Among the different mechanisms involved in oxidative stress, protein carbonylation and lipid peroxidation are both important modifications associated with the pathogenesis of several diseases, including cancer. Hematopoietic cells are particularly vulnerable to oxidative damage, as the excessive production of reactive oxygen species and associated lipid peroxidation suppress self-renewal and induce DNA damage and genomic instability, which can trigger malignancy. A richer understanding of the clinical effects of oxidative stress might improve the prognosis of these diseases and inform therapeutic strategies. The most common protein carbonylation and lipid peroxidation compounds, including hydroxynonenal, malondialdehyde, and advanced oxidation protein products, have been investigated for their potential effect on hematopoietic cells in several studies.
- oxidative stress,protein carbonylation,lipid peroxidation,hematological malignancies
1. Introduction
Oxidative stress can be defined as an imbalance between the production of reactive oxygen species (ROS) and the ability of the cells to detoxify them [1]. ROS production is a common consequence of aerobic metabolism, and can play a dual role in cells, being either beneficial or harmful. For instance, hydrogen peroxide is a major redox metabolite that operates in redox signaling, but when produced at high concentrations it can contribute to damage of biomolecules and trigger an inflammatory response [2]. High levels of ROS are associated with DNA fragmentation, lipid peroxidation, and/or protein carbonylation, leading to cellular dysfunction and even cell death [3]. Accordingly, cells rely on efficient antioxidant defenses provided by enzymes and metabolites to maintain low levels of ROS; for example, superoxide dismutase (SOD) and catalase (CAT) enzymes, and antioxidant molecules such as thiol antioxidants or vitamin E [3].
As oxidative stress has myriad consequences for cell fate, many analytical procedures have been established both for the produced ROS and their downstream effects on biomolecules. Although free radicals can be measured in biological samples, their quantification lacks sensitivity and specificity, and so much effort has been directed at quantifying their target products, including fragmented DNA, lipid peroxidation products (malondialdehyde (MDA) or 4-hydroxy-2,3-nonenal (HNE)) and protein carbonylation, an irreversible oxidative modification [4]. As DNA fragmentation does not directly correlate with ROS levels, the most useful current methods involve the quantification of lipid peroxidation and protein carbonylation [5].
Protein carbonylation is one of the most common oxidative modifications. Oxidation of proteins is of particular concern since it leads to aggregation, polymerization, unfolding, or conformational changes that may confer a loss of structural or functional activity. Oxidized protein aggregates are not readily degraded in the cell, and their accumulation causes cell dysfunction [6][7]. While many different types of protein oxidative modifications are possible, most involve protein carbonyls (aldehydes and ketones) [8]. As carbonyl groups are chemically stable, they are extremely useful for laboratory analysis, although their study is methodologically complex. The carbonyl contents of individual proteins may be assessed through derivatization of the carbonyl group with dinitrophenylhydrazine (DNPH), which forms a stable dinitrophenylhydrazone (DNP) product that can be analyzed spectrometrically or by immunoblotting [9][10]. Carbonylation research is characterized by the application of numerous protocols and proteomics workflows, which allows the measurement of compounds by several techniques. Protein carbonylation is a major final by-product of multiple oxidation pathways that occur in the cell and thus, this makes it an appropriate marker of oxidative stress [11]. In contrast, some protein modifications might just represent cellular antioxidation mechanisms as part of the oxidation-defense system [12]. To deepen into the biological implication of protein carbonylation, it is important to characterize each specific modification.
Protein carbonylation can be induced directly by the action of oxidative stress or indirectly by reactions of secondary by-products.
The main mechanism of protein carbonylation involves the direct action of ROS or the metal-catalyzed oxidation of amino acid side chains, particularly proline, arginine, lysine, and threonine. Carbonyl derivatives can also be generated through the α-amidation pathway or through the oxidation of glutamyl side chains, where the peptide is blocked in N-terminal amino acids by an α-ketoacyl derivative [13]. The indirect mechanism of protein carbonylation involves the carbonylation of lysine, cysteine, and histidine, which may be caused by their reaction with reactive carbonyl groups produced during the oxidation of carbohydrates (e.g., glyoxal (GO), methylglyoxal (MGO)) and lipids (e.g., HNE, MDA or acrolein (ACR)). This process of carbonyl generation is termed glycoxidation (the formation of advanced glycation end-products (AGEs)) and lipoxidation (the formation of ALEs), respectively [13][14][15][16][17][18]. Advanced oxidation protein products (AOPPs) are modified structures, similar to AGEs, which also serve as oxidative stress markers [19] (Figure 1).
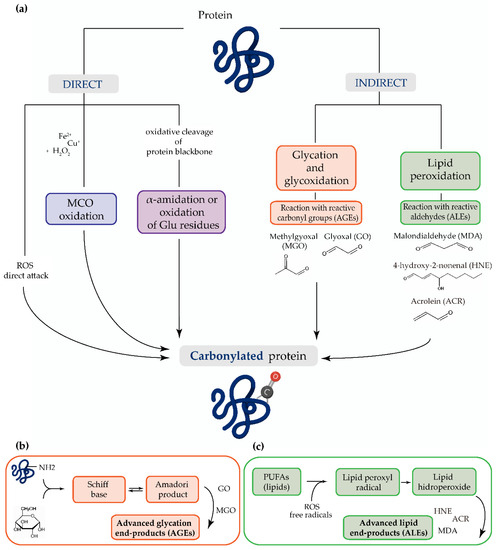
Figure 1. (a) The most common mechanisms of protein carbonylation. Direct processes include reactive oxygen species (ROS) attack, metal-catalyzed oxidation (MCO), and by oxidative cleavage of protein backbone (via the α-amidation pathway or through oxidation of glutamine side chains). The indirect mechanisms involve the reaction with (b) advanced glycation end-products (AGEs) and (c) advanced lipid peroxidation end-products (ALEs).
Lipid peroxidation is also a widely used biomarker of oxidative stress. The polyunsaturated fatty acyls (PUFAs) chains found in membranes and lipoproteins are particularly susceptible to free radical chain autoxidation, leading to a variety of unsaturated lipid hydroperoxides [20]. PUFAs may also be enzymatically oxidized, although these are regio- and stereo-controlled processes involved in normal intermediary metabolism [20]. The nonenzymatic lipoxidation-derived hydroperoxides can decompose, usually in the presence of reduced metals or ascorbate [21], to generate mono- and bifunctional reactive carbonyl-containing moieties, producing aldehydes such as MDA, GO, ACR, 4-HNE, and 4-oxo-2-nonenal (ONE) [20]. The reaction of MDA with thiobarbituric acid to form thiobarbituric reactive substances (TBARS) is a common estimator of oxidative damage. The TBARS assay is, however, nonspecific for MDA, and fatty peroxide-derived decomposition products other than MDA are thiobarbituric acid-positive [20].
The potential effects of ROS as well as their target products on hematopoietic cells are particularly relevant, as these cells are acutely sensitive to oxidative damage associated with the accumulation of free radicals [20]. The resultant lipid peroxidation produced by the excessive production of ROS and reactive nitrogen species can suppress self-renewal, limiting the number of hematopoietic stem cells, and directly induce DNA damage and genomic instability [22].
2. Lymphoma
Lymphoma are a heterogenous group of hematological malignancies derived from different types of lymphocytes and occur predominantly in lymph nodes or other lymphoid structures [23]; as such, they are considered as the solid tumors of the immune system [24]. While their etiology is not well understood, it is likely multifactorial as abnormal genetic alterations, disordered epigenetic regulation, aberrant pathway activation, or infections like Epstein–Barr virus have been reported [23][25][26].
There is growing evidence that oxidative stress and an imbalance in reduction–oxidation might play a significant role in lymphoma carcinogenesis and patient prognosis, either by generating a more favorable environment for cancer cells to proliferate or by modifying the efficiency of oncological treatments, which are largely based on the generation of ROS [27]. Hypoxia is a characteristic feature of solid tumors and, accordingly, its role in hematological malignancies was initially presumed to be inconsequential [28][29]. As mentioned above, however, lymphoma present with solid tumor-like features [30] and normal lymph nodes exhibit low oxygen tension [27][31]. Thus, ROS production might be induced in lymphoma by hypoxic stress, whereas in other hematological diseases it might be due to impaired antioxidant defenses [32].
3. Multiple Myeloma
Multiple myeloma (MM) is the second most common hematological cancer after lymphoma and is characterized by the accumulation of clonal malignant plasma cells in the bone marrow (BM). In fact, the BM microenvironment (niche) plays a key role in supporting tumor cell growth, disease progression, and drug resistance of myeloma plasma cells [33]. One of the main causes of MM is indeed oxidative stress, and it has been known for almost three decades that oxidant/antioxidant parameters are misbalanced in this disease [34] which might continuously stimulate an inflammatory milieu at the tumor microenvironment [35]. The enhanced oxidative state, in turn, increases the rate of genetic mutation, leading to the acquisition of a malignant phenotype and subsequent cancer progression, as also observed in HL [36].
Commonly, MM is preceded by asymptomatic premalignant stages including monoclonal gammopathy of uncertain significance (MGUS) and/or a symptomatic stage such as smoldering multiple myeloma (SMM). Important proteins for the progression of MM, such as c-MYC, have been shown to regulate ROS levels through the modulation of mitochondrial activity [37]. Myeloma cells increase their metabolic demand when the disease progresses, and, consequently, there is a disproportionate production of free radicals or ROS.
