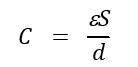This state-of-the-art entry aims to highlight advances in metal-free carbon-based supercapacitors over the last 20 years. Author discuss the various types of carbons (without metals) used (activated, nanoforms of carbon, and doped carbons) as well as key parameters in supercapacitor performance such as surface area, porosity, and functional groups.
- Carbon-based
- Supercapacitors
- metal-free
1. Introduction
Presently, fossil fuels are the primary resources consumed to meet current energy demands. For the last several years, energy demands have been increasing steadily due to an increase in the world population. As a result, fossils fuel reservoirs are depleting rapidly and the use of these resources also has a detrimental effect on the environment, such as global warming [1]. Due to the impending energy crisis, alternative and renewable resources must be used to design power generation and energy storage devices. In this regard, solar, wind, water, and geothermal resources are being considered for power generation. Batteries, fuel cells, and electrochemical supercapacitors have similarly gained much attention for effective energy generation and storage [2][3]. Among these, supercapacitors have gained considerable importance as energy storage devices for meeting many of the requirements for an alternate energy storage system.
Supercapacitors may also be referred to as ultracapacitors or double-layer capacitors. Fifty years ago, supercapacitors were recognized as the most promising approach to store energy, due to their outstanding characteristics such as high power density (100 times greater than conventional batteries [4]), long lifetime (lifecycle upwards of 10,000 cycles), and superior charge/discharge rate (up to 10–20 times faster than Lithium ion batteries). Due to their prompt charging response, supercapacitors can be used widely in digital cameras, automobiles, flashlights, elevators, portable media players, etc. Traditional capacitors cannot compete with supercapacitors since the capacitance of the latter is thousands of times higher than the traditional capacitor (μFarads vs. Farads) [5]. Supercapacitors can be used independently or they can be combined with batteries or fuel cells for various energy storage applications [6].
To date, research progress in the arena of supercapacitors has involved seeking new inexpensive materials and economical, prompt as well as facile strategies to design supercapacitors with enhanced performance. Materials that have been exploited for supercapacitor fabrications can easily be divided into three main categories, viz. (1) carbon-based materials, (2) metal oxides, and (3) conducting polymers. Zhang group [7] presented a short summarized review for supercapacitor materials, and indicated advantages and disadvantages accompanying different materials. There is no doubt that metal oxides exhibit superior performance for supercapacitors applications (upwards of 1000 F/g). Therefore, metal oxides are very popular for supercapacitor applications owing to their superior performance among the above-mentioned materials. For that reason, Zhang et al. [7] has extensively covered metal oxide-based supercapacitors in the review, whereas carbon and conducting polymers materials were briefly discussed. Although metallic supercapacitors are known for their high capacitance, there are some drawbacks associated with them, such as their extremely high cost (e.g., Ruthenium (IV) oxide precursors priced around $60 per gram), weak selectivity towards different species, and high toxicity towards the environment [8]. Whereas, the other two materials, carbon and polymers, are considered as environmentally safe compounds. However, the main disadvantage of conducting polymers is associated with their high instability due to their brittle nature [9]. Thus, an urgent need has arisen to restructure or even abandon current technologies and seek novel, green, and inexpensive materials. In this regard, carbon-based materials are gaining tremendous attention of researchers for supercapacitor applications [10], with activated carbons available commercially at about 15 USD per kilogram. The main focus of this review is to provide a detailed discussion about carbon-based supercapacitors developed in last 20 years, and novel strategies that can be utilized to improve the performance of carbon-based supercapacitors.
