Triple-negative breast cancer (TNBC) is an aggressive breast type of cancer with no expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2). It is a highly metastasized, heterogeneous disease that accounts for 10–15% of total breast cancer cases with a poor prognosis and high relapse rate within five years after treatment compared to non-TNBC cases.
- triple negative breast cancer
- future diagnosis
- breast cancer
1. Introduction
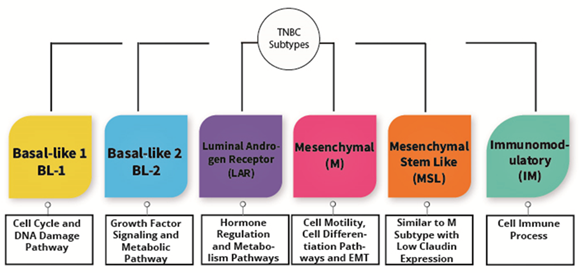
TNBC is known as a heterogeneous type of cancer that is categorized into six subtypes. The subtypes are immunomodulatory (IM), luminal androgen receptor (LAR), basal-like 1 (BL-1), basal-like 2 (BL-2), mesenchymal (M), and mesenchymal stem-like (MSL) as shown in Figure 2. These types are categorized based upon their gene expression portfolio [26]. For instance, the similarities between BL-1 and BL-2 are the substantial gene expression during cell division as well as cell cycle advancement. Nevertheless, BL-1 retains high gene expression associated with the DNA response pathway, including DNA repair activity and DNA replication, while BL-2 features high expression in growth factor signaling [26]. Alternatively, immunomodulatory (IM) possesses a high expression of genes associated with the immune cell process, specifically a natural killer cell pathway, TH1/TH2 pathway, cytokine signaling, B cell receptor (BCR), and antigen processing. Furthermore, mesenchymal stem-like and mesenchymal subtypes are undoubtedly comprehended to have a high expression of genes associated with extracellular receptor interaction, cell motility, and cell differentiation pathways. Irrespective of that, MSL shows a magnitude difference from mesenchymal subtypes, where MSL has a low expression of claudins (3, 4, 7) genes [26]. Consequently, the MSL subtype is categorized as claudin-low tumors discovered by Herschkowitz et al. [20]. Last of all, the LAR subtype shows high expression in genes related to hormonally regulated pathways and genes regarding androgen receptor as well as its co-activators [26,27]. Nevertheless, Lehmann et al. redefined TNBC molecular subtypes into four tumor-specific subtypes consisting of BL1, BL2, M, and LAR when they found that the IM together with MSL TNBC subtypes were presented from infiltrating lymphocytes and tumor-associated mesenchymal cells [28].
Figure 2. The subtypes of triple-negative breast cancer.
2. Current Diagnosis
A two-step procedure typically employed to diagnose TNBC is imaging and immunohistochemistry (IHC) [38]. Imaging encompasses a mammogram, an ultrasound of the breast along with magnetic resonance imaging (MRI) [39]. A mammogram requires a minimal dosage of radiation that does not easily penetrate the breast tissues [40]. Breast cancer diagnosis via mammograms is determined by the presence of calcifications (white spots), growth, or tumor also known as masses [41]. The main challenge is the risk of a false-negative and positive result affecting the diagnosed patient’s treatment outcome [42]. In addition, the side effects of radiation from mammograms may contribute to breast cancer development in high-risk individuals like BRCA gene carriers or family history [43]. Finally, mammography effectiveness is operator-dependent, which may interfere with the result of imaging [44].
Diagnosis via ultrasound is performed when a lump or swelling is not detected in a mammogram but still can be felt and serve as the primary approach to distinguish between breast cysts (fluid-filled sac) and tumors if sample collection is carried out in the right area and tested for cancer [45]. What differentiates breast cysts from a solid tumor is that breast cysts are most often benign, whereas a solid tumor requires further validation to characterize its malignancy [46].
Breast cancer diagnosis by MRI, on the other hand, is opted when a patient is categorized as high risk (family history/BRCA gene mutation) and to determine the severity of the carcinoma due to the efficiency of MRI to detect the early formation of breast cancer in comparison to breast ultrasound and mammogram [47,48]. The main downside of MRI is that the imaging method cannot characterize the breast cancer types and can only confirm the presence of cancer in the breast [49].
Ideally, IHC is required for breast carcinoma typing performed by cell staining with biomarkers such as hormone receptor (progesterone receptor (PR) and estrogen receptor (ER)) as well as human epidermal growth factor receptor two (HER2) markers [50]. In order to enhance the efficacy and accuracy of IHC testing for ER HER2 and PR, there are approximately 126 latest guidelines that have been provided by the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) [51]. The primary aim of these guidelines is to improve the reliability, reproducibility and to reduce the frequency of false-positive and false-negative results from IHC testing [52,53]. Based on the recommendation, IHS testing for ER and PR is classified positive only if immunoreactive cancer cells’ presence accounts for a minimum value of 1% [52]. Next, a second confirmation of HER2 should be conducted via fluorescent in situ (FISH) after initial IHC confirmation to obviate any potential false-positive/false-negative diagnosis that will affect the treatment’s direction and effectiveness plan [54].
This entry is adapted from the peer-reviewed paper 10.3390/medicina57010062