A continuous emission monitoring system (CEMS) is a well-known tool used to analyze the concentrations of air pollutants from stationary sources. In a CEMS, the presence of a high moisture level in a sample causes a loss of analytes due to artifact formation or absorption. This issue brings about a bias in the measurement data. Thus, moisture removal is an important pretreatment step.
- moisture removal
- CEMS
- Condensation
- Cooler
Thanks for your support. The entry will be online only after submission. We will help you layout and link your article at the entry. Hence more scholars and students can benefit from it. (Please remove this note when submitting, thanks.)
1. The importance of moisture removal devices for a continuous monitoring system
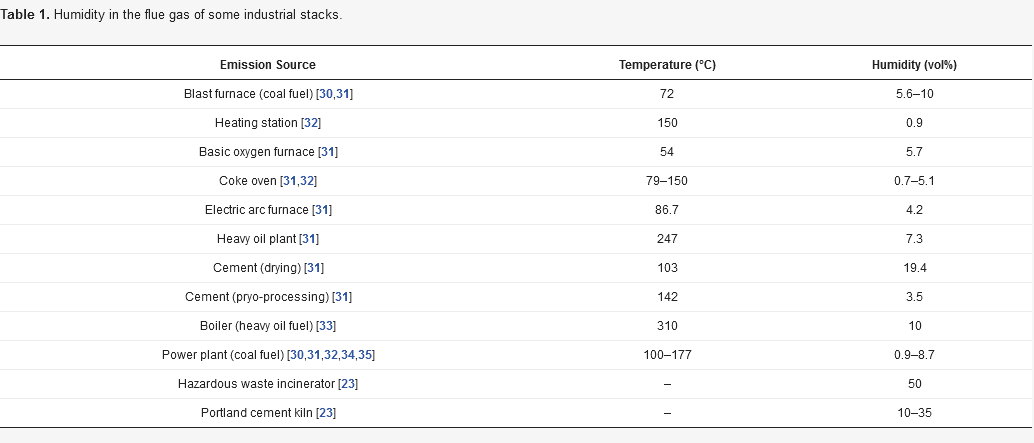
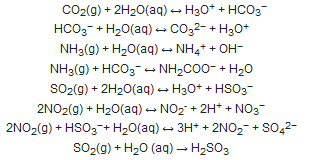
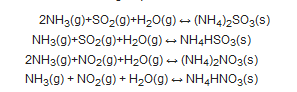
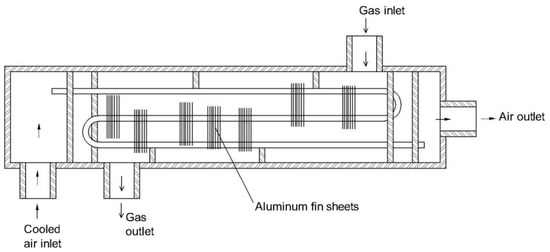
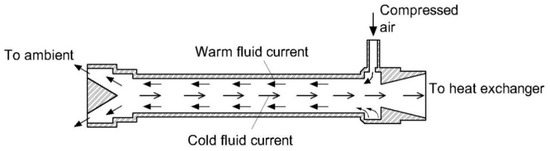
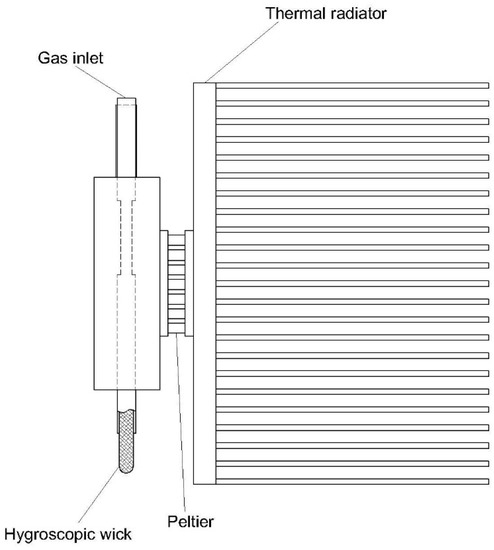
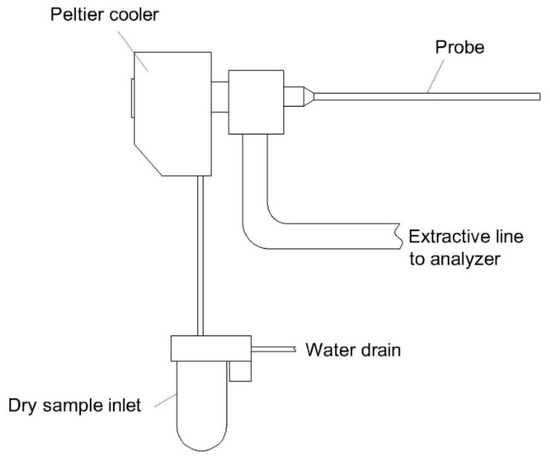
2. An overview on moisture removal devices using condensation technique in a CEMS
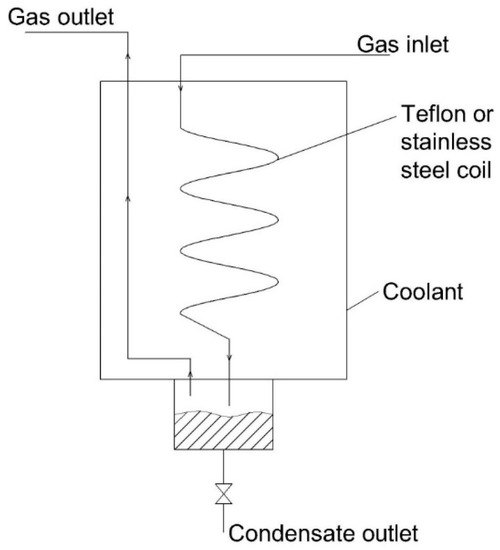
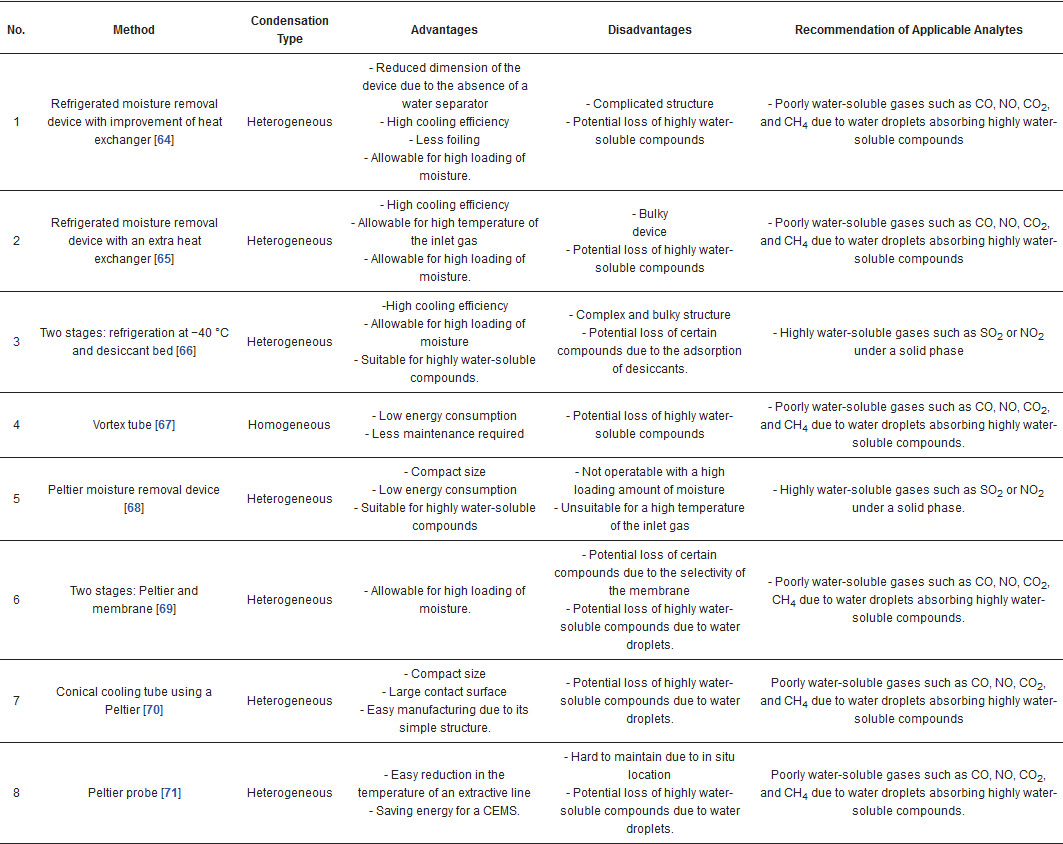
This entry is adapted from the peer-reviewed paper 10.3390/atmos12010061
References
- Jahnke, J.A. Continuous Emission Monitoring; John Wiley & Sons, Inc.: Toronto, ON, Canada, 2000; pp. 404.