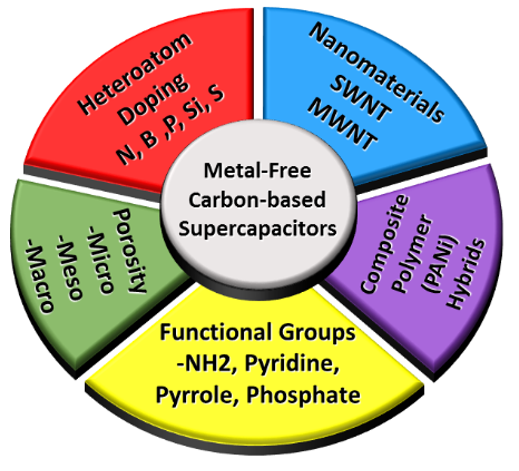
Various forms of carbon are highly sought after to obtain improved capacitance, such as carbon nanotubes, activated carbon, doped carbons, and many others. Though carbon materials usually exhibit lower capacitance than metal oxides and conducting polymers, their natural abundance in different forms and low cost make them an ideal candidate for supercapacitor applications. In addition, surface area and conductivity can easily be tuned in the natural existing forms of carbon (e.g., biomass). At present, extensive research is being performed using many different kinds carbon materials, and the main objective is to improve the performance of carbon-based supercapacitors. As a result of these studies, several examples of carbon-based materials have been made where supercapacitor performance has been significantly enhanced by various techniques such as heat treatment [11] or doping with heteroatoms [4]. Specifically, carbon nanomaterials have been demonstrated for similar or better supercapacitor properties than metal oxides [12]. A more detailed discussion regarding other strategies employed to enhance supercapacitor performance will be discussed later in this review, but are summarized in Figure 1.
Figure 1. Strategies used to improve the performance of metal free carbon-based supercapacitor.
2. Electric Double-Layer Capacitor (EDLC)
Electrochemical capacitors can be divided generally into two classes: (1) Electric double-layer capacitors (EDLCs) and (2) pseudocapacitors. Normally, all carbon-based materials operate based on the EDLC principle. Therefore, only EDLC has been explained here, briefly. The mechanism of EDLCs is based on the storage of charge in thin double-layers, which is mainly present at the interface between the electrode surface and electrolyte solution. Since no charge transfer occurs between the electrode and the electrolyte, EDLCs are also known as non-Faradaic supercapacitors. Typically, all electrode surfaces possess EDLC behavior, and the capacitance recorded from this mechanism is much higher than the conventional dielectric capacitor. Ideal capacitors show a cyclic voltammogram of rectangular shape since the current is independent of potential. However, some potential dependent current is observed in EDLC due to high congestion of electrolyte at the double layer [13].
EDLC capacitance is represented by the following formula:
where C is the capacitance, ε is the relative permittivity, S is the surface area of the electrode, and d is the thickness of the double layer present at the interface between the electrode and the electrolyte. As can be deduced from the equation, EDLC is primarily dependent upon the surface area of an electrode material, as a high specific area provides a high surface for charge accumulation at electrode electrolyte interface. Generally, the capacitance result is proportional to the specific surface area for similar kinds of materials prepared using similar methods [14]. However, the surface area is not an all-encompassing parameter for determining material performance as the pore size distribution (micro-, meso-, and macropores) also plays a joint role [15]. Thus, electrode materials with suitable properties are very important to development of EDLCs.
With regards to supercapacitor application, some key characteristics of suitable material include large active surface area (≥700 m2/g for commercial ACs [4]), excellent conductivity, suitable pore size (for the electrolyte system chosen), surface wettability, high cycle stability, rapid charge/discharge rate, etc. These characteristics are highly desirable to design an efficient EDLC capacitor [16]. Therefore, extensive fundamental studies have been performed to investigate the properties of different existing forms of carbon materials. In this regard, carbon-based electrodes are commercially utilized as an ideal choice for EDLCs due to their physicochemical properties such as high conductivity and high surface area (1–2000 m2/g), as well as high thermal and chemical stability. Furthermore, carbon materials exhibit extraordinarily high cycle stability as well as power density. Including all these properties, carbon-based materials are also highly attractive due to their significant low cost and abundant availability in nature [16]. Since carbon provides many advantages for applications such as EDLC, several designs of carbon have been developed for the purpose of energy storage capacity. Recent research of carbon materials for supercapacitor applications are classified as follows: activated carbon, doped carbon, functionalized carbon, carbon aerogels, graphene, carbon nanomaterials, and carbon quantum dots [17].
3. Carbon Materials
Carbon materials are of great importance for supercapacitor applications due to the presence of different forms of carbons and the fact that carbon is naturally abundant. Additionally, the chemical and thermal stability as well as good electrical conductivity of carbon are suitable characteristics to design low-priced supercapacitors. Furthermore, these materials can be made via environmentally friendly techniques such as pyrolysis [18] and hydrothermal treatment [19]. The final products possess a porous surface and exhibit high specific surface area.
