Co–Cr dental alloys consist of Co, Cr and also other metals (e.g., gallium, iron, molybdenum, nickel, ruthenium, tungsten). Since the 1990s, regulations have emerged to protect European patients and also monitor recent scientific knowledge. The use of metals and, therefore, dental alloys has been highly regulated by directives and regulations. Europe has, therefore, developed a regulatory package to protect all players involved in alloys, whether they are metal producers, manufacturers of alloys and medical devices, healthcare professionals and patients. Objective information is provided about Co–Cr dental alloys, with regard to both the latest toxicological data and regulatory developments from 2020–2025. The release of metal ions and the problem of wear particles are also discussed. With the recent change of regulatory status of Co, it is necessary to know the many repercussions (economic, technical etc.) of their use precisely in order to then allow actors to modify their daily work. The legislative changes also bring the need to propose new alternatives to Co-Cr dental alloys.
- cobalt-chromium dental alloys
- metallic cobalt
1. Introduction
During the 20th century, the history of dentistry has intimately been linked with metal alloys and those using cobalt (Co, CAS no. 7440-48-4, EC/List no. 231-158-0) and chromium (Cr, CAS no. 7440-47-3, EC/List no. 231-157-5) hold an important place. In 1907, the first Co–Cr alloys were designed as Co–Cr–W and Co–Cr–Mo alloys (W = tungsten and Mo = molybdenum) [1] and in the 1930s they began to be used for the preparation of removable partial denture (RPD) frameworks [2]. Moreover, this material has been also widely observed in other areas of medicine such as stents [3][4], intervertebral disc replacement, and in knee or hip arthroplasty [5][6]. The success of Co–Cr alloys is mainly due to mechanical properties such as stiffness, strength and corrosion resistance, which are regarded as excellent [2]. Moreover, this type of material achieves its corrosion resistance through the formation of Cr-based oxides on the surface; its biocompatibility then becoming extremely valuable over time [7].
Co–Cr dental alloys consist of Co, Cr and also other metals (e.g., gallium (Ga), iron (Fe), Mo, nickel (Ni), ruthenium (Ru), W). In Table 1, some Co–Cr alloys actually used are listed, but only those used in the field of dentistry. These multi-metal alloys are nevertheless called “Co–Cr” because of the very high proportion of these two metals inside alloys. For example, in the case of alloy Vi-comp II® (for fixed prostheses, Table 1), there are 10 different metals and the couple Co–Cr represents 79.9% of this alloy, the other eight metals only represent 20.1%. Co, Cr and their numerous respective complexes (e.g., cobalt(II)-porphyrin, smaltite, chromium picolinate, chromite) are naturally present in soil, water, plants, animals and humans. Briefly, metallic Co, Co(0), is a lustrous, silver-gray and hard metal after reductive smelting (found in nature bonded with other elements, extremely rare as native, still not listed as a mineral by International Mineralogical Association). Co(0) exists in two crystalline forms, namely a close-packed hexagonal (CPH) crystal structure at room temperature and a face-centered cubic (FCC) system obtained from the CPH structure at 421 °C [8]. There are two main degrees of oxidation for Co, namely Co(II) and Co(III). For example, Co(III) ions occupy the catalytic site of vitamin B12 and are essential to the biological activity of vitamins in live organisms. In bone cells, replenishing the pool of cobalt ions promotes their growth [9]. Therefore, Co and related are present in small quantities in the human body [10]. On the other hand, Cr(0) is a lustrous, brittle and hard metal, existing in its native state (e.g., Sichuan native Cr). Among the other forms of Cr, the trivalent and hexavalent Cr are the two most widespread existing states [10][11]. The form of Cr found in food is Cr(III) (CAS no. 16065-83-1, EC/List no. 605-220-6) and it is essential in human organisms for maintaining a normal glucose level and ensuring an effective functioning of metabolism. Its deficiency in humans will lead to impaired glucose tolerance, fasting hyperglycemia, glycosuria, hypoglycemia, elevated circulating insulin and decreased insulin receptor number [11]. Thus, it is clear that Cr in small amounts is a necessary element for healthy functioning of the body and all of these systems. Unlike Cr(III), Cr(VI) (CAS no. 18540-29-9, EC/List no. 606-053-1) is a toxic, strong oxidizing agent and crosses cell membranes. Cr(VI) compounds are classified as known human carcinogens by the International Agency for Research on Cancer (IARC) [12]. However, it was shown that if Cr(VI) is released from an alloy, it is only present for a very limited time because it is quickly reduced to the trivalent state in vivo [13].
Table 1. Composition of some alloys used in dentistry.
| Type | Trademark Mfr. 1 (Country) |
Composition (Mass%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Co | Cr | Mo | Si 2 | Mn 2 | C 2 | Fe | W | Other | ||
| Alloys for removable partial denture | Wironit® extrahart Bego (Germany) |
63.0 | 30.0 | 5.0 | 1.0 | 1.0 | <1.0 | |||
| Remanium® GM 800+ Dentaurum (Germany) |
58.3 | 32.0 | 6.5 | 1.0 | <1.0 | 1.5 | N 2 < 1.0 | |||
| Orthodontic wires | Alloy CoCr 3.002 Dentaurum (Germany) |
31.0–35.0 | 28.0–32.0 | 4.0–6.0 | ≤0.1 | ≤0.1 | ≤0.35 | 27.0–31.0 | ||
| Remaloy® Dentaurum (Germany) |
rest | 18.0–22.0 | 3.0–5.0 | ≤0.5 | ≤1.0 | ≤0.03 | 4.0–6.0 | 3.0–5.0 | Ni 19–23 Ti 2 0.1–2 S 2 ≤ 0.1 |
|
| Alloys for fixed prostheses | CoCr Biostar ERNST HINRICHS Dental (Germany) |
61.65 | 27.75 | 1.61 | <1.0 | <1.0 | 8.45 | |||
| Vi-comp II® Dentsply Sirona (USA) |
52.5 | 27.4 | <1.0 | 1.0 | 12.1 | Ga 2.5 Ru 2.4 Cu 2 1.0 Nb 2 < 1.0 Ta 2 < 1.0 |
||||
1 Mfr.: abbreviation for manufacturer. 2 Si: silicium; Mn: manganese; C: carbon; N: nitrogen; Ti: titanium; S: sulfur; Cu: copper; Nb: niobium; Ta: tantalum.
These two metals have their own characteristics and are, therefore, found in trace amounts in the human body where they are important for regulating biochemical functions as previously described. However, once Co and Cr are artificially implanted in the human body via the use of medical devices, metal ions and wear particles release from these alloys can also cause toxicity [10]. Therefore, it is necessary to differentiate clearly between the physiological quantities of these metals and the quantities causing medical complications. As in all types of biomaterial, Co–Cr alloys could thus cause harmful effects in the human body.
