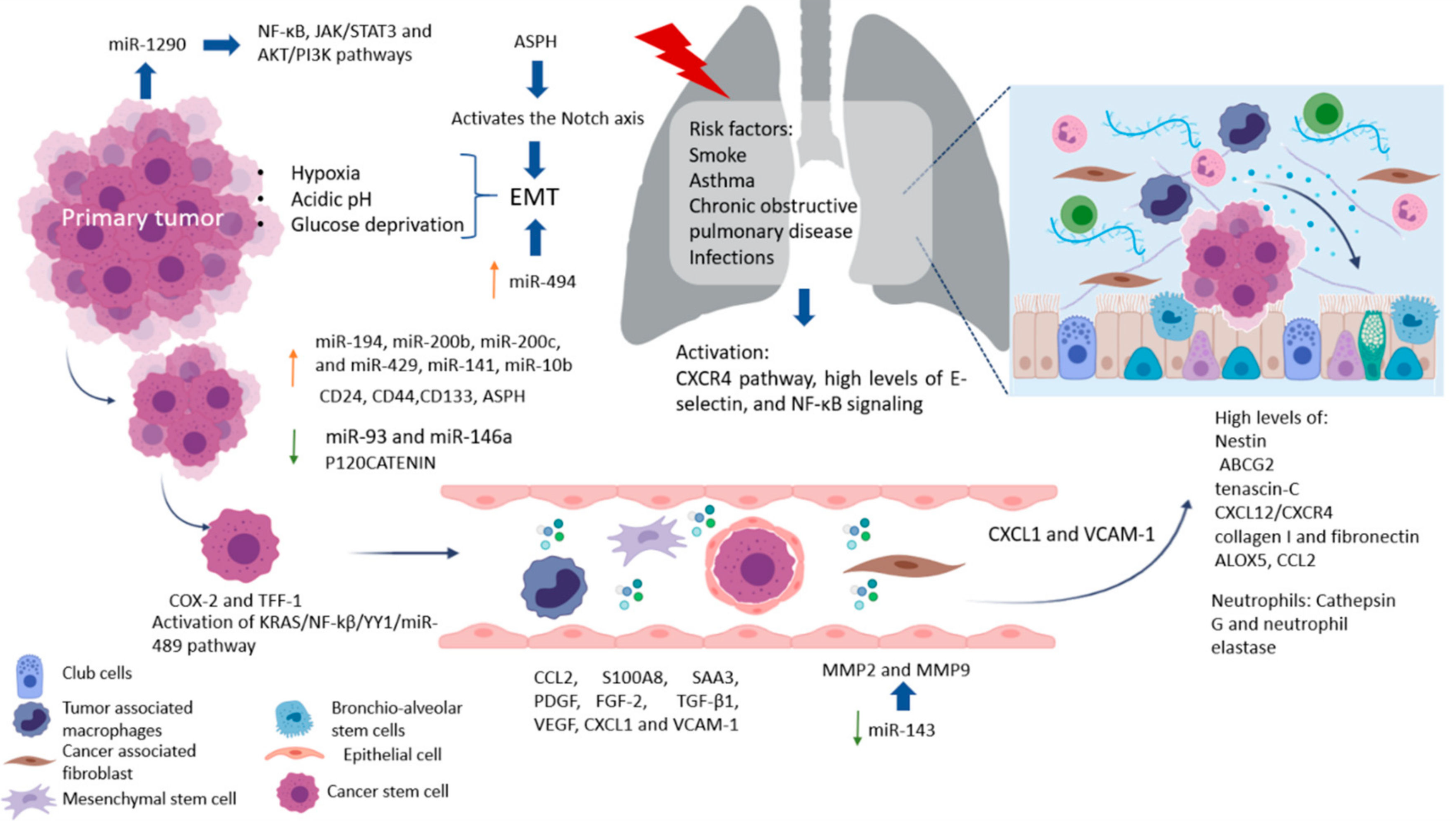
Metastasis is an inefficient process in which cells from the primary tumor spread by releasing circulating tumor cells (CTCs) into the vasculature towards a distant organ to colonize it, establishing metastasis. A process where only 0.01% of the cells that enter the circulation can successfully reestablish a new metastasis, and while this seems highly inefficient, it is more often than not a fatal step in cancer progression [1][2]. Cancer stem cells (CSC) are the subpopulation of cells responsible for promoting angiogenesis, local invasion, distant metastasis, and resistance to apoptosis. Moreover, epithelial tumor cells (mature population) gain invasiveness and migratory abilities through the process of epithelial–mesenchymal transition (EMT), which is where a complex network of interconnected factors and pathways meet, such as transforming growth factor-β (TGFβ), epidermal growth factor (EGF), insulin-like growth factor (IGF), WNT, Hedgehog, and Notch pathways, all of which regulate and promote CSC growth [3][4][5]. The spread of CSC from the primary tumor to a secondary site is a process highly dependent on signaling cues, such as hypoxia, acidic pH, and/or glucose deprivation [6]. However, only one out of 500 CSC will survive in the circulation, even though mechanisms to protect CSC from elimination by the immune system exist as the secretion of IL-4 and CD200, which represents an important role in immune escape [7]. CSC in the bloodstream or otherwise in the lymphatic system can cluster together with stromal cells (fibroblasts, endothelial, tumor-infiltrated myeloid cells, or pericytes) for improved metastasis potential. Endothelial cells (ECs) in healthy established vessels remain quiescent for years. Under certain conditions, such as hipoxia or inflammation, as occurs in pathologies such as cancer and wounds, they can rapidly switch to an angiogenic state and start to form new blood vessels by interaction with pericytes [8][9][10]. Once an angiogenic switch is turned on, factors (VEGF, PDGF, TNF-α, and IL-8) inside the tumor promote growth and metastasis [11][12]. A majority of ECs in the tumor vasculature are tumor-derived ECs (TECs) retain