Organic-inorganic hybrid perovskite materials have attracted tremendous attention as a key material in various optoelectronic devices. Distinctive optoelectronic properties, such as a tunable energy band position, long carrier diffusion lengths, and high charge carrier mobility, have allowed rapid progress in various perovskite-based optoelectronic devices (solar cells, photodetectors, light emitting diodes (LEDs), and lasers). Interestingly, the developments of each field are based on different characteristics of perovskite materials which are suitable for their own applications.
- peorvksite
- perovskite solar cell
- organic-inorganic halide structure
- perovskite light emitting diode
- perovskite photodetector
1. Introduction
With the expeditious growth of economic development and the demand for optoelectronic devices, organic-inorganic hybrid perovskites have attracted a great amount of attention as key materials in various fields, such as solar cells, light-emitting diodes [1][2][3], laser [4] and photodetectors [5][6]. By exploiting their superior properties, perovskite-based optoelectronic materials have developed at rapid speed [7][8][9][10]. Since the first demonstration was reported for perovskite solar cells (PSCs) in 2009 [11], the power conversion efficiency (PCE) of PSCs has improved considerably reaching ~25.5% within the past several years [12]. Moreover, the external quantum efficiency (EQE) of perovskite-based light-emitting diodes (PeLEDs) has now reached ~20% [13], which is comparable to that of organic light-emitting diodes (OLEDs) or quantum dot light-emitting diodes (QD-LEDs). Perovskite-based optoelectronic applications are now expanding and accomplishing astonishing performance in every field.
Perovskite material has an inorganic and organic hybrid structure with the main formula ABX3. The “A” and “B” denote two cations with different sizes, and “X” is an anion (Figure 1a). The common representative of these cations and anion is A = Cs+, CH3NH3+ (MA+), CH(NH2)2+ (FA+), CH3CH2NH3+, B = Pb2+, Sn2+, Cu2+, and X = Cl−, Br−, and I− (halogens). Based on the various combinations of A, B, and X, the perovskite material shows unique properties: a tunable band gap energy ranging from 1.6 eV (FAPbI3) to 3.1 eV (CsPbCl3) [14][15], long hole–electron diffusion length (>1 µm) [16][17], high charge carrier mobility [15][18], high photoluminescence quantum yields (PLQYs) [19][20] and low electronic trap states [21][22]. Thus, with such desirable features as light weight, flexible form, low cost, and solution-based processability, perovskite-based optoelectronic devices are leading in various fields.
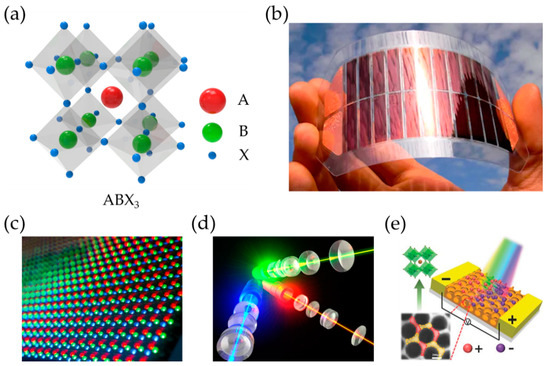
Figure 1. (a) Schematic diagram of crystal structure of hybrid perovskites and various optoelectronic applications, (b) solar cells, (c) LEDs, (d) lasers, and (d) photodetectors of perovskites. (e) Adapted with permission from [23].
Among various applications, PSCs are most widely being investigated. Most notably, PSCs with a PCE of 25.5% were produced by the Seok et al., which is comparable to the efficiency of commercialized traditional solar cells based on crystalline silicon and other combinations of inorganic semiconductors. In addition, benefitting from the near unity PL QY of perovskite nanocrystals, the EQE of PeLEDs has now reached 20% [13][24]. Since perovskite as emitting layer possesses a high degree of color purity for visible to infrared color ranges [25][26], PeLEDs promise the realization of full color displays with a wide color gamut exceeding the BT2020 standard. In laser and photodetector applications, perovskite also attracts a great deal attention due to its unique optoelectronic properties [27][28].
Halide perovskite crystals require different characteristics according to their applications. For example, in solar cells, low exciton binding energy is needed for efficient generation of electron-hole pairs, while LEDs require a highly radiative recombination process. The perovskite film properties such as layer thickness, surface characteristics, diffusion length, and trap state density largely differ from the chemical structure and film fabrication process. To identify the key issues in each perovskite-based application, we investigated the prerequisite factors in each application based on the representative studies. In this review, we briefly introduce the fundamental structure and characteristics of a halide-based perovskite layer and then highlight the key factors for achieving high performance in each application: solar cells, light-emitting diodes, lasers, and photodetectors. This is followed by a description of the remaining challenges. This review confirms the high potential of perovskite as a universal material solution for a wide range of optoelectronic applications, and enhances the understanding of key properties in each application for their practical commercialization.
