The alarming increase in antimicrobial resistance, based on the built-in abilities of bacteria to nullify the activity of current antibiotics, leaves a growing number of bacterial infections untreatable. An appealing approach, advanced in recent decades, concerns the development of novel agents able to interact with the external layers of bacteria, causing irreparable damage. Regarding this, some natural cationic antimicrobial peptides (CAMPs) have been reconsidered, and synthetic cationic polymers, mimicking CAMPs and able to kill bacteria by non-specific detrimental interaction with the negative bacterial membranes, have been proposed as promising solutions. Lately, also dendrimers were considered suitable macromolecules for the preparation of more advanced cationic biomimetic nanoparticles, able to harmonize the typical properties of dendrimers, including nanosize, mono-dispersion, long-term stability, high functionality, and the non-specific mechanism of action of CAMPs. Although cationic dendrimers are extensively applied in nanomedicine for drug or gene delivery, their application as antimicrobial agents is still in its infancy. In this first part of our overview on the main types of cationic antibacterial dendrimers, the state of the art of the potential applications of PAMAM and PPI-based agents has therefore been reviewed here, with particular attention to the innovative case studies reported in the literature
- antibiotic resistance
- novel antimicrobial agents
- cationic antimicrobial polymers
- non-specific membrane disruption
- biomimetic cationic dendrimer nanoparticles
- PAMAM and PPI antibacterial dendrimers
Introduction
The increasing growth of resistant bacterial strains, which represent a highly worrying trend that has characterized the last few years, has caused the appearance and the re-emergence of serious infections, in particular in nosocomial settings [1]. In this regard, pneumonia, bloodstream infections, wound or surgical site infections, and meningitis are often associated with the failure of antibiotic-based treatments or with the concomitant lack of new antimicrobial agents [2][3][4]. Gram-negative bacteria such as Klebsiella pneumoniae, Acinetobacter baumannii, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Burkholderia cepacia, and Escherichia coli pose a major threat to human health, since they are the most critically resistant and rapidly spreading bacteria [5][6][7]. Moreover, in addition to their intrinsic resistance mechanisms, these pathogens are rapidly becoming multidrug-or even pan-drug-resistant to most life-saving drugs. In particular, aerobic non-fermenting Gram-negative bacilli such as A. baumannii, P. aeruginosa, and S. maltophilia, are emerging as clinically relevant superbugs, contributing significantly, with their alarming resistance levels, to numerous therapeutic failures. Gram-negative bacteria, unlike Gram-positive bacteria, are characterized by high and similar resistance levels, both in Europe and in the United States.Given this situation, as well as being inspired by reports developed by a group of independent experts led by World Health Organization (WHO), the medical research community must develop new antimicrobial agents active on current resistant strains of Gram-positive and Gram-negative bacteria. Furthermore, because bacterial infections, especially if caused by biofilm production, hinder the durability, reliability, and performance of many medical devices and implants, antibiofilm strategies such as antibacterial coatings that repel bacteria and prevent biofilm formation are highly desirable.Natural cationic antimicrobial peptides (CAMPs) are a class of unconventional antimicrobial agents with a broad spectrum of action, active on a wide variety of Gram-positive and Gram-negative bacteria, fungi, protozoa and yeast. These molecules, without the need to enter the bacterium cell or to interfere with specific metabolic processes, basically act based on their positive charge. Their action is rapid and not specific and is based initially on electrostatic interactions with the bacterial surface, followed by the progressive damage of the bacterial outer and/or cytoplasmic membranes (OM and CM) that leads to the bacterial death. On the base of this mechanism of action, CAMPs kill pathogens simply through external contact, without the need to address the numerous resistance mechanisms due to genetic mutations that bacteria can develop. In other words, since these materials do not interfere with the vital processes for bacteria, which can eventually be modified—by genetic mutation—by resistant pathogens, they are generally indifferent to the multiple resistance mechanisms developed by the bacteria on which they act. Recently, cationic antimicrobial macromolecules, inspired by natural CAMPs, have gained increasing attention from the scientific community because, compared to small molecules of drug, they possess several advantages such as higher long-term activity, limited residual toxicity, chemical stability, non-volatility and the inability to permeate through the skin due to their macromolecular structure and high molecular weight (MW) [8]. Among polymers, dendrimers (Ds), a specific class of nanoscaled, hyperbranched, tree-like macromolecules, with a symmetric well-defined structure and a three-dimensional architecture [9][10][11][12], have recently shown to function as antibacterial agents and as antimicrobial surface coatings as well. The first synthesis of dendrimer materials, whose structure was conceptualized in the early 1970s, dates back to the mid-eighties. Although Ds, including positively charged ones, such as cationic poly(amidoamine) (PAMAM), polypropylenimine (PPI), dendritic polylysine, and peptide structures, have been actively investigated for a wide range of industrial and biomedical applications, their potential usages as antimicrobial agents mimicking CAMPs, both as drugs, as surface coating agents and drug-delivery systems, has been recognized only very recently.In this regard, based on the Scopus data, the scientific interest in cationic polymers as novel antimicrobials was limited until 2000, but has grown steadily and exponentially to date. On the contrary, the trend of scientific production and research in the field of antimicrobial dendrimers (ADs) over a period of 30 years definitively underscores how this was non-existent until 2007, then it started to increase, but not constantly, with the highest production in the last decade (Figure 1).