remain distinct from cancer cells, by are not immortal and their ontological endothelial identity permit participate in making up the lining of neoangiogenic vasculatures in the TME and accelerating tumor progression [13][14]. Circulating tumor endothelial cells (CTECs) with a mature phenotype, derived from vessel wall turnover, play an important role in tumor initiation, progression, metastasis, and neovascularization [15]. Moreover, it has been found clusters of endothelial cells expressing endothelial markers as vimentin and other lineage markers, such as FN1, SERPINE1, and FOXC1, that improve the survival of cancer stem cells and promote their dissemination [15]. Increased CTECs in cancer patients have a worse prognosis, indicated as potential biomarkers of angiogenesis and metastasis, and can express PDL-1 that permit the evaluation of immunotherapy efficacy [16].
Once CSC attaches and develops with success, a pre-metastatic niche will form. Pre-metastatic niches additionally require exosomes or exosomal-like extracellular vesicles (ECV) from entrained bone marrow-derived cells and macrophages to start changes in the cells. Ideally building an effective microenvironment that serves as a fertile niche for tumor cell growth [8][9][10]. Researchers estimate that between 20% and 54% of malignant tumors develop metastasis. Primarily at lymph nodes, with the liver and lungs as the second most common sites of metastasis [17]. Colorectal cancer, the third most frequent neoplasm, can progress to the liver (40–50%) and lung (10–20%) depending on the primary tumor’s stage. Clinical data show that median survival is just 5–20 months without treatment [18]. Meanwhile, pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive cancers with high metastatic potential. At the time of PDAC diagnosis, approximately 50% of patients present metastatic disease with 19–39% affected in the lungs and >50% in the liver. The median survival time is around 6–11 months in patients with metastatic pancreatic cancer [19]. Interestingly, several studies have reported that isolated lung metastases are associated with better survival outcomes than patients with other solitary metastatic organs [20][21][22].