2. Perovskite Solar Cells
With continuously increasing population along with high criteria for future renewable energy targets, there is intensive needs for environmental-friendly energies such as solar cells. Exploiting the suitable properties of light absorbing materials, such as large absorption coefficients, long charge diffusion lengths, and high carrier mobility, perovskite-based solar cells (PSCs) have achieved noticeable progress within 10 years and reached a certified efficiency of 25.5% in a single junction and of 29.1% in perovskite/Si multijunction solar cells. Since the PSCs have shown outstanding performance and cost-effective solution processability, the solar cell technology is entering the fourth generation [29][30][31][32][33]. Industrial level research, such as large-scale production, environmental-friendly production, and stable operation, for the practical use of PSCs is also investigated widely in various research institutes (e.g., The Swiss Center for Electronics and Microtechnology (CSEM) and National Institute for Materials Science (NIMS)) [34][35][36][37], which have recently achieved efficiency of 26.5% in perovskite/Si multijunction solar cells in large-area production [38]. In recent, Oxford Photovoltaics (Oxford PV) achieved 29.52% of PCEs with the perovskite on GaAs solar cells which was also certified by the US National Renewable Energy Laboratory (NREL).
Since the first demonstration by T. Miyasaka et al. in 2009 [39][40], there have been intensive studies to improve the performance of PCSs. The structure of PSCs can be separated as a mesoporous n-i-p structure, planar n-i-p (conventional), and planar p-i-n structure (inverted) [40][41]. These structures are meant to improve the charge extraction and transport characteristics, where the perovskite absorption layer is sandwiched between the electron-transport layer (ETL) and hole-transport layer (HTL). These charge transport layers (CTLs, both ETLs and HTLs) play crucial roles in extracting electron and hole charge carriers, which are generated from the perovskite absorption layer, and assisting the formation of a high crystallinity perovskite layer. Therefore, along with the uniform distribution of surface properties, the energy level of CTLs needs to be located at a barrierless position rather than at perovskite absorption layers.
The most critical factor in determining the performance of the PSCs is the perovskite film property. Various studies have been reported regarding the morphological distribution of perovskite films for high uniformity, high crystallinity, and high phase purity of the perovskite structure. Therefore, the perovskite film fabrication process is the most basic and important factor which directly affects the film quality. This section discusses the role of CTLs and the perovskite film fabrication process in the performance of PSCs.
2.1. Key Parameters for Solar Cells
The performances of solar cells are defined by the current density-voltage (J-V) characteristics under illumination. The main parameters, which indicate the performance of the device, are short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and most importantly, the power conversion efficiency (PCE). The Jsc is the current density when the devices are in zero bias conditions indicating the exciton dissociation property and charge transport property of the solar cells. Meanwhile, the Voc is the voltage difference measured between two electrodes when no current flows through the devices, indicating the built-in potential of solar cells. The FF and PCE are expressed as the following equations:
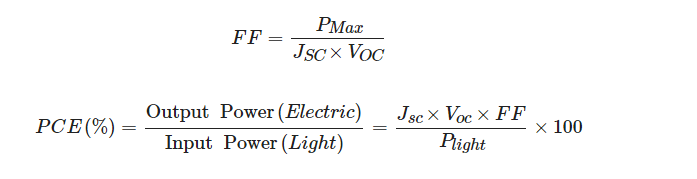
where PMax is the maximum power that the solar cells can produce and Plight is the irradiated solar energy. The FF measures the “squareness” of the J-V characteristics in solar cells, which is directly affected by the series and shunt resistances of the devices. The PCE is measured in standard conditions, which is a temperature of 25 °C and a 1 sun irradiation (100 mW/cm2) with an air mass of 1.5. The traditional silicon-based solar cells have shown an FF between 0.5 to 0.8 and a maximum PCE exceeding 25%.