3.1. Porous Activated Carbon
In order to attain high specific capacitance, porous materials that exhibit high surface area are promising candidates. Several methodologies have been employed to design porous carbon materials such as traditional chemical and physical activation and a combination of both processes, template-based methods, carbide-derived porous materials, etc. Fundamental physical characteristics such as specific surface area, electrical conductivity, pore size, shape, and their distribution are being studied in detail to determine the full potential of porous materials for supercapacitor applications.
Porous carbon materials synthesized by pyrolysis are vastly applied for industrial and laboratory applications. Biomass-derived carbon materials have acquired considerable attention in many fields, including supercapacitors [20][21][22][23][24]. Since most biomass sources are composed of carbon and oxygen, utilization of these materials for constructive applications provide a possible solution for global warming and other environmental challenges. Activated carbons are the least expensive to produce in comparison to other carbon materials. Their highly porous structure significantly enhances the surface area of the materials; thus, activated carbon has been employed as an electrode material for EDLC supercapacitor applications [16][25].
For supercapacitor applications, porous carbon materials can easily be obtained using chemical activation methods with various activating agents like potassium hydroxide (KOH), sulphuric acid (H2SO4), silver chloride (AgCl), zinc II chloride (ZnCl2), and others. Amorphous carbon materials can be chemically activated using these agents, which aid in creating porous structures in carbon materials [26]. It is well known that an electrochemical activation method, normally used to activate carbon, can remarkably enhance the capacitance of final product in comparison to the parent carbon [27][28][29]. Chen and coworkers investigated the effect of KOH activation of AC performance and saw a ~185% increase in specific capacitance value post-treatment [30]. They attribute this enhancement to a high mesopore surface area (2505.6 m2/g). Qin et al. achieved similar results using ZnCl2 as an activating agent [31]. However, they attributed the high specific capacitance to a high specific surface area with significant micropore formation.
An outstanding approach to acquiring a high surface area for activated carbon and consequently, high specific capacitance, has been reported by Yushin and coworker [32]. A synthetic polymer named polypyrrole was used to synthesize activated carbon by performing a single activation step using KOH. Thermal treatment of polypyrrole produced a high percentage of carbonaceous residue, which led to a highly porous material with the highest specific surface area (greater than 3400 m2/g) ever published for a carbon electrode. An exceptional specific capacitance of about 300 F/g was recorded in ionic liquid electrolyte for the activated carbon derived from polypyrrole. Among all other types of carbon-based electrodes, such as activated carbon, carbide derived carbon, carbon nanotube, and graphene, the activated carbon derived from polypyrrole exhibits the highest specific capacitance in ionic liquid electrolyte. Since 1-ethyl-3-methyl imidazolium tetrafluoroborate, an ionic liquid, was employed, an increase in capacitance was observed with increase in temperature due to a decrease in viscosity and an increase in conductivity of the ionic liquid.
In another report, porous activated carbon produced from waste tea leaves, a biomass source, was tested in an aqueous electrolyte [33]. The amorphous activated carbon displayed very high specific surface area with a value of 2841 m2/g. An outstanding value of specific capacitance (330 F/g) was observed in KOH electrolyte with high cycle stability. About 92% of the initial capacitance was retained after 20,000 cycles, thus demonstrating high cycling stability. Similarly, another study demonstrated a sustainable synthesis of porous activated carbon using waste tree seeds [34]. A thermal pre-carbonization and subsequent activation with KOH yielded a material with 365 F/g capacitance value and retained 92% of that value over 5000 cycles.
3.2. Graphene
Graphene, which exists as an sp2 hybridized carbon lattice, is well known for its remarkable electron delocalization characteristics. Therefore, it is widely used for numerous electrochemical applications such as supercapacitors [35][36][37][38][39], next generation electronics [40], and sensors [41][42][43][44][45]. Herein, supercapacitor applications have been discussed in more detail.