2. Lung Metastasis
The lungs are one of the most complex organs in the human body. Their function is to exchange oxygen from the external environment with carbon dioxide from the cardiovascular system. Its role in the development of metastasis can result after physical trauma that induces local inflammation (smokers, asthma, obstructive pulmonary disease, or pneumonia) and tissue damage, creating a favorable environment that attracts metastatic cells from distant sites. Also, it has been reported that lung inflammation in smokers or in people with lung diseases such as asthma, chronic obstructive pulmonary disease, and infections, such as pneumonia, are risk factors for lung metastases. Some factors related to the migration of cells and vascular permeability are CCL2, S100A8, and SAA3. Upregulation of fibronectin colocalized with LOX, and higher levels of expression of VCAM-1, a receptor for VLA-4 in patient samples of metastatic tumor nodules in the lungs and bone, have been found [23]. The pro-metastatic effect played by exposure to smoking is related to the activation of the ubiquitin-chemokine receptor type 4 (CXCR4) pathway, high tissue levels of E-selectin, activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), signaling in pneumocytes, increased chemokine ligand 2 (CCL2) expression, and macrophage infiltration in the lung microenvironment. Moreover, lung alveolar cells induce chemokine secretion, which recruits neutrophils. The latter, through the synthesis of arachidonate 5-lipoxygenase (ALOX5)-dependent leukotriene, may promote survival and proliferation of leukotriene B4-expressing metastatic clones [17]. Inflamed lungs recruit neutrophils to release Cathepsin G and neutrophil elastase and destroy the protein Thrombospondin 1 (Tsp-1), which protects lung tissue from metastasis. Another alternative, but not mutually exclusive, is that micro-metastatic foci are already present at the time of physical injury [24]. Therefore, it is believed that lung metastases depend on circulatory system involvement, which flows into the pulmonary arterial system through the subclavian vein via the thoracic duct from the lymph nodes invaded by tumor cells [25]. More recently, DPC4 loss is shown to be associated with EMT, tumor progression, and the presence pulmonary metastases [26].
Factors Crucial for the Formation of Lung Metastasis
Factors produced by cells in pre-niche can support survival and growth of disseminated tumor cells were in Figure 1. The interstitial space is the main site for lung metastasis, which is adjacent to the terminal bronchioles. Bronchioalveolar stem cells (BASC) are situated in the terminal bronchioles and present pro-surfactant apoprotein-C (SP-C), a marker for alveolar type II (AT2) cells, and CC10, a marker for club cells—both are related with the lung pre-metastatic niche. Bone marrow cells, club cells, and alveolar macrophages are also present in pre-metastatic lung [23].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13010103
References
- Valastyan, S.; Weinberg, R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147, 275–292.
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- Kuşoğlu, A.; Avcı, Ç.B. Cancer stem cells: A brief review of the current status. Gene 2019, 681, 80–85.
- Rodriguez-Aznar, E.; Wiesmüller, L.; Sainz, J.B.; Hermann, P.C. EMT and Stemness—Key Players in Pancreatic Cancer Stem Cells. Cancers 2019, 11, 1136.
- Tsubakihara, Y.; Moustakas, A. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β. Int. J. Mol. Sci. 2018, 19, 3672.
- Mendoza, M.; Khanna, C. Revisiting the seed and soil in cancer metastasis. Int. J. Biochem. Cell Biol. 2009, 41, 1452–1462.
- Codony-Servat, J.; Rosell, R. Cancer stem cells and immunoresistance: Clinical implications and solutions. Transl. Lung Cancer Res. 2015, 4, 689–703.
- Garza Treviño, E.N.; González, P.D.; Valencia Salgado, C.I.; Martinez Garza, A. Effects of pericytes and colon cancer stem cells in the tumor microenvironment. Cancer Cell Int. 2019, 19, 1–12.