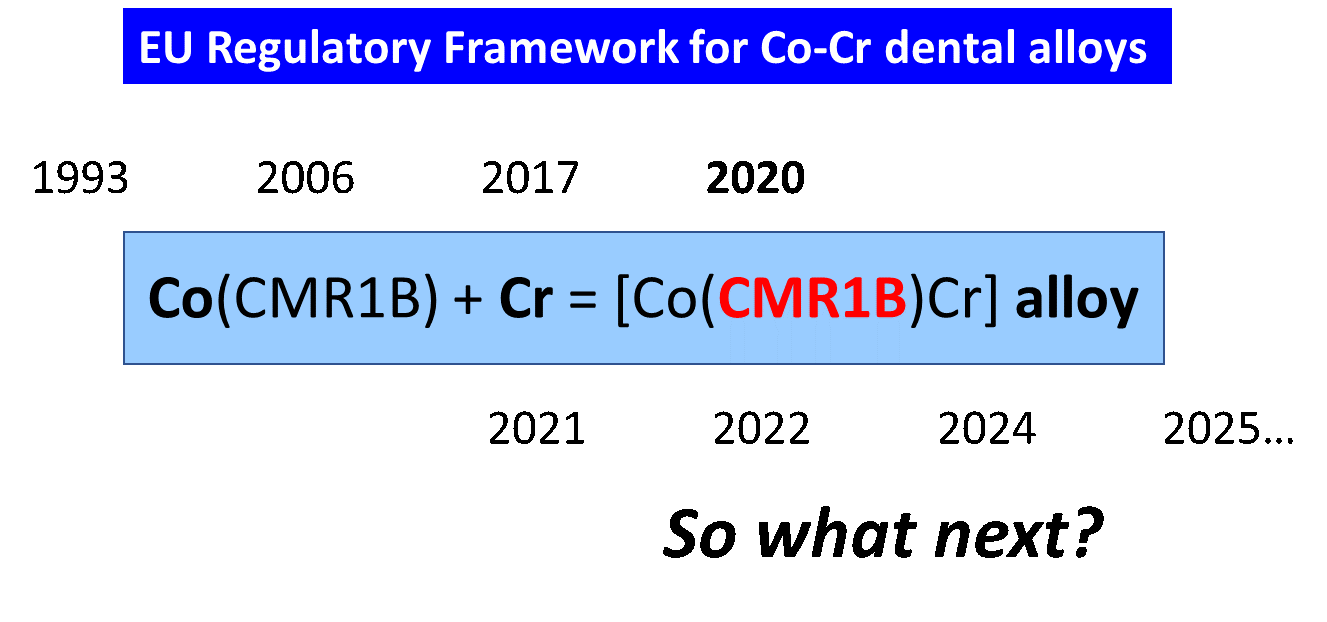
Since the 1990s, regulations have emerged to protect European patients and also monitor recent scientific knowledge. The use of metals and, therefore, dental alloys has been highly regulated by directives and regulations. Europe has, therefore, developed a regulatory package [14] to protect all players involved in alloys, whether they are metal producers, manufacturers of alloys and medical devices, healthcare professionals and patients. This regulatory package will also evolve with advances and scientific knowledge. In 2020, an important event affected the regulation of Co–Cr alloys because Co was listed as a CMR (carcinogenic, mutagenic and toxic for reproduction) substance (Figure 1) and now marketing an alloy has become complicated!
Figure 1. A new mathemathical-regulatory equation.
2. Co–Cr Dental Alloys: Metal Ions and Wear Particles Release
A dental biomaterial should satisfy diverse criteria to be declared compliant for clinical use. Among the main characteristics retained, we can cite mechanical properties, biocompatibility, non-toxic, high corrosion resistance and high wear resistance [15]. The last two criteria are discussed in this section.
2.1. Corrosion and Metal Ions Release
Co and Cr dental alloys have the essential characteristic of being resistant to corrosion and oxidation in the body when they are used as inputs for a finished product (e.g., alloys for orthodontic wires). However, we cannot negate the fact that all materials are susceptible to corrosion in the human body and especially in the mouth [16][17][18]. Corrosion is the result of the oxidation of the metal parts. This phenomenon is multifactorial, and many types of corrosion can be occurred including fretting, pitting and galvanic corrosion.
Under normal conditions of use, without particular signs of corrosion, the presence of Cr allows the formation of a Cr-based oxide layer on the surface [19]. This layer is valuable, among other things, to protect the Co from oxidation and then to improve the resistance of Co–Cr alloys against galvanic corrosion, for example. Unfortunately, over time several phenomena can lead to the corrosion of metallic biomaterials such as mechanical abrasion of the protective passivated layer and attack by local acidification due to the presence of certain germs (e.g., Streptococcus mutans) [20]. Corrosion can be also related to fragile areas (e.g., lack of material, cracks) due to internal defects during physical metallurgy and processing of Co–Cr alloys [21]. The environment of the oral cavity is thus very favorable to corrosion, with many factors including at least natural agents (air and water, saliva), food contents (chloride ions), sugary drinks, dental plaque, microorganisms, very frequent temperature and pH variations, and the presence of diverse dental prosthetic devices. When the corrosion process starts, Co and Cr ion metals are then released in the oral cavity. Depending on the composition of the alloy (Table 1), other metal ions may also be released such as Cu(II), Fe(II), Fe(III), Mn(II), Mo(VI), Ni(II), etc. [17][22][23]. A recent study [24] compared the corrosion resistance of cast Co– and Ni–Cr dental alloys. Two alloys were tested in contact with three different environments, namely deionized water, artificial saliva and acidified artificial saliva. The Co–Cr alloy tested was largely superior to that of Ni–Cr, offering more stability in an acid environment. Nevertheless, Co and Cr ions were released into solution during testing.
2.2. Wear of Dental Materials
A second very important aspect of Co–Cr alloys for their use in restorative materials is their high wear resistance. It is not conceivable to develop alloys which release numerous wear particles. As developed in Section 2.1, these wear particles could also generate metal ions. Moreover, it is well known that certain metals are considered toxic to human health. For example, since the 1990s, nickel has been considered as carcinogenic to humans since the work of the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) [25]. One of the immediate consequences was the European parliament’s decision to restrict the use of Ni and then to develop new biomaterials [26]. For manufacturers and users, the release of Ni from nickel-based alloys and related materials has become problematic. In the same way, the release of metals from Co–Cr alloys is extremely important to determine and then to confirm or not their low wear properties are related to a lower toxicity to the human body.
There is a great number of studies in the field of Co–Cr alloys used in arthroplasty (hip, knee) where exposure levels may be high [27][28][29]. Wear remains a highly studied phenomenon [30] to always improve existing alloys and allow them to be optimized by adding additional metals [31]. On the other hand, in the specific field of dentistry, a recent review brings together the important elements around the wear of restorative materials [31]. All these studies concerning the wear levels of biomedical equipment are of course linked to the toxicity that the released particles can generate afterwards [27][31].
More recently, preoccupations have been raised about the size of particles generated from the wear of prostheses and implants using (Co–Cr) alloys. Kovochich et al. [32] studied the size of wear particles released from metal-on-metal (MoM) hip implants during normal and edge-loading conditions. The analysis of the wear debris showed widely varying sizes and composition:
- (1) Mean primary particle size by volume was 35 nm (under normal wear, range = 9–152 nm) and 95 nm (under edge-loading conditions, range = 6–573 nm).
- (2) Hydrodynamic diameter analysis by volume gave mixed results, namely particles from normal wear ranged from nano- (<100 nm) to submicron (<1000 nm) in size; from edge-loading conditions, the size range of particles was comprised between <100 nm and up to 3000–6000 nm.
- (3) The nature of the isolated particles also varied according to the study conditions, the vast majority of particles under normal use was Cr (98.5%).
- (4) Under edge-loading conditions, wear particles contained more Co (≈640-fold) than Cr.
On reading these first results around the double characterization (physical and chemical) of MoM wear particles, we must ask what about the wear particles generated from dental restorative materials? Do the nano- and micro-sized wear debris have the same clinical impact?
All the studies mentioned above, around the issue of the release of ions and wear particles, require access to a wide range of tools in order to characterize them as precisely as possible.