Graphene is broadly used to fabricate supercapacitor devices due to their amazing mechanical strength, large surface area (2630 m2/g), and excellent electrical properties such as high electrical and thermal conductivity, wide electrochemical window, and a high charge carrier mobility, ca. 20 m2/V/s [46]. Due to these outstanding features, these materials have been exploited in different forms for energy storage devices. However, extraordinary characteristics of graphene showed specific capacitance results in various electrolytes (aqueous (135 F/g), organic (99 F/g), and ionic liquid (75 F/g) electrolyte), which are much lower than the expected theoretical capacitance value of 520 F/g [47][48]. These low capacitance values are ascribed to a decreased double layer formation. Therefore, it has been suggested that the theoretical capacitance value can only be attained when all active sites of the graphene electrode surface are accessible to electrolytes. In this regard, several methodologies have been utilized to design effective graphene-based materials for supercapacitor applications. The main goal of these studies is to prepare a graphene material with the maximum number of active sites. In these reports, researchers sought to enhance the specific surface area of graphene materials in addition to achieving tunable pore size with high electric conductivity [48][49][50]. Graphene prepared using different approaches have been tested in different electrolytes since the capacitance is dependent on electrolyte nature as well.
Methodologies employed to synthesize excellent graphene include epitaxial growth of graphene on substrate using chemical vapor deposition [51], exfoliation of graphite using AFM or in conventional organic solvent [52][53], gas phase synthesis of graphene platelets without any substrate [54], multilayered graphene synthesis [55], and many others [51]. Among these, exfoliation of graphite to graphene oxide is one of the most popular methods to fabricate supercapacitor electrodes based on graphene materials. This method is cost-effective and can be used easily for large scale commercialization. In addition, chemical modifications can easily be made on graphene oxide due to the availability of oxygen-containing functional groups. Furthermore, it allows easy tuning of the nanostructured size, while retaining the intrinsic specific surface area and electrical conductivity of the graphene.
Reducing agents employed to reduce graphene oxide directly impact the capacitance value of the resultant graphene materials. Reducing agents utilized at room temperature include hydrazine [56], dimethylhydrazine [57], hydrogen iodide [57], hydroquinone [58], sodium borohydride [59], etc. Besides their toxic nature, some of these reducing agents used for chemical reduction of graphene may introduce additional functional groups on the graphene surface during long processed reduction reactions.
Weak reducing agents cannot reduce all of the oxygen on the graphene oxide that enables easy penetration of aqueous electrolytes. Chen et al. [60] used hydrobromic acid, a weak reductant, to reduce graphene oxide. Hydrobromic acid was unable to reduce some stable oxygen groups present on graphene oxide. The presence of oxygen groups on graphene surface improves the wettability and facilitates the penetration of electrolyte into electrode pores. The presence of oxygen functionalities also yields pseudocapacitance and it was proven by observing the reduction signal of oxygen during cyclic voltammetry measurements. Hence, both EDLC and pseudocapacitance contribute to enhance the overall supercapacitor performance of the graphene. A maximum capacitance value of 348 F/g was observed using this graphene, where partial oxygen functionalities were reduced in 1 M aqueous H2SO4. Moreover, Chen et al. observed a continuous increase in capacitance value up until 2000 cycles. The 120% increase in capacitance from the initial value is attributed to the reduction of remaining oxygen during the cycling processes that improve the capacitor performance.
Recently, chemical reduction using various metal oxides in hydrochloric acid solution has been demonstrated as a more environmentally friendly approach to reduce graphene [61]. However, an electrode prepared by thermal and chemical reduction of graphene still suffers from small pore size, metallic impurity, and agglomeration [62]. Thus, it is necessary to design new methodologies to restore the graphene material with less agglomeration and higher pore size. A highly conductive graphene material (1000–3000 S m−1) was obtained by thermal nitridation of reduced graphene oxide [25]. The resulting materials are highly porous and exhibit high thermal stability. However, a significantly low value for the Brunauer-Emmett-Teller (BET) surface area (630.6 m2/g) has been reported. These results demonstrate the great tendency of agglomeration of the nitrogen doped graphene material. Thus, small values for the specific capacitance are observed ca. 138.1 F/g. However, the capacitance performance can be tailored by altering the current density. Nevertheless, high electric conductivity, high porosity, and high connectivity of the nitrogen doped graphene sheets exhibit excellent stability. Moreover, these devices can be recharged within a minute.