2.2. Role of Charge Transport Layers
During the developmental progress of PSCs, much attention has focused on the development of the perovskite light harvesting layer. However, the CTLs are also crucial factors that determine both formation of perovskite film and performance of the devices. In the early stage of developing PSCs, H. Kim et al. [42] adopted mesoscopic TiO2 film as ETLs and 2,20,7,70-tetrakis-(N,N-di-4-methoxyphenylamino)-9,90-spirobifluorene (spiro-MeOTAD) as HTLs, achieving high efficiency (PCE ~ 10%) and stable PSCs (long-term stability tests for over 500 h). Their results emphasized the importance of CTLs in determining the performance of a PSC. There are several prerequisite factors [43][44] to function as ideal CTLs in PSCs: no energetic barrier for electrons and holes, high thermal stability, and non-toxicity. Until now, various ETLs (e.g., TiO2, SnO2, and ZnO) and HTLs (e.g., Spiro-OmeTAD, NiO, CuO, and PTAA) have been reported to achieve high performance PSCs.
The most important characteristic of CTLs is the energy level alignment with the perovskite absorption layer. For the energetically barrierless device configuration, the lowest unoccupied molecular orbital (LUMO) of the ETL should be located at a lower energy state than the perovskite layer and the highest occupied molecular orbital (HOMO) of the HTL should be located at a higher energy state than the perovskite layer. Moreover, the transmittance of CTLs in the UV-Vis region should be taken into consideration to maintain the number of photons during light exposure. The bottom layer of perovskite should have a smooth and uniform surface to obtain high crystallinity of the perovskite film, and the upper layer of the perovskite should use non-polar solvent not to damage the perovskite layer.
By taking all these considerations, transition metal oxide TiO2 is the most widely used for high performance PSCs [45]. Since TiO2 shows good chemical stability, a suitable energy level position compared with perovskites, and cost-efficient solution processability, various studies have reported the successful implementation of TiO2 as the ETL in PSCs. Recently, M. J. Paik et al. [46] proposed the high quality TiO2 layer by combining the TiO2 underlayer, which was formed by chemical bath deposition, and sprayed TiO2 colloids (Figure 2b). The dense and uniform TiO2 ETL-based PSCs have shown high performance with a PCE of 22.7%. Various studies have also widely investigated TiO2 with a bilayer ETL (e.g., TiO2/WO3, TiO2/SnO2, and TiO2/ZnO) [46][47][48] or applying metal doped TiO2 ETL (e.g., Li, Al, Nb, and Ni) to improve the optoelectrical property of the TiO2 ETL. Although a high annealing temperature has been raised as a challenging issue in the TiO2 ETL, various low temperature processable TiO2 ETLs with alternative fabrication methods (e.g., atomic layer deposition (ALD) and E-beam) have been reported [49][50][51].
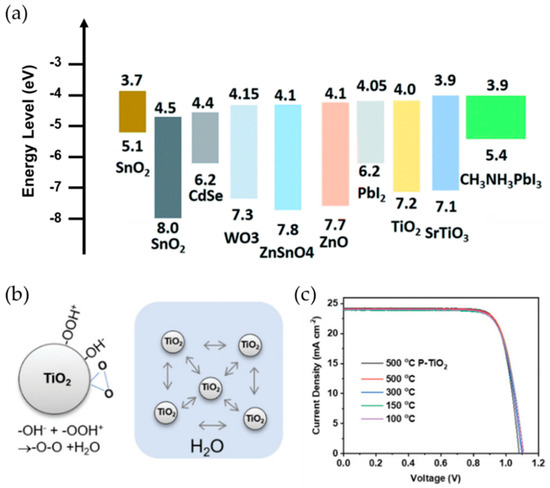
Figure 2. (a) Energy band diagram of various ETLs with respect to MAPbI3 perovskite crystals. Adapted with permission from [52]. (b) The preparation of a TiO2 ETL for high performance PSCs at low temperatures based on the synthesis of a stable TiO2 colloidal aqueous solution. (c) Current density−voltage characteristics of PSCs fabricated by TiO2 ETLs with varying annealing temperature. Adapted with permission from [53].
2.3. Deposition Method of Perovskite Film
Two deposition processes have been most widely investigated in perovskite film fabrication: Solution process and thermal evaporation deposition process. In this section, we discuss perovskite film fabrication methods (solution process and evaporation process) and their effect on morphology and performance in PSCs.
2.3.1. Solution Process
In the solution spin coating process, one step and two step spin coating processes are the most commonly used in solution processes of perovskite film fabrication (Figure 3a). The one step spin coating process has the advantages of simplicity and low-cost processability, which is conducted using a perovskite precursor solution which is dissolved in polar solvents (e.g., DMF, DMAc, DMSO, and GBL) [54][55]. During the spin coating process and annealing process, the crystal structure of perovskite is determined by ionic interaction between anions and cations in the precursor. However, due to the slow crystallization process, partial aggregations and pinholes are generated, which results in low reproductivity in the film formation.