2.3. Toolbox to Detect Metals
Since the generalization of the use of orthopedic implants (e.g., hip, knee, ankle, tooth), numerous studies have been published to observe the impact of corrosion and wear particles on the human body [23][24][33]. In the very specific case of dental use, studies are difficult to conduct because there are so many situations that lead to wear restorative material. Crothers defined a large series of oral events leading to wear situations (e.g., sliding, bruxism, toothbrush and dentifrice, scaling and cleaning, polishing pastes) [34]. Nevertheless, great efforts have been made to propose methods and tools to study the phenomena of corrosion and wear and thus be able to anticipate any problem.
First, to identify and quantify metal ion release generated from Co–Cr alloys, different methods and techniques can be used to determine the corrosion rate linked with the release of metal ions and then their biocompatibility [17][35][36][37][38][39][40]. The toolbox to explore the release of metal ions and possible consequences such as corrosion is presented in Table 2. Another way to check/verify the ion release is to use “patch testing” [41][42][43].
Table 2. Methods and techniques to explore the release of metal ions and corrosion.
| Metal Ions Release and Corrosion | Methods and Techniques | Remarks |
|---|---|---|
| Testing methods | alloys shaped into discs/cylinders and polished static immersion test (chemical corrosion) |
variation of parameters: artificial saliva solution, presence or absence of bacteria (e.g., Eikenella corrodens), pH, time, altered conditions, etc. |
| dynamic immersion test (biocorrosion) | ||
| Release of ions | atomic absorption spectroscopy (AAS) electrochemical impedance spectroscopy (EIS) inductively coupled plasma optical emission spectrometry (ICP-OES) |
to identify released elements, to determine ion concentrations |
| inductively coupled plasma mass spectrometry (ICP–MS) | ||
| polarization test by potentiostat | ||
| Characterization techniques | energy dispersive spectroscopy (EDS) optical interferometry scanning electron microscopy (SEM) |
to compare the corrosion resistance, to evaluate porosity, to analyze surface topography |
| X-ray diffraction (XRD) |
Second, tribological performances of Co–Cr alloys and related materials can be also studied. The main methods and techniques are summarized in Table 3 using general and specific references [15][30][44][45][46][47]. For some years, in situ testing have also been developed [48]. This constitutes a very interesting complementary approach to improve the correlation between the results obtained by in vitro tests and those arising from in vivo studies.
Table 3. Methods and techniques to study the tribological behavior of metallic biomaterials.
| Wear of Metallic Biomaterials | Methods and Techniques | Remarks |
|---|---|---|
| Testing methods | alloys shaped into discs/cylinders and polished ball and crater, ball-on-disc block-on-disc one-way slide and static end load |
variation of parameters: temperature, magnitude of biting force, simulated body fluids, etc. |
| pin-on-disc, pin-on-flat | ||
| Surface roughness | optical profilometer 3D-profilometer |
to test the cross-sectional profile, to calculate the wear volume |
| atomic force microscope (AFM) | ||
| X-ray photoelectron spectroscopy (XPS) | ||
| Characterization techniques | energy dispersive spectroscopy (EDS) scanning electron microscopy (SEM) transmission electron microscopy (TEM) |
for particles and/or surface |
| X-ray absorption spectroscopy (XRAS) | ||
| X-ray diffraction (XRD) |
For more information, a recent review described methods to evaluate the wear simulation in the laboratory [31]. The authors also highlighted the difficulty of adopting a harmonized protocol for testing the wear of dental materials. Many industrialists and researchers have developed their own techniques according to the alloys manufactured. Attempts have been made around International Organization for Standardization (ISO) standards, but a lot of work remains to be done in the near future. At present, comparisons between biomaterials are difficult and require some hindsight with the great variability of the results obtained by the different teams.
In conclusion, the use of metallic biomedical materials could lead to the release of metal ions and debris which can lead to the appearance of local or systemic toxicity. Therefore, it is important to know the toxicological risks linked to Co and Cr exposures, the two main metals present in the corresponding alloys.
3. Toxicological Risks of Co–Cr Dental Alloys
A selection of the latest research on the toxicological risks of Co–Cr dental alloys is summarized in this section. In parallel, a systematic review was very recently prepared [49] to analyze the full literature of the last 25 years on the toxicity of Co–Cr dental alloys. It should be noted that exposure to metals, and more particularly Co and Cr, occurs mainly through three routes of exposure, namely inhalation, ingestion and skin contact. In the case that concerns us, these metals are also in contact with the mucous membrane of the mouth.
Another important point to remember is the fact that Co and Cr are naturally present in the human body (Table 4) [50]. Co is an essential trace element and as for Cr, it is rather referred to as a microelement. As seen in the introduction, Co and Cr have beneficial effects on human health but also, they can have harmful effects. Therefore, it is necessary to respect a daily intake for each of the two elements (Table 4) [50].
Table 4. Amounts of Co and Cr for an adult human body.
| Element | Total Average Quantity (g) | Daily Requirement (mg/day) |
|---|---|---|
| Co | 1.1 | 0.0001 |
| Cr | 0.006 | 0.0050 |
3.1. Recent Toxicological Studies
Globally, Co–Cr alloys are widely used for dental applications because they offer the opportunity to use suitable materials that are characterized by a good biocompatibility [51]. In particular, their corrosion resistance plays an important part in reducing complications with surrounding tissues. Co–Cr is sufficiently chemically inert to be significantly relevant in reducing irritation, allergic reactions and general immune system resistance [52]. Despite everything, local and systemic effects can appear [53][54]. It is well known that metal ions and debris can cause hypersensitivity reactions and affect the immune response system. It should also be borne in mind that Co–Cr dental alloys mainly consist of Co and Cr, but other metals are also present (e.g., Ni, Mo, Mn) and can thus be responsible for undesirable effects [41]. Few clinical studies focusing on its biological impacts in dentistry have been performed until now. For example, there are very few case reports in the field of Co–Cr alloys and their dental use. A case report from the year 2010 demonstrated an association between the release of Co from dental reconstructions and an oral hypersensitivity reaction in a patient allergic to Co. In this case, the patient had a severe and constant burning pain in the mouth, tongue and lips two months after insertion of new dental implants in the upper and lower jaws, but the symptoms disappeared after the implants were replaced [55]. In another report [56], the patient has suffered for one year from pustules, blisters and scaly erythema on the hands and feet after treatment with Cr–Co crowns, although there were no symptoms in the oral cavity. These symptoms disappeared 3 weeks after removal of the crowns. Therefore, we wish to shed light on recent studies published (2016–2020) dealing with toxicological problems related to Co–Cr alloys.
In a non-randomized clinical-controlled trial, with 80 patients classified into two equal groups, conducted from January 2013 to January 2015, Arafa’s study [57] compared the effects of Ti and Co–Cr connectors in RPD. For each parameter analyzed (tooth mobility, bone loss and tissue reaction), Co–Cr alloy was less efficient. For example, at 24 months, the tissue reaction (mm) by Benson index in the Ti alloy group was ranged from 0 to 0.02. On the other hand, in the Co–Cr group the range obtained was from 0 to 0.16.
Between 2014 and 2016, Linauskienė et al. [58] conducted a retrospective study of allergic contact dermatitis (ACD) in Lithuania, which evaluated the sensitization of 546 patients to Ni, Co, Cr, gold (Au), palladium (Pd) and Ti. It is interesting to note that among a subpopulation of 87 patients tested (dentists or patients with oral symptoms), 35.6% (n = 31/87) and 14.9% (n = 13/87) of patients were sensitized to Au(I) sodium thiosulfate 2.0% and Pd(II) chloride, respectively. More globally, Co sensitization is often accompanied by sensitization to Ni. Sensitization to Ti was not found.