Ruoff and coworkers prepared porous carbon with a BET surface area of up to 3100 square meters per gram. This was the highest surface area reported at the time of publication [63]. In this study, microwave-treated and thermally-exfoliated graphene oxide were chemically activated using KOH to enhance the surface area of carbon due to a high number of pores formed. This method can easily be scaled to an industrial level. These three dimensional sp2 hybridized materials have pores with widths in the range of 0.6–5 nm size that exhibit high electrical conductivity, with low oxygen and hydrogen content. These supercapacitor electrodes have been tested with organic as well as with ionic liquid electrolytes and have been proven to show high values of gravimetric capacitance and energy density.
Graphene electrodes are also prepared by thermal reduction since it is a known green synthetic method [64][65]. Using thermal reduction, exfoliation of graphene was performed at a very high temperature to prepare a reduced graphene-based supercapacitor. The thermal reduction process is a comparatively expensive approach to reduce graphene because it requires high temperatures and a tediously long time. Furthermore, reduction at elevated temperatures generates carbon dioxide gas that can cause structural impairments on the graphene surface. Therefore, continuous research has been conducted to design new alternative approaches. To date, exfoliation reduction at room temperature is considered to be the best method to acquire high specific capacitance.
It is well established that graphene materials possess a high affinity to agglomerate themselves. Due to this restacking, pores on the graphene structure cannot be accessed by the electrolyte. Therefore, it significantly reduces surface area and minimizes the capacitance value. In order to solve this issue, Liu et al. [66] presented the curved morphology of a graphene sheet. The curved shape inhibits the graphene sheet’s tendency to restack, and thus the electrolyte can easily reach into the pores, wet the graphene, and efficiently form an electric double layer. The graphene sheets with pore sizes in the range of 2–25 nm, were tested with ionic liquid electrolyte. The curved graphene sheets exhibited very high capacitance (100–250 F/g) with no contribution from pseudocapacitance. Typical flat shape graphene sheet examined under the same conditions with ionic liquid electrolyte, exhibited less than 10 F/g capacitance. This breakthrough research indicates the potential of graphene in energy storage devices. Hence, it is imperative to develop new strategies to expose the surface area of graphene, which will inhibit the restacking of graphene sheet. Thus, the electrolyte can access the exposed surface area and form a double layer, which consequently enhances the performance of the material for supercapacitor application.
3.3. Carbon Nanotubes
Carbon nanotubes (CNTs) are low-cost durable materials, which possess high surface areas. Carbon nanotubes have been explored for supercapacitor electrode applications due to their outstanding mechanical, chemical, electronic, and optical properties [6][67][68]. As a result of these interesting properties, these materials are being utilized in other applications as well, which influences the significant growth of commercial production of CNTs. In the literature, a number of papers and patents that describe CNTs are continuously increasing [68]. CNTs exhibit comparable capacitance values to activated carbon, although activated carbon possesses a larger surface area. The great performance using CNTs is ascribed to the utilization of maximum surface area of CNTs for continuous charge distribution [69]. Furthermore, their mesoporous characteristics allow electrolyte to diffuse more easily that reduce the equivalent series resistances and thus improve the power output [70][71].
Mainly, CNTs have been classified into two subclasses, viz., single wall carbon nanotubes (SWNTs) and multi wall carbon nanotubes (MWNTs). Highly conductive single walled and multi walled carbon nanotubes offer a large accessible pore surface area. In addition, the pore sizes of single wall and multiwall carbon nanotubes can easily be tuned for efficient diffusion of electrolyte ions. Due to this flexibility, both SWNTs and MWNTs have been employed for EDLC electrode application to acquire maximum power of the electrode [69]. Both SWNT and MWNT supercapacitor performance have been studied and capacitances are reported to be 180 F/g and 102 F/g for SWNT and MWNT, respectively [70][72]. Researchers are introducing new methodologies to use unbundled SWNTs to improve their performance. The important feature of a MWNT is the rapid discharge time of 7 ms for 10 MWNTs, up to 10 V. However, their low energy density due to their much smaller surface area limits their applications [6]. Rey-Raap et al. investigated the use of MWNTs as additive for biomass-derived activated carbons and found that at 2% CNT content the specific capacitance is increased from 139 F/g to 190 F/g [73].