- Lillesand, T.; Kiefer, R.W.; Chipman, J.. Remote Sensing and Image Interpretation; John Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 736.
- Trieu-Vuong Dinh; In-Young Choi; Youn-Suk Son; Jo-Chun Kim; A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sensors and Actuators B: Chemical 2016, 231, 529-538, 10.1016/j.snb.2016.03.040.
- Youwen Sun; Wen-Qing Liu; Yi Zeng; Shi-Mei Wang; Shu-Hua Huang; Pinhua Xie; Xiao-Man Yu; Water Vapor Interference Correction in a Non Dispersive Infrared Multi-Gas Analyzer. Chinese Physics Letters 2011, 28, 073302, 10.1088/0256-307x/28/7/073302.
- Barouch Giechaskiel; M Matti Maricq; Leonidas Ntziachristos; Christos Dardiotis; Xiaoliang Wang; Harald Axmann; Alexander Bergmann; Wolfgang Schindler; Review of motor vehicle particulate emissions sampling and measurement: From smoke and filter mass to particle number. Journal of Aerosol Science 2014, 67, 48-86, 10.1016/j.jaerosci.2013.09.003.
- Simone Simões Amaral; João A. Carvalho Jr.; Maria Angélica Martins Costa; Cleverson Pinheiro; An Overview of Particulate Matter Measurement Instruments. Atmosphere 2015, 6, 1327-1345, 10.3390/atmos6091327.
- J. Leskinen; J. Tissari; O. Uski; A. Virén; T. Torvela; T. Kaivosoja; H. Lamberg; I. Nuutinen; T. Kettunen; J. Joutsensaari; et al. Fine particle emissions in three different combustion conditions of a wood chip-fired appliance – Particulate physico-chemical properties and induced cell death. Atmospheric Environment 2014, 86, 129-139, 10.1016/j.atmosenv.2013.12.012.
- Ruoting Jiang; Michelle L. Bell; A Comparison of Particulate Matter from Biomass-Burning Rural and Non-Biomass-Burning Urban Households in Northeastern China. Environmental Health Perspectives 2008, 116, 907-914, 10.1289/ehp.10622.
- Tiina Torvela; J. Tissari; Olli Sippula; T. Kaivosoja; Jani Leskinen; A. Virén; Anna Lahde; Jorma Jokiniemi; Effect of wood combustion conditions on the morphology of freshly emitted fine particles. Atmospheric Environment 2014, 87, 65-76, 10.1016/j.atmosenv.2014.01.028.
- Patricia Krecl; Johan Ström; Christer Johansson; Carbon content of atmospheric aerosols in a residential area during the wood combustion season in Sweden. Atmospheric Environment 2007, 41, 6974-6985, 10.1016/j.atmosenv.2007.06.025.
- Daniel Mellon; Simon J. King; Jin Kim; Jonathan P. Reid; Andrew J. Orr-Ewing; Measurements of Extinction by Aerosol Particles in the Near-Infrared Using Continuous Wave Cavity Ring-Down Spectroscopy. The Journal of Physical Chemistry A 2011, 115, 774-783, 10.1021/jp109894x.
- Anders Pettersson; Edward R. Lovejoy; Charles A. Brock; Steven S. Brown; A.R. Ravishankara; Measurement of aerosol optical extinction at with pulsed cavity ring down spectroscopy. Journal of Aerosol Science 2004, 35, 995-1011, 10.1016/j.jaerosci.2004.02.008.
- M. Elsasser; M. Crippa; Jurgen Orasche; Peter F Decarlo; Markus Oster; Mike Pitz; Josef Cyrys; T. L. Gustafson; J. B. C. Pettersson; Jurgen Schnellekreis; et al. Organic molecular markers and signature from wood combustion particles in winter ambient aerosols: aerosol mass spectrometer (AMS) and high time-resolved GC-MS measurements in Augsburg, Germany. Atmospheric Chemistry and Physics 2012, 12, 6113-6128, 10.5194/acp-12-6113-2012.
- S. Hosseini; Q. Li; D. Cocker; D. Weise; A. Miller; M. Shrivastava; J. W. Miller; S. Mahalingam; M. Princevac; H. Jung; et al. Particle size distributions from laboratory-scale biomass fires using fast response instruments. Atmospheric Chemistry and Physics 2010, 10, 8065-8076, 10.5194/acp-10-8065-2010.