4. Leukemia
Leukemia is known to be a heterogeneous disease and four major subtypes are recognized: acute lymphoblastic leukemia (ALL), chronic lymphoblastic leukemia (CLL), acute myeloid leukemia (AML), and chronic myeloid leukemia (CML). ALL is the most frequent cause of death from cancer before the age of 20 [38] and presents genomic alterations implicated in the proliferation and maturation of lymphoid progenitor cells [39]. CLL is the most common leukemia worldwide and is characterized by the accumulation of B (B-CLL) or T (T-CLL) cells arrested in the early phase of cell division [40], although its ontogeny is unknown [41]. AML is the most common acute leukemia in adults and is defined by the clonal expansion of abnormally differentiated blasts of the myeloid lineage [42]. Finally, CML is a myeloproliferative disorder with a unique genetic rearrangement, the Philadelphia chromosome (BCR-ABL1) which causes the disease [43].
Oxidative stress is a prominent feature in many leukemias [4], and they are characterized by a higher level of ROS than nonleukemic cells. The implication of ROS in the course of leukemias has been scarcely studied [44][32][45][46].
5. Myelodysplastic Syndromes
Myelodysplastic syndromes (MDS) are also a heterogeneous group of onco-hematological cell disorders that are characterized by the presence of immature myeloid precursors (blasts), dysplastic hematopoiesis in the BM, and peripheral cytopenias [47][48][49]. Approximately one-third of MDS cases progress to AML [50][51].
Although its origin is not well understood, the role of oxidative stress in the pathogenesis of the MDS have been investigated in several studies [47][52][53][54]. Approximately 60–80% of patients experience symptomatic anemia and 80–90% require red blood cell transfusion support [55]. For this reason, many patients with MDS develop transfusion-dependent iron overload (IOL) [56]. When present in excess, cellular iron leads to toxicity and cell death via free radical formation and lipid peroxidation [57]. It has been reported that the development of IOL significantly worsens the survival of patients with MDS, and it is associated with a higher risk of leukemic transformation [58]. While the role of ROS in MDS is established, the role played by lipoxidation products in the disease and its progression is unclear [9]. High concentrations of the HNE adduct have significant cytotoxic effects on DNA synthesis and mitochondrial activity in leukemic cells, but not in normal hematopoietic precursor cells [59]. However, a recent study examining protein carbonylation in MDS found no significant differences in HNE adducts in BM samples between the MDS and the control group [60]. Analysis of the lipid peroxidation products MDA and nitrite revealed significantly higher levels in patients with MDS and IOL compared with peers without IOL and the control group, and both parameters positively correlated with the levels of ferritin [61] (Figure 2). The same authors later confirmed the increase in MDA levels and higher levels of antioxidant enzymes [22], suggesting that increases in lipid peroxidation is followed by an increase in antioxidant capacity.
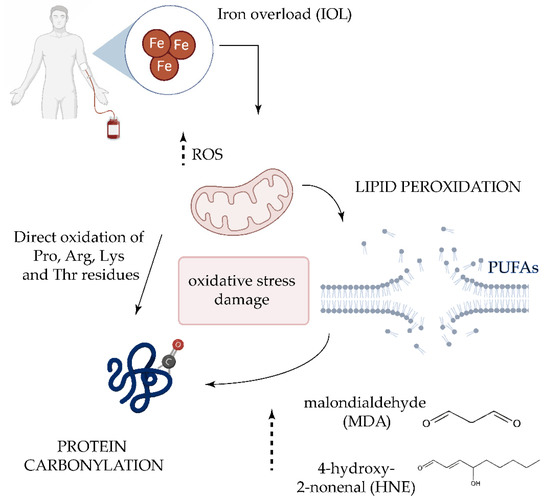
Figure 2. Oxidative damage in transfusion-dependent patients with MDS. Frequent transfusions in patients with MDS leads to iron overload in serum, which plays a key role in the generation of highly reactive oxygen species (ROS) [62]. The increase of ROS could directly oxidize proline (Pro), Arginine (Arg), Lysine (Lys), and Threonine (Thr) residues. ROS-induced lipid peroxidation of long-chain polyunsaturated fatty acids (PUFAs) also promotes protein carbonylation by reaction with lipid peroxidation end-products such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE).
6. BCR/ABL Negative Myeloproliferative Neoplasms
BCR/ABL negative myeloproliferative neoplasms (MPNs) are unique hematopoietic stem-cell disorders that share mutations that constitutively activate the physiologic signal-transduction pathways responsible for hematopoiesis [63]. MPNs are clonal disorders that are mainly characterized by hyperproliferative BM with varying degrees of reticulin/collagen fibrosis, extramedullary hematopoiesis, abnormal peripheral blood count, and constitutional symptoms. They include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) [64].
An imbalanced oxidative status, higher ROS levels, and lower total antioxidant capacity levels compared with controls was found in patients with myelofibrosis by Verner et al. Additionally, the oxidative stress and MDA levels were increased, whereas the total antioxidant status was lower [65]. Following therapy, oxidative stress index and MDA values were significantly lower than the pretreatment values [66]. Higher plasma levels of MDA together with significantly higher protein carbonyls content was also recently reported in patients with MPNs compared with healthy subjects [67].
In patients with PV and ET, Musolino et al. evaluated oxidative stress, finding higher levels of advanced oxidated protein products and S-nitrosylated proteins in both diseases and an increase of AGEs in patients with ET with respect to controls. The authors found a correlation between S-nitrosylated proteins and hemoglobin values in patients with PV, and between AGEs and thrombotic events in patients with ET, suggesting a potential role of ROS in the onset of myeloproliferative-associated thrombotic risk [68].
7. Oxidative Stress Modulators for the Treatment of Hematological Malignancies
As discussed earlier, the misbalance of ROS production in cancer cells can alter cell survival mechanisms. Accordingly, a number of studies have focused on developing new treatment strategies by targeting the redox system in tumor cells. Interestingly, while some of the tested agents exert antioxidant properties, a large number of them are also documented to increase the levels of intracellular ROS in addition to blocking a biochemical target [69][70]. Given the large number of targeted therapies tested, we focused only on those agents that have a modulatory effect on oxidative stress, by affecting either protein carbonylation or lipid peroxidation (Figure 3).
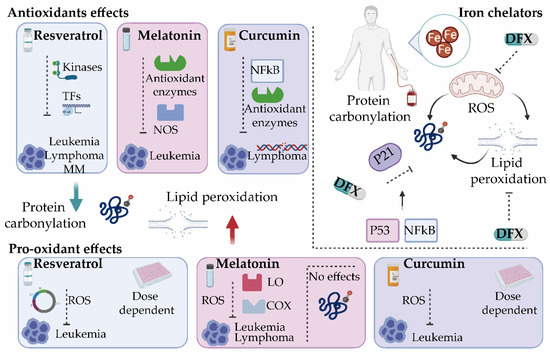
Figure 3. Oxidative stress modulators for the treatment of hematological malignancies. Several antioxidants such as resveratrol, melatonin, and curcumin exert both antioxidant and pro-oxidant activities. By modulating different antioxidant enzymes and transcription factors (TFs), these compounds reduce protein carbonylation and/or lipid peroxidation and inhibit tumor progression. They present cytotoxic effects by enhancing reactive oxygen species (ROS) production. Iron chelators such as deferasirox (DFX) inhibit ROS production directly or indirectly by suppressing the active redox forms of iron and regulating mitochondrial activity and can significantly decrease lipid peroxidation and protein carbonylation in a mechanism dependent on the cell cycle and p21. It will be interesting to explore whether DFX exerts its control on p21 through NF-κB [71] or by inhibiting signaling pathways activated by oxidative stress that control the cell cycle via p53 [72]. COX, cyclooxygenase; LO, lipoxygenase; MM, multiple myeloma; NOS, nitric oxide synthase.
7.1. Potential Antioxidant Drugs
As the total cellular antioxidant capacity is generally compromised in hematologic malignancies, administration of antioxidant drugs might represent a successful way to restore the redox balance. Several antioxidants have been already evaluated as potent anticarcinogenic agents in different kinds of tumors, both in monotherapy or in combination with other antioxidants or classical chemotherapeutics and some of them even revealed promising results in clinical trials. Compared to classical treatment, these compounds possess several important advantages. Beside lower costs they do not exert serious side effects on normal tissues and can be used for chemoprevention [70].
Antioxidants, such as spirulina [73], Enhydra fluctuans extracts [74], and selenium [75][76] can re-establish hematological parameters while simultaneously reducing protein carbonylation and/or lipid peroxidation levels.
In the context of hematological tumors, the antitumor activity of the natural polyphenol resveratrol has been tested in virtually all types of blood cancer cells, including leukemias, lymphomas, and MM [77][78][79][80]. Resveratrol protects phospholipids from oxidation [70], although it is able to inhibit all stage of carcinogenesis (e.g., initiation, promotion, and progression) [81][82][83] by modulating transcription factors, upstream kinases, and their regulators [84]. As resveratrol has no impact on hematopoiesis [85] its potential utility for ex vivo pharmacological purging of leukemia cells from BM autografts before transplantation has been proposed [86][87]. Resveratrol has also been shown to reverse drug resistance in a broad range of in vitro cell systems by sensitizing tumor cells to drug-mediated effects in combination with other chemotherapeutic agents [82]. Nevertheless, a phase 2 clinical trial of resveratrol with or without bortezomib for patients with relapsed and/or refractory MM highlighted side effects and an unacceptable safety profile in combination with bortezomib in these patients [88].
