Secretory IgA (SIgA) is the dominant antibody class in mucosal secretions, it is also present in saliva and breast milk. The majority of plasma cells producing SIgA are located within mucosal membranes lining the intestines, airway and reproductive tracts, as well as mammary gland. SIgA protects against the adhesion of pathogens and their penetration into the mucosal barriers. Moreover, SIgA regulates microbiota composition at mucosa sites and provides local homeostasis.
- secretory immunoglobulin A,gut,microbiota,immune homeostasis,mucosal secretions,tolerance
1. Introduction
Immunoglobulin (Ig) is a protein composed of two identical heavy (H) and light (L) chains connected via disulfide bonds. Both chains are composed of variable (V) domains and constant (C) domains. Functionally, Ig is divided into the antigen-binding fragment (Fab) region (paired VHL-domains responsible for specific epitope binding with CL and CH1 domains) connected through a hinge region to the crystallizable region fragment (Fc, made with remaining CH domains). Differences between Fc constant domains enable an immunoglobulin classification to five isotypes: IgG, IgA, IgM, IgE and IgD [1]
Immunoglobulin A (IgA) is present in all mammals and birds. It is found in large amounts in the mucosal secretions of gastrointestinal tract and in other secretions, including saliva and breast milk [1][2]. However, IgA is also present in serum at lower concentration (about 2–3 mg per mL) [2][3]. In humans, daily IgA production is higher than any other immunoglobulin isotype (up to ~60 mg per kg of body weight) [4].
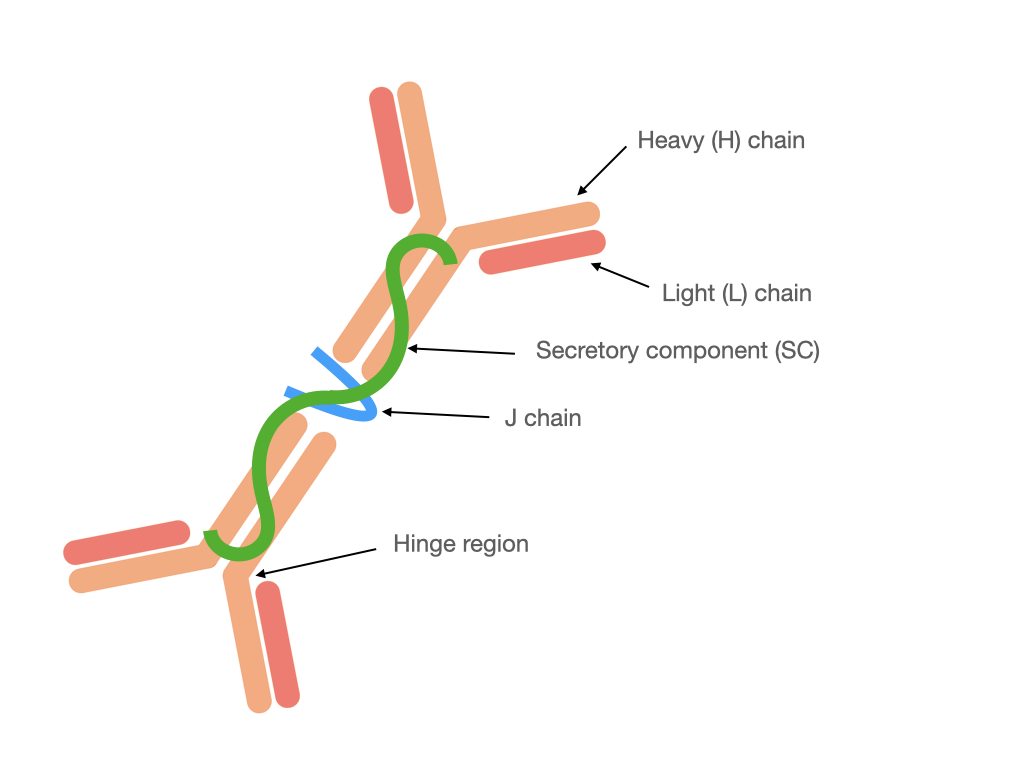
Monomeric IgA is present in serum, whereas in mucosal secretions is found secretory IgA (SIgA). It is different from the structure of IgA present in the serum because SIgA (Fig. 1) generally occurs in a polymeric form stabilized by joining chain (J chain), in particular in dimeric or tetrameric setup. Additionally, SIgA contains a secretory component (SC) derived from polymeric Ig receptor (pIgR) utilized for transcytosis through epithelial cells during secretion [2][5]. In humans, there are two subclasses of IgA: IgA1 and IgA2 [6]. In serum subclass IgA1 dominates, whereas in mucosal secretions the proportion between IgA1 and IgA2 depends on the site of production, e.g., up to: 60% IgA1 in saliva, 90% IgA1 in nasal and 60% IgA2 in intestinal secretions [5]. In the human colostrum approximately 48% of immunoglobulins correspond to IgA2 and 40% to the IgA1 subclass [7] that confers an adaptation to protect against potentially harmful pathogens, and which is also a way to regulate the colonization of the microbiota in newborns.