- Vincent, J.H. . Aerosol Sampling; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 636.
- Nicolas Coudray; Alain Dieterlen; Estelle Roth; Gwenaëlle Trouvé; Density measurement of fine aerosol fractions from wood combustion sources using ELPI distributions and image processing techniques. Fuel 2009, 88, 947-954, 10.1016/j.fuel.2008.12.013.
- Beatrice Castellani; Elena Morini; Mirko Filipponi; Andrea Nicolini; Massimo Palombo; Franco Cotana; Federico Rossi; Comparative Analysis of Monitoring Devices for Particulate Content in Exhaust Gases. Sustainability 2014, 6, 4287-4307, 10.3390/su6074287.
- D. A. Lundgren; D. W. Cooper; Effect of Humidify on Light-Scattering Methods of Measuring Particle Concentration. Journal of the Air Pollution Control Association 1969, 19, 243-247, 10.1080/00022470.1969.10466482.
- Paul Zieger; R. Fierz-Schmidhauser; Ernest Weingartner; U. Baltensperger; Effects of relative humidity on aerosol light scattering: results from different European sites. Atmospheric Chemistry and Physics 2013, 13, 10609-10631, 10.5194/acp-13-10609-2013.
- Rohan Jayaratne; Xiaoting Liu; Phong Thai; Matthew Dunbabin; Lidia Morawska; The influence of humidity on the performance of a low-cost air particle mass sensor and the effect of atmospheric fog. Atmospheric Measurement Techniques 2018, 11, 4883-4890, 10.5194/amt-11-4883-2018.
- Wenjia Shao; Hongjian Zhang; Hongliang Zhou; Fine Particle Sensor Based on Multi-Angle Light Scattering and Data Fusion. Sensors 2017, 17, 1033, 10.3390/s17051033.
- US EPA. An Operator’s Guide to Eliminating Bias in CEM Systems; United States Environmental Protection Agency: Washington, DC, USA, 1994.
- US EPA. EPA Handbook–Continuous Emission. Monitoring Systems for Non-Criteria Pollutants; United States Environmental Pro-tection Agency: Washington, DC, USA, 1997.
- US EPA. Ammonia CEMS Background; United States Environmental Protection Agency: Washington, DC, USA, 1993.
- Josep M. Anglada; Marilia T. C. Martins-Costa; Joseph S. Francisco; Manuel F. Ruiz-López; Triplet state promoted reaction of SO2 with H2O by competition between proton coupled electron transfer (pcet) and hydrogen atom transfer (hat) processes. Physical Chemistry Chemical Physics 2019, 21, 9779-9784, 10.1039/c9cp01105f.
- Kangkang Li; Hai Yu; Guojie Qi; Paul Feron; Moses Tade; Jingwen Yu; Shujuan Wang; Rate-based modelling of combined SO 2 removal and NH 3 recycling integrated with an aqueous NH 3 -based CO 2 capture process. Applied Energy 2015, 148, 66-77, 10.1016/j.apenergy.2015.03.060.
- Jong-Beom Seo; Soo-Bin Jeon; Won-Joon Choi; Jae-Won Kim; Gou-Hong Lee; Kwang-Joong Oh; The absorption rate of CO2/SO2/NO2 into a blended aqueous AMP/ammonia solution. Korean Journal of Chemical Engineering 2010, 28, 170-177, 10.1007/s11814-010-0332-2.
- Yi Zhao; Runlong Hao; Tianhao Wang; Chunyan Yang; Follow-up research for integrative process of pre-oxidation and post-absorption cleaning flue gas: Absorption of NO2, NO and SO2. Chemical Engineering Journal 2015, 273, 55-65, 10.1016/j.cej.2015.03.053.
- Susianto; Mathieu Pétrissans; Anélie Pétrissans; André Zoulalian; Experimental study and modelling of mass transfer during simultaneous absorption of SO2 and NO2 with chemical reaction. Chemical Engineering and Processing - Process Intensification 2005, 44, 1075-1081, 10.1016/j.cep.2005.03.001.
- Shaofei Kong; Jianwu Shi; Bing Lu; Weiguang Qiu; Baosheng Zhang; Yue Peng; Bowen Zhang; Zhipeng Bai; Characterization of PAHs within PM10 fraction for ashes from coke production, iron smelt, heating station and power plant stacks in Liaoning Province, China. Atmospheric Environment 2011, 45, 3777-3785, 10.1016/j.atmosenv.2011.04.029.