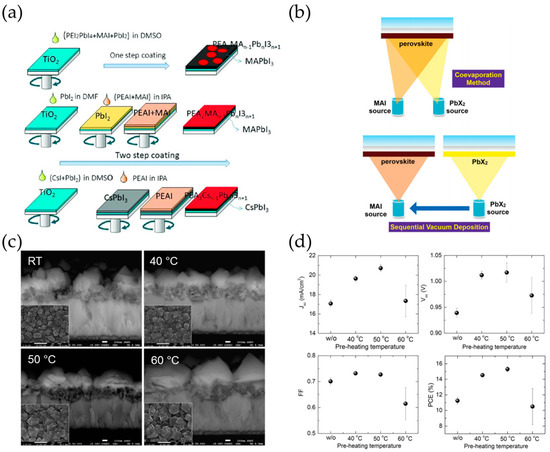
Figure 3. (a) Typical solution process (One step and two step) of perovskite crystals. Adapted with permission from [Stability Issue of Perovskite Solar Cells under Real-World Operating Conditions]. (b) Schematic illustration of perovskite fabrication by using thermal evaporation process. Adapted with permission from [56]. Effect of pre-heating temperature on (c) the grain size of perovskite and (d) the PSCs performance. Adapted with permission from [57].
To enhance the uniform distribution of the perovskite film quality, a two-step spin coating process was proposed by K. Liang et al. [58] who used a two-step solution deposition method. They successfully fabricated CH3NH3BI3 (B = Pb, Sn) perovskite films by depositing BI2 film in advance and converting the film into a perovskite layer by adopting a CH3NH3I (MAI) solution. These sequential steps of BX2 and AX were investigated. J. Im et al. [59] reported high performance CH3NH3PbI3 PSCs with varying the MAI concentration. The concentration of MAI highly influences the cuboid size of perovskite films, which influences the optoelectronic characteristics of the perovskite absorption layer by altering the packing density of the crystal population and the light harvesting property. Therefore, by optimizing the cuboid size of MAPbI3 perovskite films, the PSCs achieved a high level of light harvesting and charge carrier extraction properties resulting a PCE of 17%. Similar studies have been reported on enhancing the enhancing film morphology. H. Ko et al. [60] and N. Ahn et al. [61] reported the effect of PbI2 concentration and annealing temperature in grain growth of perovskite film, resulting in a PCE of 15.8% and 14.3%, respectively (Figure 3c,d). Later, the crystal growth mechanism of perovskite in two step fabrication was quantitively investigated by Y. Fu et al. [62][63]. By adopting sufficient concentration of MAI in PbI2 films, the interface reaction initially formed a MAPbI3 structure along with producing PbI42−. After the saturation amount in the MAI solution, the PbI42− reacted with CH3NH3+ and slowly recrystallized to form a single crystal MAPbI3 perovskite structure. However, along with incomplete conversion of PbI2 residues, a long reaction time still remained as challenging issues which easily resulted in uneven formation of large crystals.
In both one step and two step processes, controlling the nucleation and crystal growth is highly emphasized for uniform formation of perovskite film morphology. One efficient way to solve this issue is inducing an anti-solvent during the formation of perovskite crystals. M. Xiao et al. [64] deposited chlorobenzene (CBZ) as an anti-solvent solution during one step spin coating of CH3NH3PbI3 (dissolved in DMF solvent) and achieved large crystalline perovskite grains with micron size. Compared to the conventional one step process, the PSCs with that used the anti-solvent process showed 192% enhancement in PCE. The role of the anti-solvent during the spin coating process controls the crystallization process by removing the residual precursor and enhancing uniformity of the perovskite film. Various kinds of solvents are adopted in anti-solvent processes. D. Son et al. [65] and N. J. Jeon et al. [66] used diethyl ether on MAPbI3 and toluene on FAPbI3, respectively, which showed remarkable improvement in performance of PSCs. With a similar principle, a mixture of anti-solvent was also investigated to form high quality perovskite film. J. Liu et al. [67] used a mixture of ethyl acetate (EA) and isopropyl alcohol (IPA) for spin coating a MAPbI3 perovskite precursor and achieved high PCE of 18.9%.
2.3.2. Thermal Evaporation Process
Fabricating a perovskite layer by the solution process inevitably accompanies a thermal annealing process on the substrates. However, the thermal annealing process limits the feasibility of choosing a substrate, which is especially essential in flexible optoelectronic devices. The thermal evaporation process can solve this issue and is also an efficient method to fabricate uniform and smooth perovskite film. Similar to the solution process, two methods are widely used to fabricate perovskite film via thermal evaporation: single source and dual source (or multiple-source) evaporation.