Figure 1. Structure of dimeric SIgA.
Mucosal membranes lining gastrointestinal, respiratory and genitourinary tracts are exposed to permanent contact with a vast variety of microorganisms. The gastrointestinal tract (GIT) is colonized by numerous and diverse microbial communities, up to ~1014 microbial cells per gram of colonic content represented by ~500–1000 bacterial species, archaea and fungi [8]. To prevent the invasion of pathogenic microbes and to regulate interactions between host and bacteria, the mucosal immune system is stimulated to produce SIgA [8][9].
2. Synthesis of SIgA in the Intestines
The synthesis of serum IgA generally occurs in the bone marrow, where the immunoglobulin is produced as a monomer. There are also other places of monomeric IgA production by plasma cells (PCs), e.g., spleen, lymph nodes, peripheral blood or even intestinal lamina propria (LP), however in much lower proportion compared to the production of polymeric IgA [10][11].
The SIgA is secreted mainly by PCs located within mucosal membranes lining the GIT, and in much lower extent, respiratory and genitourinary tracts [2]. Newborn humans and germ-free (GF) mice have few or no IgA-producing plasma cells (IgA+ PCs) in the LP. Bacterial colonization induces IgA+ PCs generation, and produced IgA includes specificity for the luminal microbiota [6]. The vast majority of IgA+ PCs are present (~80%) in LP of intestinal villi [4]. Studies showed that the small intestinal LP contains up to 15-fold more IgA+ PCs than the colonic LP [12]. Abundant production of SIgA in the intestines is one of the mechanisms that gut-associated lymphoid tissue (GALT) runs in response to constant contact of gut mucosal membranes with a large number of diverse microorganisms, mainly bacteria and other food-derived antigens [8]. GALT contains various lymphoid structures (germinal centers, GCs) localized within mucosal surfaces of the intestines, such as highly structured Peyer's patches (PPs) or isolated lymphoid follicles (ILFs), which are places of SIgA origin [13][14][15]. Of note, these lymphoid structures are predominantly associated with the small intestine [12]. Colonic GALT contains less GCs than small intestinal GALT. Interestingly, the increase in the GCs presence in colonic GALT is associated with colon tumors [16].
The IgA+ PCs can be created through T cell-dependent (TD, adaptive response) and -independent (TI, innate response) mechanisms in mice and humans [17]. However, a variety of immune and non-immune cells are indispensable in the process of IgA+ PCs generation. In humans, B2 cells, which are bone marrow-derived population of B cells, undergo differentiation to IgA+ PCs. Of note, in mice, IgA+ PCs originate from two B cell populations, i.e., primitive B1 and B2 cells, whereas in humans, there has not been found any direct equivalent to B1 cells so far [6][18].
3. Functions of SIgA in Gut Mucosal Secretions
SIgA is produced in response to microbial- and food-derived antigens and plays different roles in intestinal mucosal secretions (Table 1). It acts as the first line of defense against pathogens and facilitates mucus surface colonization by commensal microbiota and regulates immune homeostasis [19].