The pituitary hormone melatonin (N-acetyl-5-methoxytryptamine) is of great interest as an endogenous redox modulator with anticancer activity. Melatonin acts directly as a chelator of ROS [89][90][91][92][93], and indirectly by regulating the expression and activities of antioxidant enzymes and nitric oxide synthase [94][95][96][97]. Melatonin has been shown to have a synergistic cytotoxicity effect in combination with lymphoblastic leukemia drugs such as doxorubicin, decreasing ROS and carbonyl formation. Interestingly, this combination is safe for normal lymphocytes, pointing to melatonin as a promising adjuvant for anticancer therapy by allowing lower doses of the anticancer drugs, minimizing their side-effects [98].
In in vitro models of lymphoma, a reduction in lipid peroxidation and/or protein carbonylation products have also been reported using a variety of antioxidants, including 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO), ascorbic acid (vitamin C), α-tocopherol, β-carotene [99], flavonols such as quercetin and rutin [100], canthaxanthin [101] or mangiferin, a C-glycosyl xanthone [102]. Interestingly, extracts of Cocculus hirsutus [103], β-carotene [104], and α-Tocopherol [105] increased survival time in vivo.
In addition, in a murine lymphoma model, ellagic acid inhibits lipid peroxidation and protein carbonylation, decreases PKCα c-Myc expression, and improves TGF-β1 expression in addition to decreasing cell viability, supporting its anticarcinogenic action [106][107].
Another natural product belonging to the group of polyphenols is curcumin or diferuloylmethane, which inhibits free radicals from mediating peroxidation of membrane lipids or oxidative DNA damage [70]. The chemotherapeutic potential of curcumin has been also tested in mice with lymphoma. Results showed that curcumin administration leads to a decrease in lipid peroxidation and protein carbonylation levels, and an increase in the expression and activity of antioxidant enzymes, which in turn modulate the activation of NF-κB, overall reducing lymphoma growth [108].
In conclusion, antioxidants are promising drugs in the management of hematological malignancies. Nevertheless, further studies are necessary in order to confirm their role as anticancer compounds. It should be noted that there are conflicting opinions on the administration of antioxidants during cancer therapy. It is still largely unaccepted by the clinical community as some oncologists believe that it may reduce the effectiveness of chemotherapies, which are mostly based on increasing oxidative stress [36].
7.2. Potential Pro-Oxidant Drugs
In addition to antioxidants, several pro-oxidant drugs capable of modulating cellular ROS contents are currently being tested for their possible use in hematological malignancies. Some of them exert both antioxidant and pro-oxidant properties. Remarkably, the aforementioned antioxidants, melatonin, curcumin, and resveratrol, have also been described as potent pro-oxidants in cancer treatment [70][109][110].
Resveratrol has also dose-dependent pro-oxidant effects, measured as protein carbonylation, which is followed by apoptosis and cell damage [111]. Likewise, Gautam et al. demonstrated that resveratrol induces apoptotic DNA fragmentation in three leukemia cell lines (32Dp210, L1210, HL-60) but not in normal BM cells [87]. The pro-oxidant activity of resveratrol has also been linked to the induction of cell cycle arrest [112]. Resveratrol also suppresses growth of myeloid cells. Lee et al. demonstrated that resveratrol inhibits proliferation of promyelocytic leukemia cells and nonmalignant B-cell lymphoblastoid cells by blocking cell cycle progression in G0/G1, and also induces apoptosis in promyelocytic leukemia cells and acute lymphocytic leukemia cells [113][114]. Interestingly, leukemic lymphoblasts isolated from pediatric patients with ALL undergo apoptosis when treated with resveratrol [70][115].
Melatonin seems to stimulate the production of ROS in human myeloid HL-60 cells, eliciting cytotoxic effects [116]. It also increases the activity of lipoxygenases and cyclooxygenase and promotes the production of ROS in Burkitt lymphoma BL41 cells [117]. Similarly, it enhances cell death in Jurkat leukemia cells via a pro-oxidant pathway [118]. When used on tumoral leukocytes, melatonin produces a rapid and transient stimulation of intracellular ROS, but does not lead to oxidative stress, as revealed by absence of protein carbonylation and the maintenance of free thiols [119][120].
Curcumin also shows pro-oxidant anticarcinogenic mechanisms that are concentration dependent: whereas low concentrations decrease ROS production in human leukemia cells, higher concentrations have the opposite effect and favor ROS generation, measured as increased MDA levels [121]. Curcumin exerts cytotoxic activity on human T-cell leukemia cells without affecting normal cells [122]. Mechanistically, curcumin seems to affect histone acetyltransferase [123] and thioredoxin reductase, converting the latter into a pro-oxidant [124]. Moreover, curcumin induces an increase in GSH levels, responsible for the induction of an apoptotic death pathway in lymphoid Jurkat cells [70][125].
Despite the promising anticancer effects of these natural products, contradictory results responsible for its dual effects are continuously described [126]. Chemically unstable structure, feeble pharmacokinetic, and low bioavailability are reasons for the broad bioactivity profile of these compounds, blocking them from reaching maturity as a drug lead. Moreover, the potential health benefits are still questioned. It is important to highlight there is an urgent need to better characterize the polypharmacology of their degradation products. The lack of chemical standardization of potential drugs like resveratrol and curcumin limits an adequate control of biological assays, leading to unpredictable or potentially irreproducible results [127][128].
A novel redox active mediator that selectively targets tumor cells is motexafin gadolinium, which is a synthetic compound that directly inhibits the activity of Trx and protects against protein carbonylation and lipid peroxidation by inducing apoptosis in malignant cells through oxidative stress. Motexafin gadolinium is being tested clinically for the treatment of lymphoma (NCT00089284, NCT00086034) and leukemia [129][130][131].
Arsenic trioxide is used successfully for the treatment of APL, and both induction and consolidated therapy have resulted in complete remission. Kumar et al. demonstrated that arsenic trioxide induces significant oxidative stress (lipid peroxidation), DNA damage, and caspase 3 activity in HL-60 cells in a dose-dependent manner, and reduces GSH levels [132]. It also activates the intrinsic pathway of apoptosis by modulating the translocation of apoptotic molecules such as Bax and cytochrome c and decreasing the mitochondrial membrane potential.
Finally, targeting copper in cancer cells can also serve as an effective anticancer strategy. Copper is an important metal ion associated with the chromatin DNA. Unlike normal cells, cancer cells have elevated copper levels, which play an integral role in angiogenesis. The interaction between Cu(II) and the phytoestrogen coumestrol in lymphocytes results in lipid peroxidation, protein carbonylation, DNA fragmentation, and apoptosis [133].
These data reinforce the necessity of studying the role of oxidative stress modulating compounds in hematological tumors, characterizing not only their pro/antioxidant effects, but also their molecular mechanism. Although the anticancer potential of some natural products is controversial, some compounds (e.g., motexafin gadolinium) present promising results. Moreover, the clinical efficacy of these agents would be assessed by using biomarkers such as carbonylation and lipid peroxidation.
7.3. Iron Chelators
Iron homeostasis is an effective target in the treatment of different hematological tumors, particularly MDS. As discussed earlier, most patients with MDS develop transfusion dependence and IOL, which has a negative impact increasing oxidative stress parameters [9][53][134][135][136]. In this setting, iron chelation, mainly by Deferasirox (DFX) appears to improve survival in patients with lower-risk MDS and in stem cell transplant settings [135][137]. Moreover, it has been shown to reduce mortality and cytopenia and improve the hematological response [9][138][139][140][141].
Deferasirox is an iron chelator commonly used as a treatment in patients with MDS relying on blood transfusions [142]. DFX is a powerful NF-κB inhibitor in myelodysplastic cells acting independently of cell iron deprivation by chelation, and ROS scavenging [71] and the inhibition of the de novo generation of free radicals through the suppression of the active redox forms of iron [62]. DFX constrains ROS damage in hematopoietic progenitor cells by activating transcription factors and mitochondrial biogenesis [143], the dysfunction of which has been observed in cases of MDS with IOL [144]. Interestingly, a significant decrease in the mean levels of ROS and membrane lipid peroxidation has been reported during DFX therapy [145][146]. In addition, patients under DFX treatment have lower levels of protein carbonylation in BM with respect to untreated patients, which is accompanied by a reduction in the expression of the p53 target gene, p21 [60]. It would be interesting to explore whether DFX exerts its control on p21 through NF-κB [71] or, alternatively, by inhibiting signaling pathways activated by oxidative stress that control the cell cycle via p53 [60][72].
Despite the paucity of studies investigating the beneficial effects of iron chelation on protein carbonylation in other hematological diseases, its effect on ROS levels, and thus, on disease control seem robust.
Iron chelating therapy in myeloid leukemias induces the differentiation of leukemia blasts and normal BM precursors into monocytes/macrophages, in a manner involving the modulation of ROS expression [147]. Moreover, the cytotoxic effects of iron chelating therapy on myeloid blasts has been reported in vitro, in vivo, and ex vivo [148][149][150][151][152][153], presenting a synergistic effect with AML drugs such as decitabine and 5-azacytidine [154][155]. In contrast to the effects of decitabine, DFX decreases the ROS levels to varying degrees [154]. Human studies have demonstrated a protective role of DFX after allogenic-hematopoietic stem cell transplantation in AML [156][157] and in a patient with chemotherapy-resistant AML [158].