During this new investigation conducted by Al-Imam et al. [59], the problems reported included signs of inflammation of the oral mucosa (n = 11/66), oral candidiasis (n = 2/66) and ill-fitting prostheses (n = 16/66). For all subjects, they had insufficient oral hygiene. None of the 66 patients (with 84 dental prostheses) reported allergies to Co symptoms. These functional prostheses released no Co until 5 years after insertion. In parallel, 32 non-functional prostheses were also investigated and the authors noted Co release (n = 24/32) during the fabrication stages.
The observational study of Olms et al. [60] involving 86 subjects determined the frequencies and symptoms of allergies to dental materials. All patients had oral symptoms of contact allergy. Out of all studied patients, 52 (60.5%) shown signs of allergies at first contact. Most of these allergies were directed to metals (n = 27/86). Here, again the highest sensitization rate was observed in contact with Co and Ni. 35% of the patients (n = 18/52) reported a Ni allergy, and in parallel 19% of the patients (n = 10/52) had a Co allergy. Next to metal allergies, sensitivity towards some cosmetic ingredients were also observed, particularly allergies to methacrylate-containing denture resins (8% of the population studied). Moreover, incompatibilities were also observed in association with dental materials such as toothpastes, fluoride gels, eugenol, ceramics and polyamides. These findings are important to consider when evaluating reaction to dental materials. In case of combined allergies or allergies towards only one ingredient, it can be difficult to identify the allergen involved. If the patient had already developed allergies or other kinds of sensitivity issue and had also allergies to various materials used in dental care, this could cause difficulties in relating some symptoms to solely the use of Co–Cr alloys.
In a study published in 2020 [40], next to classical investigations on ions release and surface roughness, the authors also studied the cytotoxicity of selected Co–Cr alloys. The authors concluded that all five tested alloys were within the limits of cell viability according to standards for both mouse fibroblasts (L929) and the human bronchial epithelial cell line (BEAS-2B). It was even observed that the cytotoxic effect on BEAS-2B cells was slightly higher for Ti6Al4V ELI alloy than cast Co–Cr specimens.
Concerning the differential effects of metals, works have been published for the first time [61]. It is necessary to consider the different metals released into the oral microenvironment and their dissociated effects. In the coming years, new investigations will make it possible to learn even more about the effects of each of the metals released.
In general, the main effects reported on Co and Cr concern ACD and most studies require further clinical investigation.
3.2. Carcinogenicity, Mutagenicity and Toxicity for Reproduction of Co and Cr Metals
Certain metals such as beryllium (Be), cadmium (Cd) and Cr(VI) are listed as known human carcinogens by the IARC (monographs on the identification of carcinogenic hazards to humans) [62] and US National Toxicology Program (NTP, 14th report on carcinogens) [63]. For other metals, these two international organizations use the mention “probable carcinogens”. Among them, we can cite Co metal with tungsten carbide (from both IARC and NTP), and Co and Co compounds that release Co ions in vivo (from NTP only). The NTP and IARC act independently and their conclusions may thus diverge depending on the element studied. The scientific community and national/international authorities closely monitor metal exposures, for example, in the context of occupational exposures (e.g., metallurgical industries) [64] and exposures following orthopedic treatment (e.g., surgical implants) [12][65]. Recently, in September 2019, the U.S. Food and Drug Administration (FDA) published a report presenting many aspects on their in vitro and in vivo evaluations whose clinical observations on cancer [66].
Concerning the specific case of Co–Cr alloys, studies have been carried out for years to demonstrate if Cr [67] and Co [68] may or may not be considered as CMR substances. At present, in the Cr group, Cr(VI) is considered as CMR substance [25] when Cr(VI) is inhaled. In humans, the associated cancer is that of the lungs. On the other hand, Cr(VI) is not identified to be a stomach cancer hazard in humans, probably because it is quickly converted to Cr(III) in gastric fluid [64]. Some studies could conclude with regard to the cytotoxicity of Co–Cr alloys [69][70] but cytotoxicity is not carcinogenicity!
Other studies such as the one by Fernández-Miñano E. et al. demonstrated DNA damage in oral mucosa cells of children with fixed orthodontic appliances [71]. Nevertheless, which metal(s) present in complex mixtures of metals is/are the cause of the observed effects? In another article published in 2016 [72], the authors analyzed a large number of studies on exposure to metals, some of which are very specific to dentistry. They concluded that it is extremely difficult to make definitive conclusions on exposure to metal mixtures. Many parameters must be considered such as complex mixtures of metals, variable concentrations, and dietary habits. Therefore, for Co metal, the studies are not as clear-cut as those found for Cr(VI). The correlation between potentially cytotoxic in vitro studies and proven clinical observations in humans is not as clearly established in the dental field.
A systematic review has just been prepared to make a precise point on this issue of the toxicological profile of Co–Cr dental alloys [49].
3.3. Year 2017, a Pivotal Year for Co–Cr Dental Alloys
On 22 September 2017, based on investigations carried out by the European Chemicals Agency (ECHA), the Committee for Risk Assessment (RAC) adopted an opinion on the proposal for harmonised classification of the metal Co [73]. Consequently, the metal Co should be reclassified as CMR 1B substance. This opinion may have surprised the industrial world because other broader studies (not only for dental use) did not lead to such marked toxicities [65].
If we add the new regulation (European Union, EU) 2017/745 on medical devices regulation (MDR) published on 5 April 2017 [74], the year 2017 was pivotal for Co–Cr dental alloys. How can the production of Co–Cr alloy be managed for the manufacture of dental prostheses and restorative materials containing a substance declared CMR? How will European regulations impact the entire Co–Cr dental alloys sector? What will be the future of alloys containing Co?
4. European Union (EU) Regulatory Framework
For the last few decades, the use of Co–Cr based alloys increased because they were considered an economic alternative to precious alloys and a safer alternative to non-precious Ni-based alloys. In 1990, the IARC classified metallic Ni in group 2B of possible carcinogens to humans [75]. Since 2017, new legislative changes also affect metallic Co. Therefore, it is necessary to understand the European regulatory framework, both on medical devices and chemicals, and more particularly metals such as Co.