Don Futaba et al. [74] presented a controlled fabrication method to acquire more highly dense and aligned SWNTs, which possesses high surface area. The performance of these newly synthesized SWNTs were compared with carbon solid electrodes and activated carbon. The results demonstrate a superior capacitance performance with a very small loss of capacitance as well as high power operation of SWNTs as compared to other two carbon electrodes mentioned earlier. This tremendous performance was attributed to ion diffusivity in porous aligned SWNTs.
This entry is adapted from the peer-reviewed paper 10.3390/electrochem1040028
References
- Shafiee, ; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189.
- Dicks, L. The role of carbon in fuel cells. J. Power Sources 2006, 156, 128–141.
- Kim, K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical supercapacitors for energy storage and conversion. Handb. Clean Energy Syst. 2015, 1–25.
- Gao, ; Zhang, J.; Luo, X.; Wan, Y.; Zhao, Z.; Han, X.; Xia, Z. Energy density-enhancement mechanism and design principles for heteroatom-doped carbon supercapacitors. Nano Energy 2020, 72, 104666.
- Kötz, ; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498.
- Vangari, ; Pryor, T.; Jiang, L. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng. 2012, 139, 72–79.
- Wang, ; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828.
- Su, S.; Zhang, J.; Frank, B.; Thomas, A.; Wang, X.; Paraknowitsch, J.; Schlögl, R. Metal‐Free Heterogeneous Catalysis for Sustainable Chemistry. ChemSusChem 2010, 3, 169–180.
- Zhang, ; Zhao, X. Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 2012, 116, 5420–5426.
- Zhang, L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531.
- Inal, I.G.; Aktas, Z. Enhancing the performance of activated carbon based scalable supercapacitors by heat treatment. Appl. Surf. Sci. 2020, 514, 145895.
- Yu, ; Nagelli, E.; Du, F.; Dai, L. Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173.
- Rauda, E.; Augustyn, V.; Dunn, B.; Tolbert, S.H. Enhancing pseudocapacitive charge storage in polymer templated mesoporous materials. Acc. Chem. Res. 2013, 46, 1113–1124.
- Halama, ; Szubzda, B.; Pasciak, G. Carbon aerogels as electrode material for electrical double layer supercapacitors—Synthesis and properties. Electrochim. Acta 2010, 55, 7501–7505.
- Wang, ; Zhu, F.; Jia, W.; Wu, Y.; Zhao, L.; Yu, L.; Chen, Y.; Shen, Z. Electrochemical performance of activated carbons with different specific surface area as supercapacitor electrode materials. Int. J. Electrochem. Sci. 2016, 11, 6688–6695.
- Faraji, ; Ani, F.N. The development supercapacitor from activated carbon by electroless plating—A review. Renew. Sustain. Energy Rev. 2015, 42, 823–834.
- Inagaki, ; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903.
- Yin, ; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods 2020, 4, 1900853.
- Altinci, C.; Demir, M. Beyond Conventional Activating Methods, A Green Approach for the Synthesis of Bio-Carbon and its supercapacitor electrode performance. Energy Fuels 2020.
- Gaddam, R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352, doi:10.1016/j.nanoen.2016.05.047.
- Deng, ; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854, doi:10.1039/C6GC01172A.
- Su, -L.; Chen, J.-R.; Zheng, G.-P.; Yang, J.-H.; Guan, X.-X.; Liu, P.; Zheng, X.-C. Three-dimensional porous activated carbon derived from loofah sponge biomass for supercapacitor applications. Appl. Surf. Sci. 2018, 436, 327–336.
- Han, ; Jiang, H.; Zhou, Y.; Hong, W.; Zhou, Y.; Gao, P.; Ding, R.; Liu, E. A high performance nitrogen-doped porous activated carbon for supercapacitor derived from pueraria. J. Alloy. Compd. 2018, 744, 544–551.
- Inal, I.G.; Holmes, S.M.; Yagmur, E.; Ermumcu, N.; Banford, A.; Aktas, Z. The supercapacitor performance of hierarchical porous activated carbon electrodes synthesised from demineralised (waste) cumin plant by microwave pretreatment. J. Ind. Eng. Chem. 2018, 61, 124–132.
- Pandolfo, ; Hollenkamp, A. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27.