DFX has also been reported to inhibit mantle cell lymphoma cell proliferation [159][160], and iron deprivation is cytotoxic to malignant B- and T-cells [161][162]. Moreover, in ALL and T-cell lymphoma, DFX displayed synergistic activity with three ALL-specific drugs: dexamethasone, doxorubicin, and L-asparaginase. Iron chelation appears to act through a ROS-dependent DNA damage response and potentiates the action of an inhibitor of the PARP pathway of DNA repair [163].
Finally, in the context of MM, chelation of intracellular iron induces cell death in myeloma cells [164]. Deferasirox also induces apoptosis in MM cells by targeting oncogenic Pyk2/β-catenin signaling [165]. Conversely, iron loading impairs cell proliferation in MM and increases the efficacy of bortezomib, as iron causes lipid oxidation and inhibits proteasome function [166].
This entry is adapted from the peer-reviewed paper 10.3390/antiox9121212
References
- Singh, R.K.; Tripathi, A.K.; Tripathi, P.; Singh, S.; Singh, R.; Ahmad, R. Studies on biomarkers for oxidative stress in patients with chronic myeloid leukemia. Hematol Oncol Stem Cell Ther 2009, 2, 285–288, doi:10.1016/s1658-3876(09)50039-8.
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol 2017, 11, 613–619, doi:10.1016/j.redox.2016.12.035.
- Battisti, V.; Maders, L.D.K.; Bagatini, M.D.; Santos, K.F.; Spanevello, R.M.; Maldonado, P.A.; Brulé, A.O.; Araújo, M. do C.; Schetinger, M.R.C.; Morsch, V.M. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin. Biochem. 2008, 41, 511–518, doi:10.1016/j.clinbiochem.2008.01.027.
- Ahmad, R.; Tripathi, A.K.; Tripathi, P.; Singh, S.; Singh, R.; Singh, R.K. Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. In Vivo 2008, 22, 525–528.
- Ahmad, R.; Tripathi, A.K.; Tripathi, P.; Singh, R.; Singh, S.; Singh, R.K. Studies on lipid peroxidation and non-enzymatic antioxidant status as indices of oxidative stress in patients with chronic myeloid leukaemia. Singapore Med J 2010, 51, 110–115.
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of Amyloid β-Peptide-Associated Oxidative Stress and Brain Protein Modifications in the Pathogenesis of Alzheimer’s Disease and Mild Cognitive Impairment. Free Radic Biol Med 2007, 43, 658–677, doi:10.1016/j.freeradbiomed.2007.05.037.
- Cecarini, V.; Gee, J.; Fioretti, E.; Amici, M.; Angeletti, M.; Eleuteri, A.M.; Keller, J.N. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim. Biophys. Acta 2007, 1773, 93–104, doi:10.1016/j.bbamcr.2006.08.039.
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol Med 2003, 9, 169–176, doi:10.1016/s1471-4914(03)00031-5.
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants (Basel) 2018, 7, doi:10.3390/antiox7080102.
- Linares, M.; Marín-Garcíía, P.; Méndez, D.; Puyet, A.; Diez, A.; Bautista, J.M. Proteomic approaches to identifying carbonylated proteins in brain tissue. J. Proteome Res. 2011, 10, 1719–1727, doi:10.1021/pr101014e.
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Meth. Enzymol. 1994, 233, 346–357, doi:10.1016/s0076-6879(94)33040-9.
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spec Rev 2014, 33, 79–97, doi:10.1002/mas.21381.
- Rudzińska, M.; Parodi, A.; Balakireva, A.V.; Chepikova, O.E.; Venanzi, F.M.; Zamyatnin, A.A. Cellular Aging Characteristics and Their Association with Age-Related Disorders. Antioxidants (Basel) 2020, 9, doi:10.3390/antiox9020094.
- Morabito, F.; Cristani, M.; Saija, A.; Stelitano, C.; Callea, V.; Tomaino, A.; Minciullo, P.L.; Gangemi, S. Lipid peroxidation and protein oxidation in patients affected by Hodgkin’s lymphoma. Mediators Inflamm. 2004, 13, 381–383, doi:10.1080/09629350400008760.
- Miranda, C.L.; Reed, R.L.; Kuiper, H.C.; Alber, S.; Stevens, J.F. Ascorbic acid promotes detoxification and elimination of 4-hydroxy-2(E)-nonenal in human monocytic THP-1 cells. Chem. Res. Toxicol. 2009, 22, 863–874, doi:10.1021/tx900042u.
- Previati, M.; Lanzoni, I.; Corbacella, E.; Magosso, S.; Guaran, V.; Martini, A.; Capitani, S. Cisplatin-induced apoptosis in human promyelocytic leukemia cells. Int. J. Mol. Med. 2006, 18, 511–516.
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20, doi:10.1038/sj.bjp.0707395.
- Adams, S.; Green, P.; Claxton, R.; Simcox, S.; Williams, M.V.; Walsh, K.; Leeuwenburgh, C. Reactive carbonyl formation by oxidative and non-oxidative pathways. Front. Biosci. 2001, 6, A17-24, doi:10.2741/adams.
- Gangemi, S.; Allegra, A.; Aguennouz, M.; Alonci, A.; Speciale, A.; Cannavò, A.; Cristani, M.; Russo, S.; Spatari, G.; Alibrandi, A.; et al. Relationship between advanced oxidation protein products, advanced glycation end products, and S-nitrosylated proteins with biological risk and MDR-1 polymorphisms in patients affected by B-chronic lymphocytic leukemia. Cancer Invest. 2012, 30, 20–26, doi:10.3109/07357907.2011.629383.
- Sayre, L.M.; Lin, D.; Yuan, Q.; Zhu, X.; Tang, X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab. Rev. 2006, 38, 651–675, doi:10.1080/03602530600959508.
- Lee, S.H.; Oe, T.; Blair, I.A. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science 2001, 292, 2083–2086, doi:10.1126/science.1059501.
- De Souza, G.F.; Ribeiro, H.L.; De Sousa, J.C.; Heredia, F.F.; De Freitas, R.M.; Martins, M.R.A.; Gonçalves, R.P.; Pinheiro, R.F.; Magalhães, S.M.M. HFE gene mutation and oxidative damage biomarkers in patients with myelodysplastic syndromes and its relation to transfusional iron overload: an observational cross-sectional study. BMJ Open 2015, 5, doi:10.1136/bmjopen-2014-006048.
- Sun, R.; Medeiros, L.J.; Young, K.H. Diagnostic and predictive biomarkers for lymphoma diagnosis and treatment in the era of precision medicine. Mod. Pathol. 2016, 29, 1118–1142, doi:10.1038/modpathol.2016.92.
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-Hodgkin lymphoma. Lancet 2012, 380, 848–857, doi:10.1016/S0140-6736(12)60605-9.
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin 2018, 68, 116–132, doi:10.3322/caac.21438.
- Intlekofer, A.M.; Younes, A. Precision therapy for lymphoma--current state and future directions. Nat Rev Clin Oncol 2014, 11, 585–596, doi:10.1038/nrclinonc.2014.137.
- Bur, H.; Haapasaari, K.-M.; Turpeenniemi-Hujanen, T.; Kuittinen, O.; Auvinen, P.; Marin, K.; Koivunen, P.; Sormunen, R.; Soini, Y.; Karihtala, P. Oxidative stress markers and mitochondrial antioxidant enzyme expression are increased in aggressive Hodgkin lymphomas. Histopathology 2014, 65, 319–327, doi:10.1111/his.12389.
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers (Basel) 2019, 11, doi:10.3390/cancers11081191.
- Irigoyen, M.; García-Ruiz, J.C.; Berra, E. The hypoxia signalling pathway in haematological malignancies. Oncotarget 2017, 8, 36832–36844, doi:10.18632/oncotarget.15981.
- Matolay, O.; Méhes, G. Sustain, Adapt, and Overcome-Hypoxia Associated Changes in the Progression of Lymphatic Neoplasia. Front Oncol 2019, 9, 1277, doi:10.3389/fonc.2019.01277.
- Bhalla, K.; Jaber, S.; Nahid M, N.; Underwood, K.; Beheshti, A.; Landon, A.; Bhandary, B.; Bastian, P.; Evens, A.M.; Haley, J.; et al. Role of hypoxia in Diffuse Large B-cell Lymphoma: Metabolic repression and selective translation of HK2 facilitates development of DLBCL. Sci Rep 2018, 8, 744, doi:10.1038/s41598-018-19182-8.
- Al-Gayyar, M.M.H.; Eissa, L.A.; Rabie, A.M.; El-Gayar, A.M. Measurements of oxidative stress status and antioxidant activity in chronic leukaemia patients. J. Pharm. Pharmacol. 2007, 59, 409–417, doi:10.1211/jpp.59.3.0011.
- Camiolo, G.; Barbato, A.; Giallongo, C.; Vicario, N.; Romano, A.; Parrinello, N.L.; Parenti, R.; Sandoval, J.C.; García-Moreno, D.; Lazzarino, G.; et al. Iron regulates myeloma cell/macrophage interaction and drives resistance to bortezomib. Redox Biol 2020, 36, doi:10.1016/j.redox.2020.101611.
- Zima, T.; Spicka, I.; Stípek, S.; Crkovská, J.; Pláteník, J.; Merta, M.; Tesar, V. Antioxidant enzymes and lipid peroxidation in patients with multiple myeloma. Neoplasma 1996, 43, 69–73.