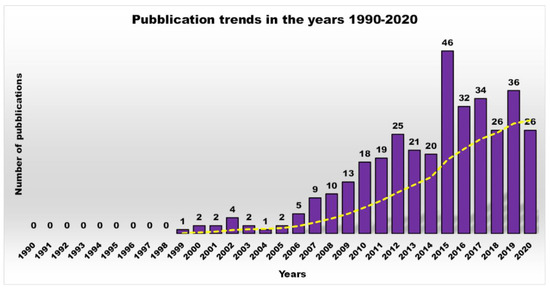
Figure 1. Number of publications as a function of time, obtained by typing the key words “antimicrobial dendrimers” in Scopus.
Furthermore, as shown in Figure 2, a more restricted investigation of the scientific output of the last decade concerning cationic antibacterial dendrimers (CADs) (yellow bars), the subject of this work, revealed that the actual number of studies is far lower than that reported in Figure 1 (purple bars), which generally depicts the number of publications on ADs.
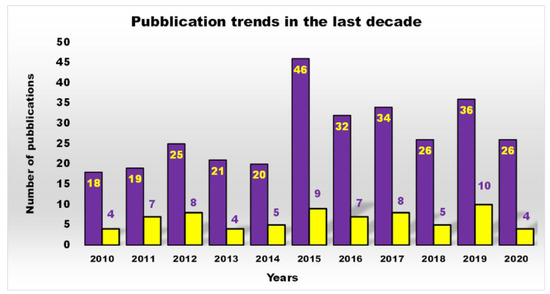
Figure 2. Number of publications, as a function of time, obtained by typing the key phrase “antimicrobial dendrimers” in Scopus (purple bars) and that obtained by typing “cationic antibacterial dendrimers” (yellow bars), topic of the review.
However, different types of Ds, including cationic ones, have been developed [13] for the treatment of infections sustained by multidrug-resistant bacteria, mainly during the last decade. In this context, some synthetized antimicrobial Ds also proved to have antibiofilm effects [14] or capabilities to act in synergism with commonly used antibiotics [15][16], and in some case studies in vivo evaluations were also performed [17]. Commercially available PAMAM and PPI dendrimers (PAMAM Ds and PPI Ds) are the most investigated, but despite their considerable broad-spectrum activity observed in vitro, if not opportunely modified to tone down several issues, such as low biodegradability, susceptibility to opsonization, toxicity to mammalian cells, including hemolytic toxicity, cytotoxicity, and hematological toxicity, as well as fast clearance, in their native form they are not suitable for clinical applications. To address these issues, as in other biomedical applications, uncharged dendrimer matrices, decorated with protonable residues, are worthy of consideration as a less toxic alternative. In this context, amino acid-modified, polyester-based dendrimer scaffolds should be the most attractive, also because of their good biodegradability [18][19]. In this first entry on cationic antibacterial dendrimers, after a short overview of the different classes of antimicrobial Ds developed up to 2010, the state of the art of PAMAM and PPI-based ones developed in the last decade, has been reviewed, highlighting the relationships structure/activity with particular attention to how dendrimer surface groups may affect the antimicrobial activity and their capability to inhibit the biofilm formation. Case studies have been also included and summarized.