4.1. Medical Device Regulatory Requirements—Period 1990–2020
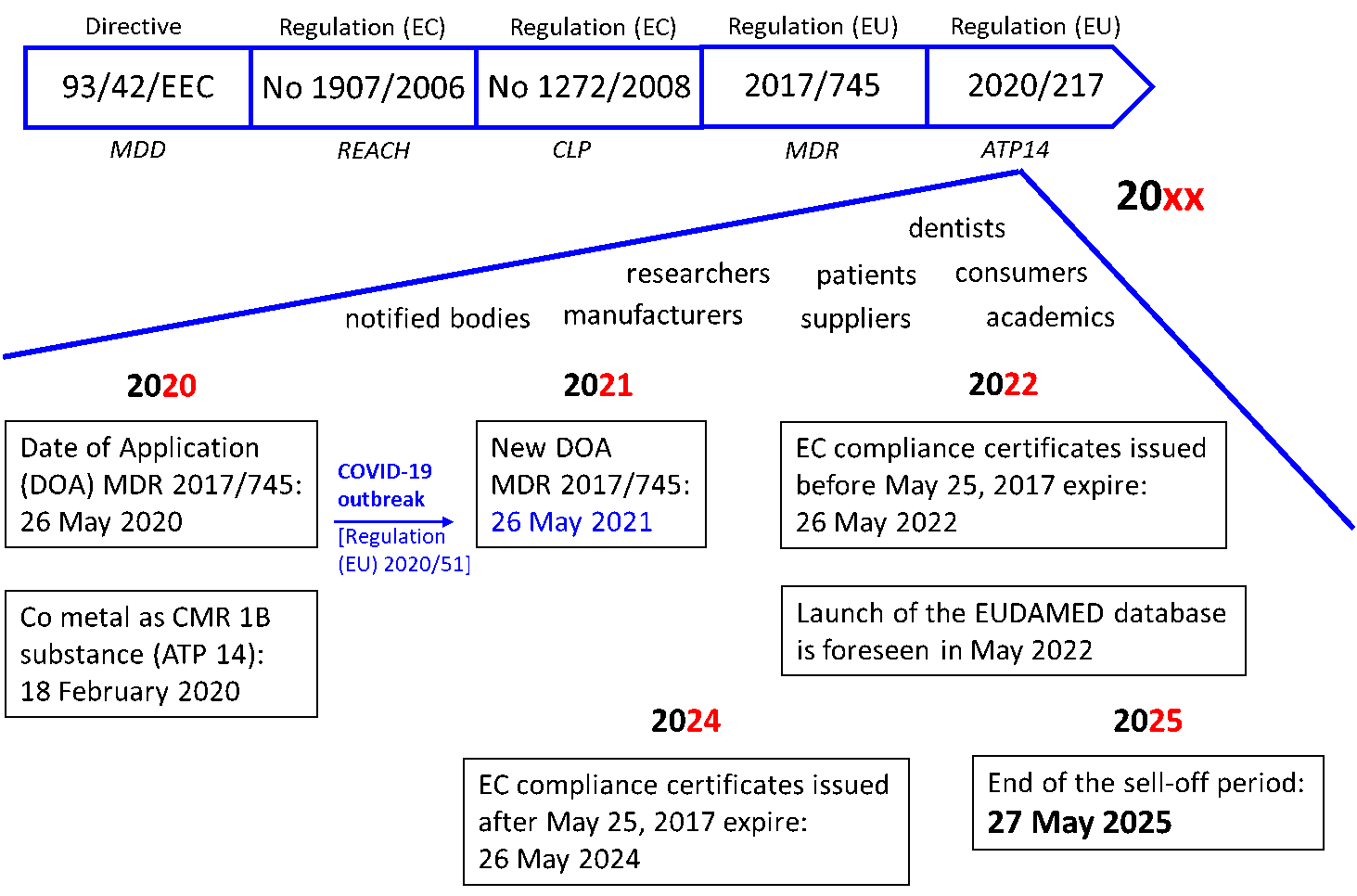
Since the 1990s, the European legislature has focused on the regulation of medical devices in order to harmonize the legislation of the member countries of the EU. Then in 1993, EU issued the Directive 93/42/EEC (also known as the Medical Devices Directive—MDD) (Figure 2) [76]. Until these major texts, the legislation had changed very little until 2017 and the entry into force of the MDR (26 May 2017). After a transition time of three years, the MDR (EU) 2017/745 was to apply as of 26 May 2020. However, the COVID-19 outbreak has been there! The new date of the MDR application (DOA) is now fixed for 26 May 2021 by a decision published on 24 April 2020. The regulation (EU) 2020/561 thus modified the regulation (UE) 2017/745 [77].
Figure 2. European Union (EU) medical device regulation timeline until 2020 and beyond.
All medical devices fall under the new regulation. Then, all players in medical devices, namely suppliers, manufacturers, academics, researchers and dentists are fully required to apply this regulation. Regulation (EU) 2017/745 revised all classification rules and specified new ones. Medical devices are classified according to the level of risk associated with their use (class I, class IIA, class IIB and class III, according to an increasing risk). If more than one rule applies, the classification to be retained is the highest. Consumer and patient protection measures are at the heart of this new regulatory system. The main benefits for all consumers and patients are to give better protection in terms of public health and patient safety. For example, for high-risk devices, stricter pre-market control is required. A new EU database on medical devices (EUDAMED) is also created and will propose a continuing view of the lifecycle of all products being available on the EU market. An identification system based on a unique device identifier (UDI) will optimize traceability of medical devices. Finally, in the case of defective products, a new financial mechanism should allow patients to be effectively compensated. Overall, this EU update aims to increase the credibility and reputation of the oversight system after the occurrence of a few incidents (e.g., breast implants).
The commission implementing regulation (EU) 2017/2185 of 23 November 2017 [78] gave the list of codes and corresponding types of device for the purpose of specifying the scope of the designation as notified bodies (NB) in the field of medical devices under regulation (EU) 2017/745. In the case of non-active dental implants and dental materials, the code is MDN 1103. The important step for all manufacturers is to affix the CE (“Conformité Européenne”) mark on it. According to the product’s type, a specific CE marking route will have to be followed. The declaration of conformity allowing the CE mark and NB number to be affixed on the product involves a NB. Generally, the first three steps include preparation of technical documentation, audit by NB, and declaration of conformity. At the end, the medical device could be placed on the EU market.
4.2. Chemicals Legislation—Period 2007–2020
In parallel with the MDR (EU) 2017/745, Europe focused on optimized management of chemicals. EU issued the regulation (EC) No 1907/2006 of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and establishing ECHA (Figure 2) [79]. REACH entered into force on 1 June 2007. The global purpose of this regulation is to ensure a high level of protection of both human health and the environment within the internal market. Different major deadlines were scheduled and the last one, 1 June 2018, ended the REACH implementation period. Companies are responsible for collecting information on the properties and uses of the substances they manufacture or import in quantities equal to or greater than one ton per year. They have to assess the dangers and risks that the substance may present. This information is sent to ECHA through a registration dossier. Registration is based on the principle “one substance, one registration”. Registration therefore applies to substances and related such as substances contained in mixtures and to certain cases of substances listed in articles.
In order to complement REACH, regulation (EC) No 1272/2008 on Classification, Labelling and Packaging (CLP) was issued and entered in force on 20 January 2009 [80]. This regulation harmonized the criteria for the classification of substances and mixtures, and the rules on labelling and packaging for hazardous substances and mixtures. It also aims at establishing a classification and labeling inventory of substances. Over the years, other chemicals are also listed in the ECHA database such as “Intermediate Use Only”. At the last update (13 November 2020), the database contained 23,032 unique substances and contains information from 101,006 dossiers. Metals Co (231-158-0) and Cr (231-157-5) were registered on 17 September 2010 and 8 September 2010, respectively [81].
This CLP legislation defined new CMR categories (1A, 1B and 2). Only substances classified as CMR of category 1A/1B are restricted by REACH. Since 2009 and the publication of the first Adaptation to Technical Progress (ATP), many updates and corrections have been made. As previously mentioned, the RAC proposed a reclassification of metal Co as a CMR 1B substance [73]. The 14th ATP to the CLP Regulation was published in the EU official journal on 18 February 2020, including the harmonized classification for cobalt metal as CMR (Annex VI to CLP, in force from 9 September 2021) [82].
4.3. Co-Existence and Grace Period 2017–2025
As with the introduction of the REACH regulation, the implementation of the MDR (EU) 2017/745 is being carried out through a transition period of four years (26 May 2017—26 May 2021). During this period, medical devices can be evaluated under either MDD or MDR legislation. After this point in time, all medical devices must only be approved according to the MDR process. Starting in March 2021, the the first module of EUDAMED for the registration of the economic operators (manufacturers, authorized representatives and importers) will be available. It will allow these operators to obtain the famous Single Registration Number (SRN) which will give access to the other functionalities of the database. This SRN will also be requested by the NB. In 2022, the massive database EUDAMED should be fully effective. Moreover, certificates and devices already on the market—known as legacy devices—will remain valid for a further five years until May 2022 (EC compliance certificates issued before 25 May 2017) or for a further seven years until May 2024 (EC compliance certificates issued after 25 May 2017) (Figure 2). From 27 May 2025, the only MDR (EU) 2017/745 will apply.