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G.; et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067, doi:10.1007/s00011-012-0498-7.
- Imbesi, S.; Musolino, C.; Allegra, A.; Saija, A.; Morabito, F.; Calapai, G.; Gangemi, S. Oxidative stress in oncohematologic diseases: an update. Expert Rev Hematol 2013, 6, 317–325, doi:10.1586/ehm.13.21.
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Tonissen, K.F. TrxR1 inhibition overcomes both hypoxia-induced and acquired bortezomib resistance in multiple myeloma through NF-кβ inhibition. Cell Cycle 2016, 15, 559–572, doi:10.1080/15384101.2015.1136038.
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552, doi:10.1056/NEJMra1400972.
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162, doi:10.1016/S0140-6736(19)33018-1.
- Jabłońska, E.; Kiersnowska-Rogowska, B.; Ratajczak, W.; Rogowski, F.; Sawicka-Powierza, J. Reactive oxygen and nitrogen species in the course of B-CLL. Adv Med Sci 2007, 52, 154–158.
- Milne, K.; Sturrock, B.; Chevassut, T. Chronic Lymphocytic Leukaemia in 2020: the Future Has Arrived. Curr Oncol Rep 2020, 22, 36, doi:10.1007/s11912-020-0893-0.
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute myeloid leukaemia. Lancet 2018, 392, 593–606, doi:10.1016/S0140-6736(18)31041-9.
- Gale, R.P.; Apperley, J. What Does Chronic Myeloid Leukaemia Tell Us About Other Leukaemias? Curr Hematol Malig Rep 2019, 14, 477–479, doi:10.1007/s11899-019-00555-3.
- Devi, G.S.; Prasad, M.H.; Saraswathi, I.; Raghu, D.; Rao, D.N.; Reddy, P.P. Free radicals antioxidant enzymes and lipid peroxidation in different types of leukemias. Clin. Chim. Acta 2000, 293, 53–62, doi:10.1016/s0009-8981(99)00222-3.
- Er, T.-K.; Tsai, S.-M.; Wu, S.-H.; Chiang, W.; Lin, H.-C.; Lin, S.-F.; Wu, S.-H.; Tsai, L.-Y.; Liu, T.-Z. Antioxidant status and superoxide anion radical generation in acute myeloid leukemia. Clin. Biochem. 2007, 40, 1015–1019, doi:10.1016/j.clinbiochem.2007.05.013.
- Antoszewska-Smith, J.; Pawlowska, E.; Blasiak, J. Reactive oxygen species in BCR-ABL1-expressing cells - relevance to chronic myeloid leukemia. Acta Biochim. Pol. 2017, 64, 1–10, doi:10.18388/abp.2016_1396.
- Richardson, C.; Yan, S.; Vestal, C. Oxidative Stress, Bone Marrow Failure, and Genome Instability in Hematopoietic Stem Cells. International Journal of Molecular Sciences 2015, 16, 2366–2385, doi:10.3390/ijms16022366.
- Nimer, S.D. Myelodysplastic syndromes. Blood 2008, 111, 4841–4851, doi:10.1182/blood-2007-08-078139.
- Cazzola, M.; Malcovati, L. Myelodysplastic syndromes--coping with ineffective hematopoiesis. N. Engl. J. Med. 2005, 352, 536–538, doi:10.1056/NEJMp048266.
- Chung, Y.J.; Robert, C.; Gough, S.M.; Rassool, F.V.; Aplan, P.D. Oxidative stress leads to increased mutation frequency in a murine model of myelodysplastic syndrome. Leukemia Research 2014, 38, 95–102, doi:10.1016/j.leukres.2013.07.008.
- Tefferi, A.; Guglielmelli, P.; Larson, D.R.; Finke, C.; Wassie, E.A.; Pieri, L.; Gangat, N.; Fjerza, R.; Belachew, A.A.; Lasho, T.L.; et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014, 124, 2507–2513, doi:10.1182/blood-2014-05-579136.
- Farquhar, M.J.; Bowen, D.T. Oxidative stress and the myelodysplastic syndromes. Int. J. Hematol. 2003, 77, 342–350, doi:10.1007/BF02982641.
- Ghoti, H.; Amer, J.; Winder, A.; Rachmilewitz, E.; Fibach, E. Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur. J. Haematol. 2007, 79, 463–467, doi:10.1111/j.1600-0609.2007.00972.x.
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Pereira, S.; Pereira, A.; Costa, J.M.N.; et al. Oxidative stress levels are correlated with P15 and P16 gene promoter methylation in myelodysplastic syndrome patients. Clinical and Experimental Medicine 2016, 16, 333–343, doi:10.1007/s10238-015-0357-2.
- Shenoy, N.; Vallumsetla, N.; Rachmilewitz, E.; Verma, A.; Ginzburg, Y. Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome. Blood 2014, 124, 873–881, doi:10.1182/blood-2014-03-563221.
- Chai, X.; Li, D.; Cao, X.; Zhang, Y.; Mu, J.; Lu, W.; Xiao, X.; Li, C.; Meng, J.; Chen, J.; et al. ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Scientific Reports 2015, 5, doi:10.1038/srep10181.
- Britton, R.S.; Ramm, G.A.; Olynyk, J.; Singh, R.; O’Neill, R.; Bacon, B.R. Pathophysiology of Iron Toxicity. In Progress in Iron Research; Hershko, C., Konijn, A.M., Aisen, P., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, 1994; pp. 239–253 ISBN 978-1-4615-2554-7.
- Malcovati, L.; Porta, M.G.D.; Pascutto, C.; Invernizzi, R.; Boni, M.; Travaglino, E.; Passamonti, F.; Arcaini, L.; Maffioli, M.; Bernasconi, P.; et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J. Clin. Oncol. 2005, 23, 7594–7603, doi:10.1200/JCO.2005.01.7038.
- Semlitsch, T.; Tillian, H.M.; Neven, Z.; Martin, P. Differential influence of the lipid peroxidation product 4-hydroxynonenal on the growth of human lymphatic leukaemia cells and human periopherial blood lymphocytes. Anticancer Res. 2002, 22(3), 1689–97.
- Rodríguez-García, A.; Morales, M.L.; Garrido-García, V.; García-Baquero, I.; Leivas, A.; Carreño-Tarragona, G.; Sánchez, R.; Arenas, A.; Cedena, T.; Ayala, R.M.; et al. Protein Carbonylation in Patients with Myelodysplastic Syndrome: An Opportunity for Deferasirox Therapy. Antioxidants (Basel) 2019, 8, doi:10.3390/antiox8110508.
- De Souza, G.F.; Barbosa, M.C.; Santos, T.E. de J.; Carvalho, T.M. de J.P.; de Freitas, R.M.; Martins, M.R.A.; Gonçalves, R.P.; Pinheiro, R.F.; Magalhães, S.M.M. Increased parameters of oxidative stress and its relation to transfusion iron overload in patients with myelodysplastic syndromes. J. Clin. Pathol. 2013, 66, 996–998, doi:10.1136/jclinpath-2012-201288.
- Gattermann, N.; Rachmilewitz, E.A. Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann. Hematol. 2011, 90, 1–10, doi:10.1007/s00277-010-1091-1.
- Spivak, J.L. Myeloproliferative Neoplasms. N. Engl. J. Med. 2017, 376, 2168–2181, doi:10.1056/NEJMra1406186.
- Iurlo, A.; Cattaneo, D.; Gianelli, U. Blast Transformation in Myeloproliferative Neoplasms: Risk Factors, Biological Findings, and Targeted Therapeutic Options. Int J Mol Sci 2019, 20, doi:10.3390/ijms20081839.
- Vener, C.; Novembrino, C.; Catena, F.B.; Fracchiolla, N.S.; Gianelli, U.; Savi, F.; Radaelli, F.; Fermo, E.; Cortelezzi, A.; Lonati, S.; et al. Oxidative stress is increased in primary and post-polycythemia vera myelofibrosis. Exp. Hematol. 2010, 38, 1058–1065, doi:10.1016/j.exphem.2010.07.005.
- Durmus, A.; Mentese, A.; Yilmaz, M.; Sumer, A.; Akalin, I.; Topal, C.; Alver, A. Increased oxidative stress in patients with essential thrombocythemia. Eur Rev Med Pharmacol Sci 2013, 17, 2860–2866.
- Djikic, D.; Markovic, D.; Bogdanovic, A.; Mitrovic-Ajtic, O.; Suboticki, T.; Diklic, M.; Beleslin-Cokic, B.; Bjelica, S.; Kovacic, M.; P Cokic, V. Oxidative and nitrosative stress in myeloproliferative neoplasms: the impact on the AKT / mTOR signaling pathway. J BUON 2018, 23, 1481–1491.
- Musolino, C.; Allegra, A.; Saija, A.; Alonci, A.; Russo, S.; Spatari, G.; Penna, G.; Gerace, D.; Cristani, M.; David, A.; et al. Changes in advanced oxidation protein products, advanced glycation end products, and s-nitrosylated proteins, in patients affected by polycythemia vera and essential thrombocythemia. Clin. Biochem. 2012, 45, 1439–1443, doi:10.1016/j.clinbiochem.2012.07.100.
- Chandra, J. Oxidative Stress by Targeted Agents Promotes Cytotoxicity in Hematologic Malignancies. Antioxidants & Redox Signaling 2009, 11, 1123–1137, doi:10.1089/ars.2008.2302.