Table 1. The primary functions of SIgA in gut mucosal secretions.
|
Function |
Mechanism |
Reference |
|
Immune exclusion |
Retention of bacteria in the intestinal lumen. |
|
|
Niche occupancy |
Binding bacteria to mucus. |
|
|
Immobilization |
Impairing mobility by targeting flagella, cell agglutination. |
[13] |
|
Modulation of bacterial gene expression |
Targeting bacterial surface end extracellular proteins. |
[25] |
|
Neutralization |
Targeting adhesins and toxins. |
|
|
Antigen uptake |
Facilitated transcytosis. |
Two mechanisms of SIgA binding to antigens are possible: canonical and non-canonical. Canonical (Fab-dependent) SIgA binding to its antigens occurs via the complementarity-determining regions (CDRs), whereas non-canonical via glycan moieties of this molecule. The process of IgA+ B cell SHM causes alterations in CDR sequence and structure and leads to the generation of affinity-matured IgA [30]. SHM occurs mainly during T cell-dependent, but also during T cell-independent IgA+ B cell maturation [30][31]. Secreted forms of IgA, but also J chain, and SC are heavily glycosylated. O-glycans located between Cα1 and Cα2 domains of SIgA heavy chain are involved in bacteria binding. The sugar moieties also protect the hinge from bacterial protease digestion. The wide range of N-linked glycans on SC target bacterial adhesins as well, and anchor SIgA to the mucosal lining of the epithelium entrapping microorganisms [21].
Pathogenic strains and toxins are predominantly neutralized by high-affinity SIgA produced through TD mechanism, whereas gut commensal microbiota is coated with low-affinity IgA produced through TI mechanism [13]. However, there are some exceptions to this rule. For instance, Bunker et al. (2015) observed that some commensal bacteria, such as segmented filamentous bacteria (SFB, Candidatus Arthromitus) and Mucispirillum sp. elicited IgA responses in TD manner [12].
According to studies, SIgA exhibits cross-species reactivity. It means that SIgA has the ability to bind to various but specified species of microorganisms [30]. In studies conducted on intestinal IgA plasmablasts isolated from patients with ascending colon cancer, Benckert et al. (2011) observed that about half of IgA clones do not react with a panel of commensal and pathogenic microbial strains, third of the clones were cross-species reactive, fifth were self-reactive and the small fraction of clones reacted with single target of prepared panel of microbial strains [19][32]. However, this ability of SIgA to bind to multiple microbial species might result from its polyreactivity. Highly glycosylated molecules of SIgA can interact with glycans exposed by intestinal bacteria, such as O-antigens, polysaccharide capsules, and teichoic acids [30]. Although the structure of many bacterial surface glycans is unknown, it was observed that almost identical glycan motifs are present on commensal Escherichia coli and pathogenic Haemophilus influenzae. This result suggests that cross-species binding of SIgA might rely on recognition of similar surface glycans present on even unrelated species [30][33].
4. Conclusions
SIgA plays a crucial role in the mucosal surfaces lining the GIT. A large number of studies have shown that SIgA is involved in maintaining intestinal homeostasis by neutralization of pathogenic microorganisms and toxins, downregulation of inflammatory responses, regulation of gut microbiota composition and protection from improper immune responses to antigens from microbes and food [19]. Despite the fact that there are various studies on mice and humans, some mechanisms, which regulate such SIgA functions, are still unknown. SIgA plays an important role in shaping the gut microbiota structure during newborn colonization that may be crucial for proper systemic immune response development. Research of Baldassarre et al. (2016) showed the possibility of intervention to modulate colonization and microbiota development in neonatal gut by probiotic administration to women during pregnancy and breastfeeding changing the cytokine pattern in milk that enhanced the production of SIgA in neonates [34]. Dysbiosis (e.g., antibiotic induced) at an early ontogenetic stage may be responsible for susceptibility to non-infectious diseases due to shaping aberrant gut-immune axis signaling. Wider studies, which could shed light on these processes are needed. The SIgA plays a key role in host–microbe interaction and the mechanism underlying the selective recognition of gut microbiota will allow understanding of many gastrointestinal and also systemic diseases. The microbiota regulation of B cell activity may also shed a new light on the complex regulatory mechanisms of anti-tumor responses. Several studies have revealed the different roles of two specific B cell subpopulations. The IgG+ B cells play an antitumor role via IgG-mediated antigen-presentation by DCs and activation of antitumor T cell responses, whereas the IgA+ B cell subpopulation is immunosuppressive because it induces multiple tumor-promotion mechanisms [35][36].