With the new classification of Co metal as CMR (and more precisely C1B, M2 and R1B), CLP regulation (EC) 1272/2008 has a direct impact to MDR regulation (EU) 2017/745.
5. Conclusions-Perspectives
Co–Cr alloys have been widely used for several decades in the field of restorative dentistry. Their mechanical properties combined with good biocompatibility have been clearly demonstrated. Therefore, this is counterbalanced by the occurrence of certain adverse effects, the most notable being ACD. Since 2017, regulatory pressure has become strict for Co with its registration as a CMR substance, based on in vitro and animal studies. However, it remains difficult to evaluate the impact of metal ions release and wear particles from dental alloys on the occurrence of cancer in humans. It is the precautionary principle that is adopted by administrative bodies.
In the context of the use of a CMR substance in a dental alloy and the regulatory package in force (MDR, REACH, CLP), it might be prohibited to market such invasive medical devices. Regulation MDR (EU) 2017/745 indicates that any medical device containing Co (generally in the form of an alloy in dentistry) is compliant with the regulation if the Co concentration is less than 0.1% (m/m). In Co–Cr dental alloys, Co is generally the main component, with percentages between 31% and 63% (for fixed prostheses, Table 1). Nevertheless, the medical device could be placed on the market only if the following conditions are respected: no alternatives are available and justification is provided by the NB. Now, under MDR (EU) 2017/745, all dental materials containing Co will have to label the presence of this metal, which is considered a CMR, and inform consumers.
Therefore, during the period 2021–2025, Co will be authorized for use in Co–Cr dental alloys, with the strict application of MDR (EU) 2017/745 and CLP regulation (EC) No 1272/2008. Labelling and consumer warning are mandatory. The legitimate question that may arise for dentist is the following: is it ethical to offer a prosthesis made from an alloy containing a CMR substance? Thus, Europe invites the medical device manufacturers and concerned medical specialists to be prepared to bring their devices into conformity or to find an alternative until May 2025. For medical device industries, it is still quite difficult to find other biomaterials that would have similar mechanical and physico-chemical properties as Co–Cr alloys in a short period. One alternative could be to prepare new alloys with (ultra) low quantities of the incriminated metal(s). This has been explored with Ni, also declared a CMR substance [83].
After 2025, only a reasoned justification that no substitution is possible could lead the NB to grant the CE marking to a device containing Co with a concentration superior to 0.1% (m/m). The wisest way would also be to find an alternative. Supplementary options of other metals could be used instead of Co–Cr alloys for medical purposes. For example, Ti is commonly considered to be an effective substitute to Co–Cr alloys in some dental applications. Ti is one of the most durable metals. What is more, Ti has great biocompatibility properties, which allows its usage for medical purposes and reduces the risk of allergies [84].
This entry is adapted from the peer-reviewed paper 10.3390/cryst10121151
References
- Marti, A. Cobalt-base alloys used in bone surgery. Injury 2000, 31, D18–D21. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S. Physico-mechanical properties and prosthodontic applications of Co–Cr dental alloys: A review of the literature. J. Adv. Prosthodont. 2014, 6, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.F.; Roffi, M.; Degrauwe, S.; Secco, G.G.; Aminian, A.; Windecker, S.; Pilgrim, T. Orsiro cobalt-chromium sirolimus-eluting stent: Present and future perspectives. Expert Rev. Med. Devices 2017, 14, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Gherbesi, E.; Natalini, G. The Ultimaster coronary stent system: 5-year worldwide experience. Future Cardiol. 2020, 16, 251–261. [Google Scholar] [CrossRef]
- Louwerens, J.K.; Hockers, N.; Achten, G.; Sierevelt, I.N.; Nolte, P.A.; Van Hove, R.P. No clinical difference between TiN-coated versus uncoated cementless CoCrMo mobile-bearing total knee arthroplasty; 10-year follow-up of a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2020, 1–7. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Zhou, X.; Zhang, L.; Mi, J.; Shan, Z.; Huang, B.; Chen, Z.; Chen, Z. Modulating the cobalt dose range to manipulate multisystem cooperation in bone environment: A strategy to resolve the controversies about cobalt use for orthopedic applications. Theranostics 2020, 10, 1074–1089. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Lee, B.W.; Alsenz, R.; Ignatiev, A.; Van Hove, M.A. Surface structures of the two allotropic phases of cobalt. Phys. Rev. B 1978, 17, 1510–1520. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chuang, C.; Yao, C.; Tang, C.-M. Effect of cobalt precursors on cobalt-hydroxyapatite used in bone regeneration and MRI. J. Dent. Res. 2020, 99, 277–284. [Google Scholar] [CrossRef]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.R.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2015, 4, 5729. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium as an essential nutrient for humans. Regul. Toxicol. Pharmacol. 1997, 26, S35–S41. [Google Scholar] [CrossRef]
- McGregor, D.; Baan, R.; Partensky, C.; Rice, J.; Wilbourn, J. Evaluation of the carcinogenic risks to humans associated with surgical implants and other foreign bodies—A report of an IARC monographs programme meeting. Eur. J. Cancer 2000, 36, 307–313. [Google Scholar] [CrossRef]
- Meek, R.D.; Afolaranmi, G.A.; Tettey, J.; Grant, M.H. Release of chromium from orthopaedic arthroplasties. Open Orthop. J. 2008, 2, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Overview on the Medical Devices Legislation in the EU. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices#medical-devices-legislation-section (accessed on 21 November 2020).
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear characteristics of metallic biomaterials: A review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Chitra, P.; Prashantha, G.S.; Rao, A. Long-term evaluation of metal ion release in orthodontic patients using fluoridated oral hygiene agents: An in vivo study. J. World Fed. Orthod. 2019, 8, 107–111. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K.; Woźniak, B.; Downarowicz, P. Release of metal ions from orthodontic appliances: An in vitro study. Biol. Trace Elem. Res. 2011, 146, 272–280. [Google Scholar] [CrossRef]
- De Aguiar, S.R.M.M.; Nicolai, M.; Almeida, M.; Gomes, A. Electrochemical behaviour of a cobalt–chromium–molybdenum dental alloy in artificial salivas. Bio-Med. Mater. Eng. 2015, 25, 53–66. [Google Scholar] [CrossRef]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, caries and simulation models. Nutrition 2010, 2, 290–298. [Google Scholar] [CrossRef]
- Upadhyay, D.; Panchal, M.A.; Dubey, R.; Srivastava, V. Corrosion of alloys used in dentistry: A review. Mater. Sci. Eng. A 2006, 432, 1–11. [Google Scholar] [CrossRef]
- Ciszewski, A.; Baraniak, M.; Urbanekbrychczynska, M. Corrosion by galvanic coupling between amalgam and different chromium-based alloys. Dent. Mater. 2007, 23, 1256–1261. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B. In vivo corrosion of CoCrMo alloy and biological responses: A review. Mater. Technol. 2018, 33, 127–134. [Google Scholar] [CrossRef]
- Mercieca, S.; Conti, M.C.; Buhagiar, J.; Camilleri, J. Assessment of corrosion resistance of cast cobalt- and nickel-chromium dental alloys in acidic environments. J. Appl. Biomater. Funct. Mater. 2017, 16, 47–54. [Google Scholar] [CrossRef]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Chromium, Nickel and Welding. Volume 49. Available online: https://publications.iarc.fr/67 (accessed on 27 September 2020).