- Kelkel, M.; Jacob, C.; Dicato, M.; Diederich, M. Potential of the Dietary Antioxidants Resveratrol and Curcumin in Prevention and Treatment of Hematologic Malignancies. Molecules 2010, 15, 7035–7074, doi:10.3390/molecules15107035.
- Messa, E.; Carturan, S.; Maffè, C.; Pautasso, M.; Bracco, E.; Roetto, A.; Messa, F.; Arruga, F.; Defilippi, I.; Rosso, V.; et al. Deferasirox is a powerful NF-kappaB inhibitor in myelodysplastic cells and in leukemia cell lines acting independently from cell iron deprivation by chelation and reactive oxygen species scavenging. Haematologica 2010, 95, 1308–1316, doi:10.3324/haematol.2009.016824.
- Hole, P.S.; Darley, R.L.; Tonks, A. Do reactive oxygen species play a role in myeloid leukemias? Blood 2011, 117, 5816–5826, doi:10.1182/blood-2011-01-326025.
- Gargouri, M.; Soussi, A.; Akrouti, A.; Magné, C.; El Feki, A. Potential protective effects of the edible alga Arthrospira platensis against lead-induced oxidative stress, anemia, kidney injury, and histopathological changes in adult rats. Appl Physiol Nutr Metab 2019, 44, 271–281, doi:10.1139/apnm-2018-0428.
- Dua, T.K.; Dewanjee, S.; Khanra, R. Prophylactic role of Enhydra fluctuans against arsenic-induced hepatotoxicity via anti-apoptotic and antioxidant mechanisms. Redox Rep. 2016, 21, 147–154, doi:10.1179/1351000215Y.0000000021.
- Acker, C.I.; Souza, A.C.G.; Dos Santos, M.P.; Mazzanti, C.M.; Nogueira, C.W. Diphenyl diselenide attenuates hepatic and hematologic toxicity induced by chlorpyrifos acute exposure in rats. Environ Sci Pollut Res Int 2012, 19, 3481–3490, doi:10.1007/s11356-012-0882-4.
- Soudani, N.; Ben Amara, I.; Troudi, A.; Hakim, A.; Bouaziz, H.; Ayadi Makni, F.; Zeghal, K.M.; Zeghal, N. Oxidative damage induced by chromium (VI) in rat erythrocytes: protective effect of selenium. J. Physiol. Biochem. 2011, 67, 577–588, doi:10.1007/s13105-011-0104-4.
- Espinoza, J.L.; Kurokawa, Y.; Takami, A. Rationale for assessing the therapeutic potential of resveratrol in hematological malignancies. Blood Rev. 2019, 33, 43–52, doi:10.1016/j.blre.2018.07.001.
- Quoc Trung, L.; Espinoza, J.L.; Takami, A.; Nakao, S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS ONE 2013, 8, e55183, doi:10.1371/journal.pone.0055183.
- Roccaro, A.M.; Leleu, X.; Sacco, A.; Moreau, A.-S.; Hatjiharissi, E.; Jia, X.; Xu, L.; Ciccarelli, B.; Patterson, C.J.; Ngo, H.T.; et al. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenström’s macroglobulinemia. Clin. Cancer Res. 2008, 14, 1849–1858, doi:10.1158/1078-0432.CCR-07-1750.
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, doi:10.3390/biomedicines6030091.
- Zykova, T.A.; Zhu, F.; Zhai, X.; Ma, W.-Y.; Ermakova, S.P.; Lee, K.W.; Bode, A.M.; Dong, Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008, 47, 797–805, doi:10.1002/mc.20437.
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front Nutr 2016, 3, 8, doi:10.3389/fnut.2016.00008.
- Pezzuto, J.M. Resveratrol as an Inhibitor of Carcinogenesis. Pharmaceutical Biology 2008, 46, 443–573, doi:10.1080/13880200802116610.
- Kundu, J.K.; Surh, Y.-J. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008, 269, 243–261, doi:10.1016/j.canlet.2008.03.057.
- Rimmelé, P.; Lofek-Czubek, S.; Ghaffari, S. Resveratrol increases the bone marrow hematopoietic stem and progenitor cell capacity. Am. J. Hematol. 2014, 89, E235-238, doi:10.1002/ajh.23837.
- Ferry-Dumazet, H.; Garnier, O.; Mamani-Matsuda, M.; Vercauteren, J.; Belloc, F.; Billiard, C.; Dupouy, M.; Thiolat, D.; Kolb, J.P.; Marit, G.; et al. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis 2002, 23, 1327–1333, doi:10.1093/carcin/23.8.1327.
- Gautam, S.C.; Xu, Y.X.; Dumaguin, M.; Janakiraman, N.; Chapman, R.A. Resveratrol selectively inhibits leukemia cells: a prospective agent for ex vivo bone marrow purging. Bone Marrow Transplant. 2000, 25, 639–645, doi:10.1038/sj.bmt.1702189.
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717, doi:10.1111/bjh.12154.
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Brown, G.M.; Cardinali, D.P. Melatonin in Mitochondrial Dysfunction and Related Disorders. Int J Alzheimers Dis 2011, 2011, doi:10.4061/2011/326320.
- Martín, M.; Macías, M.; Escames, G.; León, J.; Acuña-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000, 14, 1677–1679, doi:10.1096/fj.99-0865fje.
- Rodriguez, M.I.; Escames, G.; López, L.C.; García, J.A.; Ortiz, F.; López, A.; Acuña-Castroviejo, D. Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J. Endocrinol. 2007, 194, 637–643, doi:10.1677/JOE-07-0260.
- Tapias, V.; Escames, G.; López, L.C.; López, A.; Camacho, E.; Carrión, M.D.; Entrena, A.; Gallo, M.A.; Espinosa, A.; Acuña-Castroviejo, D. Melatonin and its brain metabolite N(1)-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J. Neurosci. Res. 2009, 87, 3002–3010, doi:10.1002/jnr.22123.
- Poeggeler, B.; Thuermann, S.; Dose, A.; Schoenke, M.; Burkhardt, S.; Hardeland, R. Melatonin’s unique radical scavenging properties - roles of its functional substituents as revealed by a comparison with its structural analogs. J. Pineal Res. 2002, 33, 20–30, doi:10.1034/j.1600-079x.2002.01873.x.
- León, J.; Acuña-Castroviejo, D.; Escames, G.; Tan, D.-X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38, 1–9, doi:10.1111/j.1600-079X.2004.00181.x.
- Antolín, I.; Rodríguez, C.; Saínz, R.M.; Mayo, J.C.; Uría, H.; Kotler, M.L.; Rodríguez-Colunga, M.J.; Tolivia, D.; Menéndez-Peláez, A. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 1996, 10, 882–890, doi:10.1096/fasebj.10.8.8666165.
- Escames, G.; León, J.; Macías, M.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003, 17, 932–934, doi:10.1096/fj.02-0692fje.
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004, 36, 1–9, doi:10.1046/j.1600-079x.2003.00092.x.
- Zhelev, Z.; Ivanova, D.; Bakalova, R.; Aoki, I.; Higashi, T. Synergistic Cytotoxicity of Melatonin and New-generation Anticancer Drugs Against Leukemia Lymphocytes But Not Normal Lymphocytes. Anticancer Res. 2017, 37, 149–159, doi:10.21873/anticanres.11300.
- Strasser, A.; Carra, M.; Ghareeb, K.; Awad, W.; Böhm, J. Protective effects of antioxidants on deoxynivalenol-induced damage in murine lymphoma cells. Mycotoxin Res 2013, 29, 203–208, doi:10.1007/s12550-013-0170-2.
- Ramanathan, R.; Das, N.P.; Tan, C.H. Effects of gamma-linolenic acid, flavonoids, and vitamins on cytotoxicity and lipid peroxidation. Free Radic. Biol. Med. 1994, 16, 43–48, doi:10.1016/0891-5849(94)90241-0.
- Palozza, P.; Luberto, C.; Ricci, P.; Sgarlata, E.; Calviello, G.; Bartoli, G.M. Effect of beta-carotene and canthaxanthin on tert-butyl hydroperoxide-induced lipid peroxidation in murine normal and tumor thymocytes. Arch. Biochem. Biophys. 1996, 325, 145–151, doi:10.1006/abbi.1996.0018.
- Sahoo, B.K.; Zaidi, A.H.; Gupta, P.; Mokhamatam, R.B.; Raviprakash, N.; Mahali, S.K.; Manna, S.K. A natural xanthone increases catalase activity but decreases NF-kappa B and lipid peroxidation in U-937 and HepG2 cell lines. Eur. J. Pharmacol. 2015, 764, 520–528, doi:10.1016/j.ejphar.2015.07.046.
- Thavamani, B.S.; Mathew, M.; Palaniswamy, D.S. Anticancer activity of Cocculus hirsutus against Dalton’s lymphoma ascites (DLA) cells in mice. Pharm Biol 2014, 52, 867–872, doi:10.3109/13880209.2013.871642.
- Basu, M.; Banerjee, A.; Bhattacharya, U.K.; Bishayee, A.; Chatterjee, M. Beta-carotene prolongs survival, decreases lipid peroxidation and enhances glutathione status in transplantable murine lymphoma. Phytomedicine 2000, 7, 151–159, doi:10.1016/S0944-7113(00)80088-4.