- Plaks, V.; Kong, N.; Werb, Z. The Cancer Stem Cell Niche: How Essential is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell 2015, 16, 225–238.
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948.
- Hida, K.; Maishi, N.; Annan, D.A.; Hida, Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int. J. Mol. Sci. 2018, 19, 1272.
- Salazar, N.; Zabel, B.A. Support of Tumor Endothelial Cells by Chemokine Receptors. Front. Immunol. 2019, 10, 147.
- Lin, P.P. Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): A Novel Versatile Player in Tumor Neovascularization and Cancer Metastasis. Cells 2020, 9, 1539.
- Wei, Y.F.; Yang, Q.; Zhang, Y.; Zhao, T.J.; Liu, X.M.; Zhong, J.; Ma, J.; Chen, Y.X.; Zhao, C.; Li, J.X. Plumbagin restrains hepatocellular carcinoma angiogenesis by suppressing the migration and invasion of tumor-derived vascular endothelial cells. Oncotarget 2017, 8, 15230–15241.
- Cima, I.; Kong, S.L.; Sengupta, D.; Tan, I.B.; Phyo, W.M.; Lee, D.; Hu, M.; Iliescu, C.; Alexander, I.; Goh, W.L.; et al. Tumor-derived circulating endothelial cell clusters in colorectal cancer. Sci. Transl. Med. 2016, 8, 345ra89.
- Zhang, L.; Zhang, X.; Liu, Y.; Zhang, T.; Wang, Z.; Gu, M.; Li, Y.; Wang, D.D.; Li, W.; Lin, P.P. PD-L1+ aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett. 2020, 469, 355–366.
- Stella, G.M.; Kolling, S.; Benvenuti, S.; Bortolotto, C. Lung-seeking metastases. Cancers 2019, 11, 1010.
- Jahanafrooz, Z.; Hashemzaei, M. Colon cancer therapy by focusing on colon cancer stem cells and their tumor microenvironment. J. Cell. Phys. 2019, 235, 4153–4166.
- Kulke, M.H. Metastatic Pancreatic Cancer. Curr. Treat. Options Oncol. 2002, 3, 449–457.
- Oweira, H.; Petrausch, U.; Helbling, D.; Schmidt, J.; Mannhart, M.; Mehrabi, A.; Schöb, O.; Giryes, A.; Decker, M.; Abdel-Rahman, O. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J. Gastroenterol. 2017, 23, 1872–1880.
- Bellon, E.; Gebauer, F.; Tachezy, M.; Izbicki, J.R.; Bockhorn, M. Pancreatic cancer and liver metastases: State of the art. Update Surg. 2016, 68, 247–251.
- Deeb, A.; Haque, S.-U.; Olowokure, O. Pulmonary metastases in pancreatic cancer, is there a survival influence? J. Gastrointest. Oncol. 2015, 6, E48–E51.
- Maru, Y. The lung metastatic niche. J. Mol. Med. 2015, 93, 1185–1192.
- El Rayes, T.; Catena, R.; Lee, S.; Stawowczyk, M.; Joshi, N.; Fischbach, C.; Powell, C.A.; Dannenberg, A.J.; Altorki, N.K.; Gao, D.; et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl. Acad. Sci. USA 2015, 112, 16000–16005.
- Font-Clos, F.; Zapperi, S.; La Porta, C.A.M. Blood Flow Contributions to Cancer Metastasis. iScience 2020, 23, 101073.
- Shin, S.H.; Kim, H.J.; Hwang, D.W.; Lee, J.H.; Song, K.B.; Jun, E.; Shim, I.K.; Hong, S.-M.; Kim, H.J.; Park, K.M.; et al. The DPC4/SMAD4 genetic status determines recurrence patterns and treatment outcomes in resected pancreatic ductal adenocarcinoma: A prospective cohort study. Oncotarget 2017, 8, 17945–17959.