This entry is adapted from the peer-reviewed paper 10.3390/ijms21239254
References
- Schroeder, H.W.J.; Cavacini, L. Structure and Function of Immunoglobulins (author manuscript). J. Allergy Clin. Immunol. 2010, 125, S41–S52.
- Woof, J.M.; Ken, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282.
- Woof, J.M.; Kerr, M.A. IgA function—Variations on a theme. Immunology 2004, 113, 175–177.
- Fagarasan, S.; Honjo, T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat. Rev. Immunol. 2003, 3, 63–72.
- Woof, J.M.; Russell, M.W. Structure and function relationships in IgA. Mucosal Immunol. 2011, 4, 590–597.
- Gibbons, D.L.; Spencer, J. Mouse and human intestinal immunity: Same ballpark, different players; Different rules, same score. Mucosal Immunol. 2011, 4, 148–157.
- Sánchez-Salguero, E.; Mondragón-Ramírez, G.K.; Alcántara-Montiel, J.C.; Cérbulo-Vázquez, A.; Villegas-Domínguez, X.; Contreras-Vargas, V.M.; del Thompson-Bonilla, M.R.; Romero-Ramírez, H.; Santos-Argumedo, L. Infectious episodes during pregnancy, at particular mucosal sites, increase specific IgA1 or IgA2 subtype levels in human colostrum. Matern. Health Neonatol. Perinatol. 2019, 5, 9.
- Suzuki, K.; Fagarasan, S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008, 29, 523–531.
- Cerutti, A.; Rescigno, M. The Biology of Intestinal Immunoglobulin A Responses. Immunity 2008, 28, 740–750.
- Iversen, R.; Snir, O.; Stensland, M.; Kroll, J.E.; Steinsbø, Ø.; Korponay-Szabó, I.R.; Lundin, K.E.A.; de Souza, G.A.; Sollid, L.M. Strong Clonal Relatedness between Serum and Gut IgA despite Different Plasma Cell Origins. Cell Rep. 2017, 20, 2357–2367.
- Kutteh, W.H.; Prince, S.J.; Mestecky, J. Tissue origins of human polymeric and monomeric IgA. J. Immunol. 1982, 128, 990–995.
- Bunker, J.J.; Flynn, T.M.; Koval, J.C.; Shaw, D.G.; Meisel, M.; McDonald, B.D.; Ishizuka, I.E.; Dent, A.L.; Wilson, P.C.; Jabri, B.; et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 2015, 43, 541–553.
- Cerutti, A.; Chen, K.; Chorny, A. Immunoglobulin Responses at the Mucosal Interface. Annu. Rev. Immunol. 2011, 29, 273–293.
- Suzuki, K.; Nakajima, A. New aspects of IgA synthesis in the gut. Int. Immunol. 2014, 26, 489–494.
- Nochi, T.; Denton, P.W.; Wahl, A.; Garcia, J.V. Cryptopatches Are Essential for the Development of Human GALT. Cell Rep. 2013, 3, 1874–1884.
- Spencer, J.; Sollid, L.M. The human intestinal B-cell response. Mucosal Immunol. 2016, 9, 1113–1124.
- Suzuki, K.; Fagarasan, S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009, 2, 468–471.
- Tezuka, H.; Ohteki, T. Regulation of IgA production by intestinal dendritic cells and related cells. Front. Immunol. 2019, 10, 1891.