In the thermal evaporation process, the dual source evaporation method was reported earlier by M. Liu et al. [68] They presented CH3NH3PbI3−xClx mixed halide-based PSCs by evaporating MAI and a PbCl2 precursor in dual source simultaneously. The obtained perovskite film had a smooth and uniform morphology and the PSCs achieved a high PCE exceeding 15%. C. Meanwhile, Chen et al. [69] and S. Hsiao et al. [70] proposed CH3NH3PbI3-based PSCs in sequential steps of PbI2 and MAI in the thermal evaporation process, achieving perovskite film with a fine morphology. Since all inorganic perovskite (e.g., CsPbI3 and CsPbIxBr1−x) [71][72][73] has also been demonstrated by utilizing thermal evaporation, the thermal evaporation process attracted strong attention as an alternative method of solution processing.
This entry is adapted from the peer-reviewed paper 10.3390/cryst11010039
References
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.S.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored organic/inorganic hybrid perovskite light-emitting diodes. Adv. Mater. 2015, 27, 1248–1254.
- Cho, H.; Jeong, S.H.; Park, M.H.; Kim, Y.H.; Wolf, C.; Lee, C.L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222.
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692.
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 2014, 13, 476–480.
- Lian, Z.; Yan, Q.; Lv, Q.; Wang, Y.; Liu, L.; Zhang, L.; Pan, S.; Li, Q.; Wang, L.; Sun, J.L. High-Performance Planar-Type Photodetector on (100) Facet of MAPbI3 Single Crystal. Sci. Rep. 2015, 5, 16563.
- Fang, Y.; Dong, Q.; Shao, Y.; Yuan, Y.; Huang, J. Highly narrowband perovskite single-crystal photodetectors enabled by surface-charge recombination. Nat. Photonics 2015, 9, 679–686.
- Stylianakis, M.M.; Maksudov, T.; Panagiotopoulos, A.; Kakavelakis, G.; Petridis, K. Inorganic and hybrid perovskite based laser devices: A Review. Materials 2019, 12, 859.
- Jeong, M.; Choi, W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620.
- Ren, H.; Yu, S.; Chao, L.; Xia, Y.; Sun, Y.; Zuo, S.; Li, F.; Niu, T.; Yang, Y.; Ju, H.; et al. Efficient and stable Ruddlesden–Popper perovskite solar cell with tailored interlayer molecular interaction. Nat. Photonics 2020, 14, 154–163.
- Zhang, C.; Wang, S.; Li, X.; Yuan, M.; Turyanska, L.; Yang, X. Core/Shell Perovskite Nanocrystals: Synthesis of Highly Efficient and Environmentally Stable FAPbBr3/CsPbBr3 for LED Applications. Adv. Funct. Mater. 2020, 30, 1910582.
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051.
- NREL Chart. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 22 December 2020).
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248.
- Aharon, S.; Etgar, L. Two Dimensional Organometal Halide Perovskite Nanorods with Tunable Optical Properties. Nano Lett. 2016, 16, 3230–3235.
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696.
- Wei, H.; Fang, Y.; Mulligan, P.; Chuirazzi, W.; Fang, H.H.; Wang, C.; Ecker, B.R.; Gao, Y.; Loi, M.A.; Cao, L.; et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 2016, 10, 333–339.
- Stranks, S.D.; Stranks, S.D.; Eperon, G.E.; Eperon, G.E.; Grancini, G.; Grancini, G.; Menelaou, C.; Menelaou, C.; Alcocer, M.J.P.; Alcocer, M.J.P.; et al. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344.
- Wang, C.; Zhang, Y.; Wang, A.; Wang, Q.; Tang, H.; Shen, W.; Li, Z.; Deng, Z. Controlled Synthesis of Composition Tunable Formamidinium Cesium Double Cation Lead Halide Perovskite Nanowires and Nanosheets with Improved Stability. Chem. Mater. 2017, 29, 2157–2166.
- Kumar, S.; Jagielski, J.; Kallikounis, N.; Kim, Y.H.; Wolf, C.; Jenny, F.; Tian, T.; Hofer, C.J.; Chiu, Y.C.; Stark, W.J.; et al. Ultrapure Green Light-Emitting Diodes Using Two-Dimensional Formamidinium Perovskites: Achieving Recommendation 2020 Color Coordinates. Nano Lett. 2017, 17, 5277–5284.