- Hsi-Hsien Yang; Wen-Jhy Lee; Shui-Jen Chen; Soon-Onn Lai; PAH emission from various industrial stacks. Journal of Hazardous Materials 1998, 60, 159-174, 10.1016/s0304-3894(98)00089-2.
- Jianwu Shi; Hao Deng; Zhipeng Bai; Shaofei Kong; Xiuyan Wang; Jiming Hao; Xinyu Han; Ping Ning; Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Science of The Total Environment 2015, 515, 101-108, 10.1016/j.scitotenv.2015.02.034.
- Andrzej Pawelec; A.G. Chmielewski; Janusz Licki; Bumsoo Han; Jinkyu Kim; Noushad Kunnummal; Osama I. Fageeha; Pilot plant for electron beam treatment of flue gases from heavy fuel oil fired boiler. Fuel Processing Technology 2016, 145, 123-129, 10.1016/j.fuproc.2016.02.002.
- Jo-Chun Kim; Ki-Hyun Kim; Al Armendariz; Mohamad Al-Sheikhly; Electron Beam Irradiation for Mercury Oxidation and Mercury Emissions Control. Journal of Environmental Engineering 2010, 136, 554-559, 10.1061/(asce)ee.1943-7870.0000188.
- Anees U. Malik; Fahd Al-Muaili; Mohammad Al-Ayashi; Abdelkader Meroufel; An investigation on the corrosion of flue gas sensor in boiler stack. Case Studies in Engineering Failure Analysis 2013, 1, 200-208, 10.1016/j.csefa.2013.07.003.
- Faghri, A.; Zhang, Y.. Transport Phenomena in Multiphase Systems; Academic Press: Cambridge: MA, USA, 2006; pp. 1064.
- Ming Qu; Omar Abdelaziz; Zhiming Gao; Hongxi Yin; Isothermal membrane-based air dehumidification: A comprehensive review. Renewable and Sustainable Energy Reviews 2018, 82, 4060-4069, 10.1016/j.rser.2017.10.067.
- Cromer, C.J. Cooling system. US Patent US6237354B1, 2001.
- Curtis, M. Air conditioning system with moisture control. US Patent US7191607B2, 2007.
- Endo, T.; Terada, H.; Katsumata, N.; Yagi, K.; Kawashima, K. Air conditioner and moisture removing device for use with the air conditioner. European Patent EP0816779A4, 1998.
- D. La; Yanjun Dai; Y. Li; R.Z. Wang; T.S. Ge; Technical development of rotary desiccant dehumidification and air conditioning: A review. Renewable and Sustainable Energy Reviews 2010, 14, 130-147, 10.1016/j.rser.2009.07.016.
- Pietro Mazzei; Francesco Minichiello; Daniele Palma; HVAC dehumidification systems for thermal comfort: a critical review. Applied Thermal Engineering 2005, 25, 677-707, 10.1016/j.applthermaleng.2004.07.014.
- L. Mei; Yanjun Dai; A technical review on use of liquid-desiccant dehumidification for air-conditioning application. Renewable and Sustainable Energy Reviews 2008, 12, 662-689, 10.1016/j.rser.2006.10.006.
- Wu, G.; Cur, N.O. Squeezable moisture removal device. US Patent US20090158928A1, 2009.
- Aine, H.E. Infrared optoacoustic gas analyzer having an improved gas inlet system. US Patent US4051372A, 1977.
- Baccanti, M.; Magni, P. Process and apparatus for determining total nitrogen content by elemental analysis. European Patent EP0586969A2, 1994.
- Jahn, M.D.; Griffin, M.T.; Popkie, N. Apparatus and method for water vapor removal in an ion mobility spectrometer. US Patent US7361206, 2008.
- Christine M. Karbiwnyk; Craig S. Mills; Detlev Helmig; John W. Birks; Minimization of water vapor interference in the analysis of non-methane volatile organic compounds by solid adsorbent sampling. Journal of Chromatography A 2002, 958, 219-229, 10.1016/s0021-9673(02)00307-2.
- Bruno Kolb; Gerhard Zwick; Maria Auer; A water trap for static cryo-headspace gas chromatography. Journal of High Resolution Chromatography 1996, 19, 37-42, 10.1002/jhrc.1240190107.
- Legendre, M.G.; Fisher, G.S. Inlet system for direct gas chromatographic and combined gas chromatographic/mass spec-trometric analysis of food volatiles. US Patent US4245494A, 1981.