- Sharma, R.; Vinayak, M. α-Tocopherol prevents lymphoma by improving antioxidant defence system of mice. Mol. Biol. Rep. 2013, 40, 839–849, doi:10.1007/s11033-012-2123-9.
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206, doi:10.1021/jf011275g.
- Mishra, S.; Vinayak, M. Anti-carcinogenic action of ellagic acid mediated via modulation of oxidative stress regulated genes in Dalton lymphoma bearing mice. Leuk. Lymphoma 2011, 52, 2155–2161, doi:10.3109/10428194.2011.591014.
- Das, L.; Vinayak, M. Anti-carcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-κB signalling in lymphoma-bearing mice. Biosci. Rep. 2012, 32, 161–170, doi:10.1042/BSR20110043.
- Bizzarri, M.; Proietti, S.; Cucina, A.; Reiter, R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opinion on Therapeutic Targets 2013, 17, 1483–1496, doi:10.1517/14728222.2013.834890.
- Zhang, H.-M.; Zhang, Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146, doi:10.1111/jpi.12162.
- Pasciu, V.; Posadino, A.M.; Cossu, A.; Sanna, B.; Tadolini, B.; Gaspa, L.; Marchisio, A.; Dessole, S.; Capobianco, G.; Pintus, G. Akt downregulation by flavin oxidase-induced ROS generation mediates dose-dependent endothelial cell damage elicited by natural antioxidants. Toxicol. Sci. 2010, 114, 101–112, doi:10.1093/toxsci/kfp301.
- Heiss, E.H.; Schilder, Y.D.C.; Dirsch, V.M. Chronic treatment with resveratrol induces redox stress- and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. J. Biol. Chem. 2007, 282, 26759–26766, doi:10.1074/jbc.M703229200.
- Lee, S.K.; Zhang, W.; Sanderson, B.J.S. Selective growth inhibition of human leukemia and human lymphoblastoid cells by resveratrol via cell cycle arrest and apoptosis induction. J. Agric. Food Chem. 2008, 56, 7572–7577, doi:10.1021/jf801014p.
- Dörrie, J.; Gerauer, H.; Wachter, Y.; Zunino, S.J. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001, 61, 4731–4739.
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840.
- Bejarano, I.; Espino, J.; Marchena, A.M.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B.; Pariente, J.A. Melatonin enhances hydrogen peroxide-induced apoptosis in human promyelocytic leukaemia HL-60 cells. Mol. Cell. Biochem. 2011, 353, 167–176, doi:10.1007/s11010-011-0783-8.
- Paternoster, L.; Radogna, F.; Accorsi, A.; Cristina Albertini, M.; Gualandi, G.; Ghibelli, L. Melatonin as a modulator of apoptosis in B-lymphoma cells. Ann. N. Y. Acad. Sci. 2009, 1171, 345–349, doi:10.1111/j.1749-6632.2009.04910.x.
- Jang, S.S.; Kim, W.D.; Park, W.-Y. Melatonin exerts differential actions on X-ray radiation-induced apoptosis in normal mice splenocytes and Jurkat leukemia cells. J. Pineal Res. 2009, 47, 147–155, doi:10.1111/j.1600-079X.2009.00694.x.
- Li, T.; Yang, Z.; Jiang, S.; Di, W.; Ma, Z.; Hu, W.; Chen, F.; Reiter, R.J.; Yang, Y. Melatonin: does it have utility in the treatment of haematological neoplasms? Br J Pharmacol 2018, 175, 3251–3262, doi:10.1111/bph.13966.
- Radogna, F.; Paternoster, L.; De Nicola, M.; Cerella, C.; Ammendola, S.; Bedini, A.; Tarzia, G.; Aquilano, K.; Ciriolo, M.; Ghibelli, L. Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol. Appl. Pharmacol. 2009, 239, 37–45, doi:10.1016/j.taap.2009.05.012.
- Chen, J.; Kang, J.; Da, W.; Ou, Y. Combination with water-soluble antioxidants increases the anticancer activity of quercetin in human leukemia cells. Pharmazie 2004, 59, 859–863.
- Kong, Y.; Ma, W.; Liu, X.; Zu, Y.; Fu, Y.; Wu, N.; Liang, L.; Yao, L.; Efferth, T. Cytotoxic Activity of Curcumin towards CCRF-CEM Leukemia Cells and Its Effect on DNA Damage. Molecules 2009, 14, 5328–5338, doi:10.3390/molecules14125328.
- Kang, J.; Chen, J.; Shi, Y.; Jia, J.; Zhang, Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem. Pharmacol. 2005, 69, 1205–1213, doi:10.1016/j.bcp.2005.01.014.
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005, 280, 25284–25290, doi:10.1074/jbc.M414645200.
- Piwocka, K.; Jaruga, E.; Skierski, J.; Gradzka, I.; Sikora, E. Effect of glutathione depletion on caspase-3 independent apoptosis pathway induced by curcumin in Jurkat cells. Free Radic. Biol. Med. 2001, 31, 670–678, doi:10.1016/s0891-5849(01)00629-3.
- Talib, W. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518, doi:10.3390/molecules23030518.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637, doi:10.1021/acs.jmedchem.6b00975.
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027, doi:10.1021/cr500689b.
- Pérez, V.I.; Cortez, L.A.; Lew, C.M.; Rodriguez, M.; Webb, C.R.; Van Remmen, H.; Chaudhuri, A.; Qi, W.; Lee, S.; Bokov, A.; et al. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1286–1299, doi:10.1093/gerona/glr125.
- Biaglow, J.E.; Miller, R.A. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol. Ther. 2005, 4, 6–13, doi:10.4161/cbt.4.1.1434.
- Sardina, J.L.; López-Ruano, G.; Sánchez-Sánchez, B.; Llanillo, M.; Hernández-Hernández, A. Reactive oxygen species: are they important for haematopoiesis? Crit. Rev. Oncol. Hematol. 2012, 81, 257–274, doi:10.1016/j.critrevonc.2011.03.005.
- Kumar, S.; Yedjou, C.G.; Tchounwou, P.B. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J. Exp. Clin. Cancer Res. 2014, 33, 42, doi:10.1186/1756-9966-33-42.
- Zafar, A.; Singh, S.; Naseem, I. Cu(II)-coumestrol interaction leads to ROS-mediated DNA damage and cell death: a putative mechanism for anticancer activity. J. Nutr. Biochem. 2016, 33, 15–27, doi:10.1016/j.jnutbio.2016.03.003.
- Smith, C.; Gasparetto, M.; Jordan, C.; Pollyea, D.A.; Vasiliou, V. The effects of alcohol and aldehyde dehydrogenases on disorders of hematopoiesis. Adv. Exp. Med. Biol. 2015, 815, 349–359, doi:10.1007/978-3-319-09614-8_20.
- Rose, C.; Brechignac, S.; Vassilief, D.; Pascal, L.; Stamatoullas, A.; Guerci, A.; Larbaa, D.; Dreyfus, F.; Beyne-Rauzy, O.; Chaury, M.P.; et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myélodysplasies). Leuk. Res. 2010, 34, 864–870, doi:10.1016/j.leukres.2009.12.004.
- Rassool, F.V.; Gaymes, T.J.; Omidvar, N.; Brady, N.; Beurlet, S.; Pla, M.; Reboul, M.; Lea, N.; Chomienne, C.; Thomas, N.S.B.; et al. Reactive oxygen species, DNA damage, and error-prone repair: a model for genomic instability with progression in myeloid leukemia? Cancer Res. 2007, 67, 8762–8771, doi:10.1158/0008-5472.CAN-06-4807.
- Merkel, D.G.; Nagler, A. Toward resolving the unsettled role of iron chelation therapy in myelodysplastic syndromes. Expert Rev Anticancer Ther 2014, 14, 817–829, doi:10.1586/14737140.2014.896208.
- Malcovati, L.; Hellström-Lindberg, E.; Bowen, D.; Adès, L.; Cermak, J.; Del Cañizo, C.; Della Porta, M.G.; Fenaux, P.; Gattermann, N.; Germing, U.; et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood 2013, 122, 2943–2964, doi:10.1182/blood-2013-03-492884.
- Zeidan, A.M.; Hendrick, F.; Friedmann, E.; Baer, M.R.; Gore, S.D.; Sasane, M.; Paley, C.; Davidoff, A.J. Deferasirox therapy is associated with reduced mortality risk in a medicare population with myelodysplastic syndromes. J Comp Eff Res 2015, 4, 327–340, doi:10.2217/cer.15.20.
- Angelucci, E.; Santini, V.; Di Tucci, A.A.; Quaresmini, G.; Finelli, C.; Volpe, A.; Quarta, G.; Rivellini, F.; Sanpaolo, G.; Cilloni, D.; et al. Deferasirox for transfusion-dependent patients with myelodysplastic syndromes: safety, efficacy, and beyond (GIMEMA MDS0306 Trial). Eur. J. Haematol. 2014, 92, 527–536, doi:10.1111/ejh.12300.
- Ko, B.-S.; Chang, M.-C.; Chiou, T.-J.; Chang, T.-K.; Chen, Y.-C.; Lin, S.-F.; Chang, C.-S.; Lu, Y.-C.; Yeh, S.-P.; Chen, T.-Y.; et al. Long-term safety and efficacy of deferasirox in patients with myelodysplastic syndrome, aplastic anemia and other rare anemia in Taiwan. Hematology 2019, 24, 247–254, doi:10.1080/16078454.2018.1557860.