- Du, X.; Wu, G.; Chenga, J.; Danga, H.; Maa, K.; Zhanga, Y.; Tana, P.F.; Chen, S. High-quality CsPbBr3 perovskite nanocrystals for quantum dot light-emitting diodes. RSC Adv. 2017, 7, 10391–10396.
- Wetzelaer, G.J.A.H.; Scheepers, M.; Sempere, A.M.; Momblona, C.; Ávila, J.; Bolink, H.J. Trap-Assisted Non-Radiative Recombination in Organic-Inorganic Perovskite Solar Cells. Adv. Mater. 2015, 27, 1837–1841.
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 5784.
- Zeng, J.; Li, X.; Wu, Y.; Yang, D.; Sun, Z.; Song, Z.; Wang, H.; Zeng, H. Space-Confined Growth of CsPbBr3 Film Achieving Photodetectors with High Performance in All Figures of Merit. Adv. Funct. Mater. 2018, 28, 1804394.
- Zhao, L.; Lee, K.M.; Roh, K.; Khan, S.U.Z.; Rand, B.P. Improved Outcoupling Efficiency and Stability of Perovskite Light-Emitting Diodes using Thin Emitting Layers. Adv. Mater. 2019, 31, 1805836.
- Sadhanala, A.; Ahmad, S.; Zhao, B.; Giesbrecht, N.; Pearce, P.M.; Deschler, F.; Hoye, R.L.Z.; Gödel, K.C.; Bein, T.; Docampo, P.; et al. Blue-Green Color Tunable Solution Processable Organolead Chloride-Bromide Mixed Halide Perovskites for Optoelectronic Applications. Nano Lett. 2015, 15, 6095–6101.
- Jaramillo-Quintero, O.A.; Sanchez, R.S.; Rincon, M.; Mora-Sero, I. Bright Visible-Infrared Light Emitting Diodes Based on Hybrid Halide Perovskite with Spiro-OMeTAD as a Hole-Injecting Layer. J. Phys. Chem. Lett. 2015, 6, 1883–1890.
- Zhang, Q.; Su, R.; Du, W.; Liu, X.; Zhao, L.; Ha, S.T.; Xiong, Q. Advances in Small Perovskite-Based Lasers. Small Methods 2017, 1, 1700163.
- Fu, Y.; Zhu, H.; Schrader, A.W.; Liang, D.; Ding, Q.; Joshi, P.; Hwang, L.; Zhu, X.Y.; Jin, S. Nanowire Lasers of Formamidinium Lead Halide Perovskites and Their Stabilized Alloys with Improved Stability. Nano Lett. 2016, 16, 1000–1008.
- Yin, W.J.; Yang, J.H.; Kang, J.; Yan, Y.; Wei, S.H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942.
- Wu, Y.; Chen, W.; Yue, Y.; Liu, J.; Bi, E.; Yang, X.; Islam, A.; Han, L. Consecutive Morphology Controlling Operations for Highly Reproducible Mesostructured Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 20707–20713.
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management informamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379.
- Mutalib, M.; Aziz, F.; Ismail, A.F.; wan Salleh, W.N.; Yusof, N.; Jaafar, J.; Soga, T.; Sahdan, M.Z.; Ahmad Ludin, N. Towards high performance perovskite solar cells: A review of morphological control and HTM development. Appl. Mater. Today 2018, 13, 69–82.
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; De Arquer, F.P.G.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726.
- Wenger, B.; Snaith, H.J.; Sörensen, I.H.; Ripperger, J.; Kazim, S.; Ahmad, S.; Nandayapa, E.R.; Boeffel, C.; Colodrero, S.; Anaya, M.; et al. Towards unification of perovskite stability and photovoltaic performance assessment. arXiv 2020, arXiv:2004.11590.
- Yum, J.-H.; Moon, S.-J.; Yao, L.; Caretti, M.; Nicolay, S.; Kim, D.-H.; Sivula, K. Robust Electron Transport Layers via In Situ Cross-Linking of Perylene Diimide and Fullerene for Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 6616–6623.
- Pant, N.; Kulkarni, A.; Yanagida, M.; Shirai, Y.; Miyasaka, T.; Miyano, K. Investigating the Growth of CH3NH3PbI3 Thin Films on RF-Sputtered NiOx for Inverted Planar Perovskite Solar Cells: Effect of CH3NH3+ Halide Additives versus CH3NH3+ Halide Vapor Annealing. Adv. Mater. Interfaces 2020, 7, 1901748.