- Jacek Namieśnik; Waldemar Wardencki; Water Vapour Removal from Gaseous Samples Used for Analytical Purposes. A Review. International Journal of Environmental Analytical Chemistry 1999, 73, 269-280, 10.1080/03067319908032669.
- Youn-Suk Son; Gangwoong Lee; Jo-Chun Kim; Jin-Seok Han; Development of a Pretreatment System for the Analysis of Atmospheric Reduced Sulfur Compounds. Analytical Chemistry 2013, 85, 10134-10141, 10.1021/ac401345e.
- Ahn, S.; Moon, J.; Kim, D.; Baek, S.; Ryoo, B. Clothes dryer with a dehumidifier. US Patent US20060117593A1, 2006.
- Cromer, C.J. Heat pump dryer with desciccant enhanced moisture removal. US Patent US6094835A, 2000.
- Lentz, L.E. Dehumidifier clothes dryer apparatus. US Patent US7458171B1, 2008.
- Naboulsi, Z.A. System to reduce moisture within a clothes dryer. US Patent US20150059201A1, 2015.
- Sieben, F.; Volkel, T. Air dehumidifier for use in a dryer. US Patent US20120137535A1, 2012.
- Araki, H.; Shibata, T.; Hatamiya, S.; Tsukamoto, M. Cooling apparatus, gas turbine system using cooling apparatus, heat pump system using cooling system, cooling method, and method for operating cooling apparatus. US Patent US8402735B2, 2013.
- Baudat, N.; Richardson, F. Direct turbine air chiller/scrubber system. US Patent US20010054354A1, 2001.
- Kulkarni, A.M.; Ashley Mass, R.M.; Davies, J.C. Mist/moisture removal using fixed bed trickle columns. US Patent US8419844B1, 2013.
- Balinski, B.A.; Jackson, F.G.; McAllister, K.D. Controlled moisture removal in a laundry treating appliance. US Patent US10633776B2, 2020.
- Kim, Y.-J. Washing machine having dehumidifying apparatus and dehumidifying method thereof. US Patent US20080295696A1, 2008.
- Saukahanian, G.; Kruger, M. Dehumidifier for air utilized in laundry drying. US Patent US3866333A, 1975.
- Downling, R.O. Compressed air dryer. US Patent US4235081A, 1980.
- Nanaumi, K.; Baba, K. Heat exchanger for cooling system compressed air dehumidifiers. US Patent US4193443A, 1980.
- Basseen, S.K.; Harlan, R.A. High efficiency air drying system. US Patent US4761968A, 1988.
- Tosi, S. Gas analyzer with cooling apparatus. European Patent EP2124049A1, 2009.
- Chapman, R.L.; Harman, J.N. Thermoelectric gas dryer. US Patent US4231256A, 1980.
- Groeger, H. Process and apparatus for pretreatment of a gas to be analysed. European Patent EP0291630A3, 1988.
- Groeger, C.-M.; Groeger, H. Apparatus for separating vaporous compounds from a gas stream. European Patent EP0361068A3, 1989.
- Bacharach, Inc., Technical note: Flue gas sample conditioning system (P/N 0024-7224), 2010.
- Dong-June Kim; Trieu-Vuong Dinh; Joo-Yeon Lee; In-Young Choi; Dong-Jin Son; In-Young Kim; Young Sunwoo; Jo-Chun Kim; Effects of Water Removal Devices on Ambient Inorganic Air Pollutant Measurements. International Journal of Environmental Research and Public Health 2019, 16, 3446, 10.3390/ijerph16183446.
- Joo-Yeon Lee; Trieu-Vuong Dinh; Dong-June Kim; In-Young Choi; Ji-Won Ahn; Shin-Young Park; Yoo-Jin Jung; Jo-Chun Kim; Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds. Applied Sciences 2019, 9, 4045, 10.3390/app9194045.
- M. Kondo; H. Kawanowa; Y. Gotoh; Ryutaro Souda; Hydration of the HCl and NH3 molecules adsorbed on amorphous water–ice surface. Applied Surface Science 2004, 237, 510-514, 10.1016/j.apsusc.2004.06.063.
- S. Woittequand; Céline Toubin; M. Monnerville; B. Pouilly; S. Briquez; S. Picaud; Photodissociation of a HCl molecule adsorbed on ice at T=210K. Surface Science 2007, 601, 3034-3041, 10.1016/j.susc.2007.05.006.