- Barranco, ; Lillo-Rodenas, M.A.; Linares-Solano, A.; Oya, A.; Pico, F.; Ibañez, J.; Agullo-Rueda, F.; Amarilla, J.M.; Rojo, J.M. Amorphous Carbon Nanofibers and Their Activated Carbon Nanofibers as Supercapacitor Electrodes. J. Phys. Chem. C 2010, 114, 10302–10307, doi:10.1021/jp1021278.
- Raymundo-Pinero, ; Azais, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 2005, 43, 786–795.
- Liu, ; Sreekumar, T.; Kumar, S.; Hauge, R.H.; Smalley, R.E. SWNT/PAN composite film-based supercapacitors. Carbon 2003, 41, 2440–2442.
- Takeuchi, ; Maruyama, T.; Koike, K.; Mogami, A.; Oyama, T.; Kobayashi, H. Non-porous carbon for a high energy density electric double layer capacitor. Electrochemistry 2001, 69, 487–492.
- Chen, ; Xuan, H.; Zheng, X.; Liu, J.; Dong, X.; Xi, F. N-doped mesoporous carbon by a hard-template strategy associated with chemical activation and its enhanced supercapacitance performance. Electrochim. Acta 2017, 238, 269–277.
- Qin, ; Xiao, Z.; Zhai, S.; Wang, S.; Wang, H.; Wang, G.; Cai, W.; Li, Z.; An, Q. Alginate-derived porous carbon obtained by Nano-ZnO hard template-induced ZnCl2-activation method for enhanced electrochemical performance. J. Electrochem. Soc. 2020, 167, 040505.
- Wei, ; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Polypyrrole-derived activated carbons for high-performance electrical double-layer capacitors with ionic liquid electrolyte. Adv. Funct. Mater. 2012, 22, 827–834, doi:10.1002/adfm.201101866.
- Peng, ; Yan, X.; Wang, R.; Lang, J.; Ou, Y.; Xue, Q. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408.
- Srinivasan, ; Elaiyappillai, E.; Pandian, H.P.; Vengudusamy, R.; Johnson, P.M.; Chen, S.-M.; Karvembu, R. Sustainable porous activated carbon from Polyalthia longifolia seeds as electrode material for supercapacitor application. J. Electroanal. Chem. 2019, 849, 113382.
- Strauss, ; Marsh, K.; Kowal, M.D.; El‐Kady, M.; Kaner, R.B. A simple route to porous graphene from carbon nanodots for supercapacitor applications. Adv. Mater. 2018, 30, 1704449.
- Gao, ; Niu, Q.; Tang, Q.; Guo, Y.; Wang, L. Graphene-like 2D porous carbon nanosheets derived from cornstalk pith for energy storage materials. J. Electron. Mater. 2018, 47, 337–346.
- Yang, ; Zhang, E.; Li, X.; Yu, Y.; Qu, J.; Yu, Z.-Z. Direct reduction of graphene oxide by Ni foam as a high-capacitance supercapacitor electrode. Acs Appl. Mater. Interfaces 2016, 8, 2297–2305.
- Sahu, ; Grover, S.; Tulachan, B.; Sharma, M.; Srivastava, G.; Roy, M.; Saxena, M.; Sethy, N.; Bhargava, K.; Philip, D. Heavily nitrogen doped, graphene supercapacitor from silk cocoon. Electrochim. Acta 2015, 160, 244–253.
- Wang, ; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107.
- Blake, ; Brimicombe, P.D.; Nair, R.R.; Booth, T.J.; Jiang, D.; Schedin, F.; Ponomarenko, L.A.; Morozov, S.V.; Gleeson, H.F.; Hill, E.W. Graphene-based liquid crystal device. Nano Lett. 2008, 8, 1704–1708.
- Yoon, J.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.-C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313.
- Kulkarni, S.; Reddy, K.; Zhong, Z.; Fan, X. Graphene nanoelectronic heterodyne sensor for rapid and sensitive vapour detection. Nat. Commun. 2014, 5, 4376.
- Ang, K.; Chen, W.; Wee, A.T.S.; Loh, K.P. Solution-gated epitaxial graphene as pH sensor. J. Am. Chem. Soc. 2008, 130, 14392–14393.
- Yun, ; Lim, Y.; Jang, G.N.; Kim, D.; Lee, S.-J.; Park, H.; Hong, S.Y.; Lee, G.; Zi, G.; Ha, J.S. Stretchable patterned graphene gas sensor driven by integrated micro-supercapacitor array. Nano Energy 2016, 19, 401–414.