- Shakour, M.A.; Chowdhury, T.H.; Matsuishi, K.; Bedja, I.; Moritomo, Y.; Islam, A. High-Efficiency Tin Halide Perovskite Solar Cells: The Chemistry of Tin (II) Compounds and Their Interaction with Lewis Base Additives during Perovskite Film Formation. RRL Sol. 2020, 2000606.
- A 26.5% Efficient Perovskite-Silicon Tandem Cell. Available online: https://www.pv-magazine.com/2020/12/09/a-26-5-efficient-perovskite-silicon-tandem-cell/ (accessed on 22 December 2020).
- Babu, R.; Giribabu, L.; Singh, S.P. Recent Advances in Halide-Based Perovskite Crystals and Their Optoelectronic Applications. Cryst. Growth Des. 2018, 18, 2645–2664.
- Choi, J.J.; Yang, X.; Norman, Z.M.; Billinge, S.J.L.; Owen, J.S. Structure of methylammonium lead iodide within mesoporous titanium dioxide: Active material in high-performance perovskite solar cells. Nano Lett. 2014, 14, 127–133.
- Meng, L.; You, J.; Guo, T.F.; Yang, Y. Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells. Acc. Chem. Res. 2016, 49, 155–165.
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591.
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062.
- Paik, M.J.; Lee, Y.; Yun, H.S.; Lee, S.U.; Hong, S.T.; Seok, S. Il TiO2 Colloid-Spray Coated Electron-Transporting Layers for Efficient Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2001799.
- Jiang, M.; Niu, Q.; Tang, X.; Zhang, H.; Xu, H.; Huang, W.; Yao, J.; Yan, B.; Xia, R. Improving the performances of perovskite solar cells via modification of electron transport layer. Polymers 2019, 11, 147.
- You, Y.; Tian, W.; Min, L.; Cao, F.; Deng, K.; Li, L. TiO2/WO3 Bilayer as Electron Transport Layer for Efficient Planar Perovskite Solar Cell with Efficiency Exceeding 20%. Adv. Mater. Interfaces 2020, 7, 1901406.
- Liu, Z.; Sun, B.; Liu, X.; Han, J.; Ye, H.; Tu, Y.; Chen, C.; Shi, T.; Tang, Z.; Liao, G. 15% efficient carbon based planar-heterojunction perovskite solar cells using a TiO2/SnO2 bilayer as the electron transport layer. J. Mater. Chem. A 2018, 6, 7409–7419.
- Kumari, N.; Gohel, J.V.; Patel, S.R. Optimization of TiO2/ZnO bilayer electron transport layer to enhance efficiency of perovskite solar cell. Mater. Sci. Semicond. Process. 2018, 75, 149–156.
- Shahiduzzaman, M.; Visal, S.; Kuniyoshi, M.; Kaneko, T.; Umezu, S.; Katsumata, T.; Iwamori, S.; Kakihana, M.; Taima, T.; Isomura, M.; et al. Low-Temperature-Processed Brookite-Based TiO2 Heterophase Junction Enhances Performance of Planar Perovskite Solar Cells. Nano Lett. 2018, 19, 598–604.
- Yin, G.; Ma, J.; Jiang, H.; Li, J.; Yang, D.; Gao, F.; Zeng, J.; Liu, Z.; Liu, S.F. Enhancing Efficiency and Stability of Perovskite Solar Cells through Nb-Doping of TiO2 at Low Temperature. ACS Appl. Mater. Interfaces 2017, 9, 10752–10758.
- Wang, J.T.W.; Ball, J.M.; Barea, E.M.; Abate, A.; Alexander-Webber, J.A.; Huang, J.; Saliba, M.; Mora-Sero, I.; Bisquert, J.; Snaith, H.J.; et al. Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 2014, 14, 724–730.
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062.
- Jiang, M.; Niu, Q.; Tang, X.; Zhang, H.; Xu, H.; Huang, W.; Yao, J.; Yan, B.; Xia, R. Improving the performances of perovskite solar cells via modification of electron transport layer. Polymers 2019, 11, 147.
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S. Sang Il Seok Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903.
- Brenes, R.; Guo, D.; Osherov, A.; Noel, N.K.; Eames, C.; Hutter, E.M.; Pathak, S.K.; Niroui, F.; Friend, R.H.; Islam, M.S.; et al. Metal Halide Perovskite Polycrystalline Films Exhibiting Properties of Single Crystals. Joule 2017, 1, 155–167.
- Zheng, L.; Zhang, D.; Ma, Y.; Lu, Z.; Chen, Z.; Wang, S.; Xiao, L.; Gong, Q. Morphology control of the perovskite films for efficient solar cells. Dalton Trans. 2015, 44, 10582–10593.