- Sutherland, D.B.; Fagarasan, S. IgA synthesis: A form of functional immune adaptation extending beyond gut. Curr. Opin. Immunol. 2012, 24, 261–268.
- Xiong, N.; Hu, S. Regulation of intestinal IgA responses. Cell. Mol. Life Sci. 2015, 72, 2645–2655.
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50.
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611.
- Melo-Gonzalez, F.; Kammoun, H.; Evren, E.; Dutton, E.E.; Papadopoulou, M.; Bradford, B.M.; Tanes, C.; Fardus-Reid, F.; Swann, J.R.; Bittinger, K.; et al. Antigen-presenting ILC3 regulate T cell-dependent IgA responses to colonic mucosal bacteria. J. Exp. Med. 2019, 216, 728–742.
- Bollinger, R.R.; Everett, M.L.; Palestrant, D.; Love, S.D.; Lin, S.S.; Parker, W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology 2003, 109, 580–587.
- Joglekar, P.; Ding, H.; Canales-Herrerias, P.; Pasrich, P.J.; Sonnenburg, J.L.; Peterson, D.A. Intestinal IgA regulates expression of a fructan polysaccharide utilization locus in colonizing gut commensal Bacteroides thetaiotaomicron. MBio 2019, 10, doi:10.1128/mbio.02324-19.
- Perrier, C.; Sprenger, N.; Corthésy, B. Glycans on secretory component participate in innate protection against mucosal pathogens. J. Biol. Chem. 2006, 281, 14280–14287.
- Uren, T.K.; Wijburg, O.L.C.; Simmons, C.; Johansen, F.E.; Brandtzaeg, P.; Strugnell, R.A. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur. J. Immunol. 2005, 35, 180–188.
- Forbes, S.J.; Bumpus, T.; McCarthy, E.A.; Corthésy, B.; Mantis, N.J. Transient suppression of shigella flexneri type 3 secretion by a protective O-antigen-specific monoclonal igA. MBio 2011, 2, doi:10.1128/mbio.00042-11.
- Reboldi, A.; Cyster, J.G. Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol. Rev. 2016, 271, 230–245.
- Pabst, O.; Slack, E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020, 13, 12–21.
- Lycke, N.Y.; Bemark, M. The regulation of gut mucosal IgA B-cell responses: Recent developments. Mucosal Immunol. 2017, 10, 1361–1374.
- Benckert, J.; Schmolka, N.; Kreschel, C.; Zoller, M.J.; Sturm, A.; Wiedenmann, B.; Wardemann, H. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J. Clin. Investig. 2011, 121, 1946–1955.
- Tsui, F.P.; Egan, W.; Summers, M.F.; Andrew Byrd, R.; Schneerson, R.; Robbins, J.B. Determination of the structure of the Escherichia coli K100 capsular polysaccharide, cross-reactive with the capsule from type b Haemophilus influenzae. Carbohydr. Res. 1988, 173, 65–74.
- Baldassarre, M.E.; Di Mauro, A.; Mastromarino, P.; Fanelli, M.; Martinelli, D.; Urbano, F.; Capobianco, D.; Laforgia, N. Administration of a multi-strain probiotic product to women in the perinatal period differentially affects the breast milk cytokine profile and may have beneficial effects on neonatal gastrointestinal functional symptoms. A randomized clinical trial. Nutrients 2016, 8, 677.
- Wang, J.Z.; Zhang, Y.H.; Guo, X.H.; Zhang, H.Y.; Zhang, Y. The double-edge role of B cells in mediating antitumor T-cell immunity: Pharmacological strategies for cancer immunotherapy. Int. Immunopharmacol. 2016, 36, 73–85.
- Liu, M.; Sun, Q.; Wang, J.; Wei, F.; Yang, L.; Ren, X. A new perspective: Exploring future therapeutic strategies for cancer by understanding the dual role of B lymphocytes in tumor immunity. Int. J. Cancer 2019, 144, 2909–2917.