- Uggowitzer, P.J.; Magdowski, R.; Speidel, M.O. High nitrogen steels. Nickel free high nitrogen austenitic steels. ISIJ Int. 1996, 36, 901–908. [Google Scholar] [CrossRef]
- Posada, O.M.; Gilmour, D.; Tate, R.J.; Grant, M.H. CoCr wear particles generated from CoCr alloy metal-on-metal hip replacements, and cobalt ions stimulate apoptosis and expression of general toxicology-related genes in monocyte-like U937 cells. Toxicol. Appl. Pharmacol. 2014, 281, 125–135. [Google Scholar] [CrossRef]
- Madl, A.K.; Kovochich, M.; Liong, M.; Finley, B.L.; Paustenbach, D.J.; Oberdörster, G. Toxicology of wear particles of cobalt-chromium alloy metal-on-metal hip implants Part II: Importance of physicochemical properties and dose in animal and in vitro studies as a basis for risk assessment. Nanomedicine 2015, 11, 1285–1298. [Google Scholar] [CrossRef]
- Kovochich, M.; Finley, B.L.; Novick, R.; Monnot, A.D.; Donovan, E.; Unice, K.; Fung, E.S.; Fung, D.; Paustenbach, D.J. Understanding outcomes and toxicological aspects of second generation metal-on-metal hip implants: A state-of-the-art review. Crit. Rev. Toxicol. 2018, 48, 839–887. [Google Scholar] [CrossRef]
- Cuiabc, G.; Liu, H.; Li, S.; Gao, G.; Hassani, M.; Kou, Z. Effect of Ni, W and Mo on the microstructure, phases and high-temperature sliding wear performance of CoCr matrix alloys. Sci. Technol. Adv. Mater. 2020, 21, 229–241. [Google Scholar] [CrossRef]
- Heintze, S.D.; Reichl, F.-X.; Hickel, R. Wear of dental materials: Clinical significance and laboratory wear simulation methods -A review. Dent. Mater. J. 2019, 38, 343–353. [Google Scholar] [CrossRef]
- Kovochich, M.; Fung, E.S.; Donovan, E.; Unice, K.M.; Paustenbach, D.J.; Finley, B.L. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 986–996. [Google Scholar] [CrossRef]
- Di Laura, A.; Quinn, P.D.; Panagiotopoulou, V.C.; Hothi, H.S.; Henckel, J.; Powell, J.J.; Berisha, F.; Amary, F.; Mosselmans, J.F.W.; Skinner, J.A.; et al. The chemical form of metal species released from corroded taper junctions of hip implants: Synchrotron analysis of patient tissue. Sci. Rep. 2017, 7, 10952. [Google Scholar] [CrossRef] [PubMed]
- Crothers, A. Tooth wear and facial morphology. J. Dent. 1992, 20, 333–341. [Google Scholar] [CrossRef]
- Hsu, H.C.; Yen, S.-K. Evaluation of metal ion release and corrosion resistance of ZrO2 thin coatings on the dental Co–Cr alloys. Dent. Mater. 1998, 14, 339–346. [Google Scholar] [CrossRef]
- Lucchetti, M.C.; Fratto, G.; Valeriani, F.; De Vittori, E.; Giampaoli, S.; Papetti, P.; Spica, V.R.; Manzon, L. Cobalt-chromium alloys in dentistry: An evaluation of metal ion release. J. Prosthet. Dent. 2015, 114, 602–608. [Google Scholar] [CrossRef]
- Haugli, K.H.; Syverud, M.; Samuelsen, J.T. Ion release from three different dental alloys—Effect of dynamic loading and toxicity of released elements. Biomater. Investig. Dent. 2020, 7, 71–79. [Google Scholar] [CrossRef]
- Tuna, S.H.; Karaca, E.; Aslan, I.; Pekkan, G.; Pekmez, N. Özçiçek Evaluation of corrosion resistance of Co–Cr alloys fabricated with different metal laser sintering systems. J. Adv. Prosthodont. 2020, 12, 114–123. [Google Scholar] [CrossRef]
- Ramirez-Ledesma, A.L.; Barrera, P.R.; Álvarez-Pérez, M.A.; Lopez, H.; Juárez-Islas, J.A. Corrosion assessment of an implantable dental Co–Cr alloy in artificial saliva and biocompatibility behavior. J. Mater. Eng. Perform. 2020, 29, 1657–1670. [Google Scholar] [CrossRef]
- Kassapidou, M.; Hjalmarsson, L.; Johansson, C.B.; Johansson, P.H.; Morisbak, E.; Wennerberg, A.; Stenport, V.F. Cobalt–chromium alloys fabricated with four different techniques: Ion release, toxicity of released elements and surface roughness. Dent. Mater. 2020, 36, e352–e363. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kim, W.-I.; Mun, J.-H.; Song, M.; Kim, H.-S.; Kim, B.-S.; Kim, M.-B.; Ko, H.C. Patch Testing with dental screening series in oral disease. Ann. Dermatol. 2015, 27, 389–393. [Google Scholar] [CrossRef]
- Kettelarij, J.A.B.; Lidén, C.; Axén, E.; Julander, A. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermat. 2013, 70, 3–10. [Google Scholar] [CrossRef]
- Kettelarij, J.A.B.; Nilsson, S.; Midander, K.; Lidén, C.; Julander, A. Snapshot of cobalt, chromium and nickel exposure in dental technicians. Contact Dermat. 2016, 75, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Kawasaki, H.; Yamamoto, A.; Hiromoto, S.; Imai, H.; Hanawa, T. Friction-wear properties of nickel-free Co–Cr–Mo alloy in a simulated body fluid. Mater. Trans. 2005, 46, 1588–1592. [Google Scholar] [CrossRef]
- Lewis, R.; Dwyer-Joyce, R.S. Wear of human teeth: A tribological perspective. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2005, 219, 2–19. [Google Scholar] [CrossRef]
- Pourzal, R.; Catelas, I.; Theissmann, R.; Kaddick, C.; Fischer, A. Characterization of wear particles generated from CoCrMo alloy under sliding wear conditions. Wear 2011, 271, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Koronfel, M.A.; Goode, A.E.; Weker, J.N.; Tay, S.E.R.; Stitt, C.A.; Simões, T.A.; Mosselmans, J.F.W.; Quinn, P.; Brydson, R.; Hart, A.J.; et al. Understanding the reactivity of CoCrMo-implant wear particles. NPJ Mater. Degrad. 2018, 2, 8. [Google Scholar] [CrossRef]
- Addy, M.; Hughes, J.; Pickles, M.J.; Joiner, A.; Huntington, E. Development of a method in situ to study toothpaste abrasion of dentine. J. Clin. Periodontol. 2002, 29, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Toxicological risks of the cobalt—Chromium alloys used in dentistry: A systematic review. J. Dent. 2020. in preparation. [Google Scholar]
- Chitturi, R.; Baddam, V.R.; Prasad, L.; Prashanth, L.; Kattapagari, K. A review on role of essential trace elements in health and disease. J. Dr. NTR Univ. Health Sci. 2015, 4, 75. [Google Scholar] [CrossRef]
- Han, X.; Sawada, T.; Schille, C.; Schweizer, E.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Spintzyk, S. Comparative analysis of mechanical properties and metal-ceramic bond strength of Co–Cr dental alloy fabricated by different manufacturing processes. Materials 2018, 11, 1801. [Google Scholar] [CrossRef]
- Kassapidou, M.; Stenport, V.F.; Hjalmarsson, L.; Johansson, C.B. Cobalt-chromium alloys in fixed prosthodontics in Sweden. Acta Biomater. Odontol. Scand. 2017, 3, 53–62. [Google Scholar] [CrossRef]