- Ko, H.-S.; Lee, J.-W.; Park, N.-G. 15.76% efficiency perovskite solar cells prepared under high relative humidity: Importance of PbI2 morphology in two-step deposition of CH3NH3PbI3. J. Mater. Chem. A 2015, 3, 8808–8815.
- Liang, K.; Mitzi, D.B.; Prikas, M.T. Synthesis and Characterization of Organic-Inorganic Perovskite Thin Films Prepared Using a Versatile Two-Step Dipping Technique. Chem. Mater. 1998, 10, 403–411.
- Im, J.H.; Jang, I.H.; Pellet, N.; Grätzel, M.; Park, N. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932.
- Ko, H.-S.; Lee, J.-W.; Park, N.-G. 15.76% efficiency perovskite solar cells prepared under high relative humidity: Importance of PbI2 morphology in two-step deposition of CH3NH3PbI3. J. Mater. Chem. A 2015, 3, 8808–8815.
- Ahn, N.; Kang, S.M.; Lee, J.W.; Choi, M.; Park, N.G. Thermodynamic regulation of CH3NH3PbI3 crystal growth and its effect on photovoltaic performance of perovskite solar cells. J. Mater. Chem. A 2015, 3, 19901–19906.
- Fu, Y.; Meng, F.; Rowley, M.B.; Thompson, B.J.; Shearer, M.J.; Ma, D.; Hamers, R.J.; Wright, J.C.; Jin, S. Solution growth of single crystal methylammonium lead halide perovskite nanostructures for optoelectronic and photovoltaic applications. J. Am. Chem. Soc. 2015, 137, 5810–5818.
- Huang, L.; Hu, Z.; Xu, J.; Zhang, K.; Zhang, J.; Zhu, Y. Multi-step slow annealing perovskitefilms for high performanceplanar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2015, 141, 377–382.
- Xiao, M.; Huang, F.; Huang, W.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 2014, 53, 9898–9903.
- Son, D.-Y.; Lee, J.-W.; Choi, Y.J.; Jang, I.-H.; Lee, S.; Yoo, P.J.; Shin, H.; Ahn, N.; Choi, M.; Kim, D.; et al. Self-formed grain boundary healing layer for highly efficient CH3NH3PbI3 perovskite solar cells. Nat. Energy 2016, 1, 16081.
- Jeon, N.J.; Jeon, N.J.; Noh, J.H.; Noh, J.H.; Yang, W.S.; Yang, W.S.; Kim, Y.C.; Kim, Y.C.; Ryu, S.; Ryu, S.; et al. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480.
- Liu, J.; Li, N.; Jia, J.; Dong, J.; Qiu, Z.; Iqbal, S.; Cao, B. Perovskite films grown with green mixed anti-solvent for highly efficient solar cells with enhanced stability. Sol. Energy 2019, 181, 285–292.
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398.
- Chen, C.-W.; Kang, H.-W.; Hsiao, S.-Y.; Yang, P.-F.; Chiang, K.-M.; Lin, H.-W. Efficient and Uniform Planar-Type Perovskite Solar Cells by Simple Sequential Vacuum Deposition. Adv. Mater. 2014, 26, 6647–6652.
- Hsiao, S.Y.; Lin, H.L.; Lee, W.H.; Tsai, W.L.; Chiang, K.M.; Liao, W.Y.; Ren-Wu, C.Z.; Chen, C.Y.; Lin, H.W. Efficient All-Vacuum Deposited Perovskite Solar Cells by Controlling Reagent Partial Pressure in High Vacuum. Adv. Mater. 2016, 28, 7013–7019.
- Yu, C.; Yu, C.H.; Ming, L.K.; Lun, C.W.; Ching, T.W.; Si, H.C.; Lin, T. All-vacuum-deposited stoichiometrically balanced inorganic cesium lead halide perovskite solar cells with stabilized efficiency exceeding 11%. Adv. Mater. 2017, 29, 1605290.
- Lau, C.F.J.; Deng, X.; Ma, Q.; Zheng, J.; Yun, J.S.; Green, M.A.; Huang, S.; Baillie, A.W.Y.H. CsPbIBr2 Perovskite Solar Cell by Spray-Assisted Deposition. ACS Energy Lett. 2016, 1, 573–577.
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Stevenson, K.J.; Troshin, P.A. Highly efficient all-inorganic planar heterojunction perovskite solar cells produced by thermal coevaporation of CsI and PbI2. J. Phys. Chem. Lett. 2017, 8, 67–72.