- Zhang, J.; Shi, P.; Liu, J.; Li, J.; Cao, Y. Efficacy and safety of iron chelator for transfusion-dependent patients with myelodysplastic syndrome: a meta-analysis. Hematology 2019, 24, 669–678, doi:10.1080/16078454.2019.1666218.
- Tataranni, T.; Agriesti, F.; Mazzoccoli, C.; Ruggieri, V.; Scrima, R.; Laurenzana, I.; D’Auria, F.; Falzetti, F.; Di Ianni, M.; Musto, P.; et al. The iron chelator deferasirox affects redox signalling in haematopoietic stem/progenitor cells. Br. J. Haematol. 2015, 170, 236–246, doi:10.1111/bjh.13381.
- Ivars, D.; Orero, M.T.; Javier, K.; Díaz-Vico, L.; García-Giménez, J.L.; Mena, S.; Tormos, C.; Egea, M.; Pérez, P.L.; Arrizabalaga, B.; et al. Oxidative imbalance in low/intermediate-1-risk myelodysplastic syndrome patients: The influence of iron overload. Clin. Biochem. 2017, 50, 911–917, doi:10.1016/j.clinbiochem.2017.05.018.
- Ghoti, H.; Fibach, E.; Merkel, D.; Perez-Avraham, G.; Grisariu, S.; Rachmilewitz, E.A. Changes in parameters of oxidative stress and free iron biomarkers during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndromes. Haematologica 2010, 95, 1433–1434, doi:10.3324/haematol.2010.024992.
- Kikuchi, S.; Kobune, M.; Iyama, S.; Sato, T.; Murase, K.; Kawano, Y.; Takada, K.; Ono, K.; Kaneko, Y.; Miyanishi, K.; et al. Improvement of iron-mediated oxidative DNA damage in patients with transfusion-dependent myelodysplastic syndrome by treatment with deferasirox. Free Radic. Biol. Med. 2012, 53, 643–648, doi:10.1016/j.freeradbiomed.2012.06.006.
- Callens, C.; Coulon, S.; Naudin, J.; Radford-Weiss, I.; Boissel, N.; Raffoux, E.; Wang, P.H.M.; Agarwal, S.; Tamouza, H.; Paubelle, E.; et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J. Exp. Med. 2010, 207, 731–750, doi:10.1084/jem.20091488.
- Fang, D.; Bao, Y.; Li, X.; Liu, F.; Cai, K.; Gao, J.; Liao, Q. Effects of iron deprivation on multidrug resistance of leukemic K562 cells. Chemotherapy 2010, 56, 9–16, doi:10.1159/000287352.
- Kim, J.-L.; Kang, H.-N.; Kang, M.H.; Yoo, Y.A.; Kim, J.S.; Choi, C.W. The oral iron chelator deferasirox induces apoptosis in myeloid leukemia cells by targeting caspase. Acta Haematol. 2011, 126, 241–245, doi:10.1159/000330608.
- Ohyashiki, J.H.; Kobayashi, C.; Hamamura, R.; Okabe, S.; Tauchi, T.; Ohyashiki, K. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009, 100, 970–977, doi:10.1111/j.1349-7006.2009.01131.x.
- Lee, D.-H.; Jang, P.S.; Chung, N.G.; Cho, B.; Jeong, D.C.; Kim, H.K. Deferasirox shows in vitro and in vivo antileukemic effects on murine leukemic cell lines regardless of iron status. Exp. Hematol. 2013, 41, 539–546, doi:10.1016/j.exphem.2013.02.004.
- Shapira, S.; Raanani, P.; Samara, A.; Nagler, A.; Lubin, I.; Arber, N.; Granot, G. Deferasirox selectively induces cell death in the clinically relevant population of leukemic CD34+CD38- cells through iron chelation, induction of ROS, and inhibition of HIF1α expression. Exp. Hematol. 2019, 70, 55-69.e4, doi:10.1016/j.exphem.2018.10.010.
- Rychtarcikova, Z.; Lettlova, S.; Tomkova, V.; Korenkova, V.; Langerova, L.; Simonova, E.; Zjablovskaja, P.; Alberich-Jorda, M.; Neuzil, J.; Truksa, J. Tumor-initiating cells of breast and prostate origin show alterations in the expression of genes related to iron metabolism. Oncotarget 2017, 8, 6376–6398, doi:10.18632/oncotarget.14093.
- Li, N.; Chen, Q.; Gu, J.; Li, S.; Zhao, G.; Wang, W.; Wang, Z.; Wang, X. Synergistic inhibitory effects of deferasirox in combination with decitabine on leukemia cell lines SKM-1, THP-1, and K-562. Oncotarget 2017, 8, 36517–36530, doi:10.18632/oncotarget.16583.
- Imanishi, S.; Takahashi, R.; Ohsuga, M.; Ohyashiki, K.; Ohyashiki, J.H. Effect of combined deferasirox and 5-azacytidine treatment on human leukemia cells in vitro. Ann. Hematol. 2015, 94, 1601–1602, doi:10.1007/s00277-015-2417-9.
- Cho, B.-S.; Jeon, Y.-W.; Hahn, A.-R.; Lee, T.-H.; Park, S.-S.; Yoon, J.-H.; Lee, S.-E.; Eom, K.-S.; Kim, Y.-J.; Lee, S.; et al. Improved survival outcomes and restoration of graft-vs-leukemia effect by deferasirox after allogeneic stem cell transplantation in acute myeloid leukemia. Cancer Med 2019, 8, 501–514, doi:10.1002/cam4.1928.
- Sivgin, S.; Eser, B.; Bahcebasi, S.; Kaynar, L.; Kurnaz, F.; Uzer, E.; Pala, C.; Deniz, K.; Ozturk, A.; Cetin, M.; et al. Efficacy and safety of oral deferasirox treatment in the posttransplant period for patients who have undergone allogeneic hematopoietic stem cell transplantation (alloHSCT). Ann. Hematol. 2012, 91, 743–749, doi:10.1007/s00277-011-1358-1.
- Fukushima, T.; Kawabata, H.; Nakamura, T.; Iwao, H.; Nakajima, A.; Miki, M.; Sakai, T.; Sawaki, T.; Fujita, Y.; Tanaka, M.; et al. Iron chelation therapy with deferasirox induced complete remission in a patient with chemotherapy-resistant acute monocytic leukemia. Anticancer Res. 2011, 31, 1741–1744.
- Lui, G.Y.L.; Kovacevic, Z.; Richardson, V.; Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget 2015, 6, 18748–18779, doi:10.18632/oncotarget.4349.
- Babosova, O.; Kapralova, K.; Raskova Kafkova, L.; Korinek, V.; Divoky, V.; Prchal, J.T.; Lanikova, L. Iron chelation and 2-oxoglutarate-dependent dioxygenase inhibition suppress mantle cell lymphoma’s cyclin D1. J. Cell. Mol. Med. 2019, 23, 7785–7795, doi:10.1111/jcmm.14655.
- Rodríguez, J.A.; Luria-Pérez, R.; López-Valdés, H.E.; Casero, D.; Daniels, T.R.; Patel, S.; Avila, D.; Leuchter, R.; So, S.; Ortiz-Sánchez, E.; et al. Lethal iron deprivation induced by non-neutralizing antibodies targeting transferrin receptor 1 in malignant B cells. Leuk. Lymphoma 2011, 52, 2169–2178, doi:10.3109/10428194.2011.596964.
- Moura, I.C.; Lepelletier, Y.; Arnulf, B.; England, P.; Baude, C.; Beaumont, C.; Bazarbachi, A.; Benhamou, M.; Monteiro, R.C.; Hermine, O. A neutralizing monoclonal antibody (mAb A24) directed against the transferrin receptor induces apoptosis of tumor T lymphocytes from ATL patients. Blood 2004, 103, 1838–1845, doi:10.1182/blood-2003-07-2440.
- Benadiba, J.; Rosilio, C.; Nebout, M.; Heimeroth, V.; Neffati, Z.; Popa, A.; Mary, D.; Griessinger, E.; Imbert, V.; Sirvent, N.; et al. Iron chelation: an adjuvant therapy to target metabolism, growth and survival of murine PTEN-deficient T lymphoma and human T lymphoblastic leukemia/lymphoma. Leuk. Lymphoma 2017, 58, 1433–1445, doi:10.1080/10428194.2016.1239257.
- Eberhard, Y.; McDermott, S.P.; Wang, X.; Gronda, M.; Venugopal, A.; Wood, T.E.; Hurren, R.; Datti, A.; Batey, R.A.; Wrana, J.; et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 2009, 114, 3064–3073, doi:10.1182/blood-2009-03-209965.
- Kamihara, Y.; Takada, K.; Sato, T.; Kawano, Y.; Murase, K.; Arihara, Y.; Kikuchi, S.; Hayasaka, N.; Usami, M.; Iyama, S.; et al. The iron chelator deferasirox induces apoptosis by targeting oncogenic Pyk2/β-catenin signaling in human multiple myeloma. Oncotarget 2016, 7, 64330–64341, doi:10.18632/oncotarget.11830.
- Bordini, J.; Morisi, F.; Cerruti, F.; Cascio, P.; Camaschella, C.; Ghia, P.; Campanella, A. Iron Causes Lipid Oxidation and Inhibits Proteasome Function in Multiple Myeloma Cells: A Proof of Concept for Novel Combination Therapies. Cancers (Basel) 2020, 12, doi:10.3390/cancers12040970.