- An, ; Ma, Y.; Li, W.; Su, M.; Li, F.; Song, Y. Three-dimensional multi-recognition flexible wearable sensor via graphene aerogel printing. Chem. Commun. 2016, 52, 10948–10951.
- Huang, ; Liang, J.; Chen, Y. An overview of the applications of graphene‐based materials in supercapacitors. Small 2012, 8, 1805–1834.
- Xia, ; Chen, F.; Li, J.; Tao, N. Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 2009, 4, 505–509.
- Stoller, D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502.
- Wu, ; Pisula, W.; Müllen, K. Graphenes as Potential Material for Electronics. Chem. Rev. 2007, 107, 718–747, doi:10.1021/cr068010r.
- Vivekchand, ; Rout, C.S.; Subrahmanyam, K.; Govindaraj, A.; Rao, C. Graphene-based electrochemical supercapacitors. J. Chem. Sci. 2008, 120, 9–13.
- Zhu, ; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924, doi:10.1002/adma.201001068.
- Hernandez, ; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.; Holland, B.; Byrne, M.; Gun'Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568.
- Lee, ; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388.
- Dato, ; Radmilovic, V.; Lee, Z.; Phillips, J.; Frenklach, M. Substrate-free gas-phase synthesis of graphene sheets. Nano Lett. 2008, 8, 2012–2016.
- Yoon, -M.; Choi, W.M.; Baik, H.; Shin, H.-J.; Song, I.; Kwon, M.-S.; Bae, J.J.; Kim, H.; Lee, Y.H.; Choi, J.-Y. Synthesis of multilayer graphene balls by carbon segregation from nickel nanoparticles. ACS Nano 2012, 6, 6803–6811.
- Stankovich, ; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565.
- Stankovich, ; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286.
- Wang, ; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195.
- Si, ; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682.
- Chen, ; Zhang, X.; Zhang, D.; Yu, P.; Ma, Y. High performance supercapacitors based on reduced graphene oxide in aqueous and ionic liquid electrolytes. Carbon 2011, 49, 573–580, doi:10.1016/j.carbon.2010.09.060.
- Lake, R.; Cheng, A.; Selverston, S.; Tanaka, Z.; Koehne, J.; Meyyappan, M.; Chen, B. Graphene metal oxide composite supercapacitor electrodes. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2012, 30, 03D118.
- Ambrosi, ; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188.
- Zhu, ; Murali, S.; Stoller, M.D.; Ganesh, K.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541.
- Schniepp, C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud'homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539.
- Wang, ; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327.
- Liu, ; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868.
- Baughman, H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes--the route toward applications. Science 2002, 297, 787–792.
- De Volder, F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539, doi:10.1126/science.1222453.
- Frackowiak, ; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950.
- Niu, ; Sichel, E.K.; Hoch, R.; Moy, D.; Tennent, H. High power electrochemical capacitors based on carbon nanotube electrodes. Appl. Phys. Lett. 1997, 70, 1480–1482.
- An, H.; Kim, W.S.; Park, Y.S.; Choi, Y.C.; Lee, S.M.; Chung, D.C.; Bae, D.J.; Lim, S.C.; Lee, Y.H. Supercapacitors using single-walled carbon nanotube electrodes. Adv. Mater. 2001, 13, 497–500, doi:10.1002/1521-4095(200104)13:7<497::AID-ADMA497>3.0.CO;2-H.
- An, H.; Kim, W.S.; Park, Y.S.; Moon, J.-M.; Bae, D.J.; Lim, S.C.; Lee, Y.S.; Lee, Y.H. Electrochemical properties of high-power supercapacitors using single-walled carbon nanotube electrodes. Adv. Funct. Mater. 2001, 11, 387–392, doi:10.1002/1616-3028(200110)11:53.3.CO;2-7.
- Rey-Raap, ; Enterría, M.; Martins, J.I.c.; Pereira, M.F.R.; Figueiredo, J.L.s. Influence of multiwalled carbon nanotubes as additives in biomass-derived carbons for supercapacitor applications. ACS Appl. Mater. Interfaces 2019, 11, 6066–6077.
- Futaba, N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994.