- Julander, A.; Hindsén, M.; Skare, L.; Lidén, C. Cobalt-containing alloys and their ability to release cobalt and cause dermatitis. Contact Dermat. 2009, 60, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ebadian, B.; Razavi, M.; Soleimanpour, S.; Mosharraf, R. Evaluation of tissue reaction to some denture-base materials: An animal study. J. Contemp. Dent. Pract. 2008, 9, 67–74. [Google Scholar] [CrossRef]
- Thyssen, J.; Menné, T.; Møller, P.; Jellesen, M.S.; Johansen, J.D. A cobalt spot test was useful in the diagnostic work-up of a cobalt allergic patient suffering from oral hypersensitivity to cobalt. J. Am. Acad. Dermatol. 2011, 65, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yin, W.; Ma, Q. Allergic palmoplantar pustulosis caused by cobalt in cast dental crowns: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Arafa, K.A. Comparing the effects of titanium alloy and chrome cobalt in removable partial denture connectors on tooth mobility, bone loss and tissue reaction. Saudi J. Dent. Res. 2016, 7, 112–117. [Google Scholar] [CrossRef]
- Linauskienė, K.; Malinauskienė, L.; Blažienė, A. Metals are important contact sensitizers: An experience from Lithuania. BioMed Res. Int. 2017, 2017, 3964045. [Google Scholar] [CrossRef] [PubMed]
- Al-Imam, H.; Benetti, A.R.; Øzhayat, E.B.; Pedersen, A.M.L.; Johansen, J.D.; Thyssen, J.; Jellesen, M.S.; Gotfredsen, K. Cobalt release and complications resulting from the use of dental prostheses. Contact Dermat. 2016, 75, 377–383. [Google Scholar] [CrossRef]
- Olms, C.; Yahiaoui-Doktor, M.; Remmerbach, T.W. Contact allergies to dental materials. Swiss Dent. J. 2019, 129, 571–579. [Google Scholar]
- Drynda, A.; Drynda, S.; Kekow, J.; Lohmann, C.H.; Bertrand, J. Differential effect of cobalt and chromium ions as well as CoCr particles on the expression of osteogenic markers and osteoblast function. Int. J. Mol. Sci. 2018, 19, 3034. [Google Scholar] [CrossRef]
- IARC Monographs. Available online: https://monographs.iarc.fr/cards_page/publications-monographs/ (accessed on 16 August 2020).
- National Toxicology Program. Available online: https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html (accessed on 16 August 2020).
- Suh, M.; Wikoff, D.; Lipworth, L.; Goodman, M.; Fitch, S.; Mittal, L.; Ring, C.; Proctor, D. Hexavalent chromium and stomach cancer: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2019, 49, 140–159. [Google Scholar] [CrossRef]
- Christian, W.V.; Oliver, L.D.; Paustenbach, D.J.; Kreider, M.L.; Finley, B.L. Toxicology-based cancer causation analysis of CoCr-containing hip implants: A quantitative assessment of genotoxicity and tumorigenicity studies. J. Appl. Toxicol. 2014, 34, 939–967. [Google Scholar] [CrossRef]
- Biological Responses to Metal Implants. FDA Report Published on September 2019. Available online: https://www.fda.gov/media/132446/download (accessed on 28 August 2020).
- Leonard, A.; Lauwerys, R. Carcinogenicity and mutagenicity of chromium. Mutat. Res. Genet. Toxicol. 1980, 76, 227–239. [Google Scholar] [CrossRef]
- Léonard, A.; Lauwerys, R. Mutagenicity, carcinogenicity and teratogenicity of cobalt metal and cobalt compounds. Mutat. Res. Genet. Toxicol. 1990, 239, 17–27. [Google Scholar] [CrossRef]
- Rusu, L.C.; Bortun, C.M.; Tănăsie, G.; Podariu, A.C.; Baderca, F.; Solovan, C.; Ardelean, L. The cytotoxicity of dental alloys studied on cell culture. Rom. J. Morphol. Embryol. 2014, 55, 111–115. [Google Scholar]
- Yan, T. The study of cytocompatibility of Co–Cr alloy and Ti alloy. Tianjin Med. J. 2015, 43, 526–528. [Google Scholar] [CrossRef]
- Fernández-Miñano, E.; Ortiz, C.; Vicente, A.; Calvo, J.L.; Ortiz, A.J. Metallic ion content and damage to the DNA in oral mucosa cells of children with fixed orthodontic appliances. BioMetals 2011, 24, 935–941. [Google Scholar] [CrossRef]
- Annangi, B.; Bonassi, S.; Marcos, R.; Hernández, A. Biomonitoring of humans exposed to arsenic, chromium, nickel, vanadium, and complex mixtures of metals by using the micronucleus test in lymphocytes. Mutat. Res. Mutat. Res. 2016, 770, 140–161. [Google Scholar] [CrossRef]
- RAC Opinion on Cobalt. ECHA Document Published on 22 September 2017. Available online: https://echa.europa.eu/documents/10162/b7316b11-ae65-1dd0-2e64-bb6ad3efbd82 (accessed on 28 August 2020).
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices. Available online: http://data.europa.eu/eli/reg/2017/745/oj (accessed on 11 November 2020).
- Cogliano, V.J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; et al. Preventable exposures associated with human cancers. J. Natl. Cancer Inst. 2011, 103, 1827–1839. [Google Scholar] [CrossRef]
- Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices. Available online: http://data.europa.eu/eli/dir/1993/42/oj (accessed on 11 November 2020).
- Regulation (EU) 2020/561 of the European Parliament and of the Council of 23 April 2020. Available online: http://data.europa.eu/eli/reg/2020/561/oj (accessed on 11 November 2020).
- Commission Implementing Regulation (EU) 2017/2185 of 23 November 2017. Available online: http://data.europa.eu/eli/reg_impl/2017/2185/oj (accessed on 11 November 2020).
- Regulation (EC) No 1907/2006 of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Available online: http://data.europa.eu/eli/reg/2006/1907/2014-04-10 (accessed on 11 November 2020).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures. Available online: http://data.europa.eu/eli/reg/2008/1272/oj (accessed on 15 November 2020).
- Registered Substances ECHA. Available online: https://echa.europa.eu/information-on-chemicals/registered-substances (accessed on 15 November 2020).
- Table of Harmonised Entries in annex VI to CLP. Available online: https://echa.europa.eu/documents/10162/13626/annex_vi_clp_table_atp14_en.xlsx/c767afd2-4d53-b8d5-de2b-0820680cac95 (accessed on 15 November 2020).
- Sonofuchi, K.; Hagiwara, Y.; Koizumi, Y.; Chiba, A.; Kawano, M.; Nakayama, M.; Ogasawara, K.; Yabe, Y.; Itoi, E. Quantitative in vivo biocompatibility of new ultralow-nickel cobalt-chromium-molybdenum alloys. J. Orthop. Res. 2016, 34, 1505–1513. [Google Scholar] [CrossRef]
- Mertová, K.; Palán, J.; Németh, G.; Fintová, S.; Duchek, M.; Studecký, T.; Veselý, J.; Máthis, K.; Džugan, J.; Trojanová, Z. Optimization of the mechanical performance of titanium for biomedical applications by advanced, high-gain SPD technology. Crystals 2020, 10, 422.