Antibacterial Dendrimers (Ds)
Starting from 1978 a series of differently structured dendrimer scaffolds, endowed with different physicochemical features, have been prepared and applied in several industrial and medical areas. The increase in mortality rates, related to the emergency of non-curable bacterial infectious diseases, underlines the incessant development of resistance strategies to drugs activated by bacteria through the transmission or the acquisition of new genetic material, not only between strains of the same species, but even between strains of different species. To reach this goal to counteract bacterial resistance and to replace ineffective antibiotics, various classes of Ds that can inhibit microbial pathogens (even by killing them), have been designed. Their development, nevertheless, is still limited (Figure 1 and Figure 2) and their application in therapy is still in the exploratory phase. Interestingly, such dendrimer devices can act either as antimicrobial agents, drug-delivery devices, or bacteriophobic coatings.
Principal Types of Antimicrobial Dendrimers Developed in the Previous Decade
Although the aim and main purposes of this document is to review the state of the art of cationic antimicrobial Ds, in order to have a more complete view of all the types of Ds that possess the suitable structural characteristics to function as antimicrobial and/or antibiofilm compounds, this section quickly introduces those developed in the previous decade and already reviewed by Castonguay et al. (2012) [13] and by Mintzer et al. (2012)[3]. These include glyco Ds, cationic Ds, anionic Ds, and peptide Ds, which in some cases can also be either surface-adsorbed or metal-conjugated. Interestingly, cationic PAMAM Ds, before being considered to be per se active antimicrobial Ds, were mainly investigated as drug carriers to solubilize and deliver conventional antibiotics with synergistic intents.In their relevant paper, Mintzer et al. focused on the mechanisms of action of the different types of Ds, concentrating in particular on the effects of multivalence deriving from the tree-like and generational structure of the Ds, and also on the influence of the abundance of the active functions on the antibacterial potency of the developed dendrimers.
Cationic Antibacterial Dendrimers (CADs)
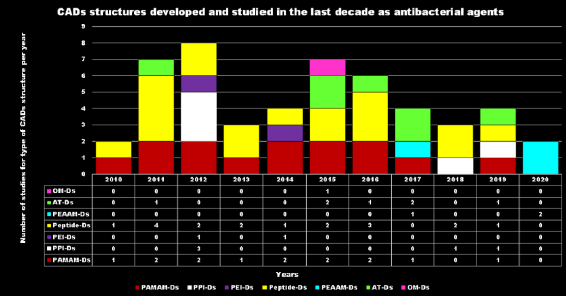
After the promising results of cationic polymers as antimicrobial agents capable of mimicking the destructive and non-specific action of CAMPs, in recent years cationic dendrimer nanoparticles have emerged as promising new antibiotic agents[20].The antibacterial activity of cationic material such as CAMPs and of their mimics depends on the electrostatic interaction between the positive charges of the device and the anionic bacteria cell surface, the progressive permeabilization of the bacterial membranes, the disruption of the lipid bilayer, the lack of cytoplasmic content, vital ions and cell death due to the disruption of the lipid bilayer. Thus, similarly to other ADs, in CADs, the multivalence in terms of positive charges plays a key role in their antimicrobial activity, and high-generation cationic Ds proved to be biocides with high activity, capability of bring localized in specific organs reduced systemic toxicity and increased duration of action[21]. Research on these agents, in fact, focuses both on their intrinsic antimicrobial activity and on the possibility they offer as drug release systems, since they are suitable for encapsulating or covalently connecting biologically active agents. This latter strategy improves the solubility of poorly soluble antibiotics, protects them from fast metabolism, increases their residence time in the circle, targets the drugs transported to specific sites of action and achieves synergistic cooperation between the cationic dendrimer carrier and the antibiotic, thus allowing a reduced dosage[22]. According to Scopus, although some sporadic case studies were reported before[23][24], the interest in cationic Ds started in 2005 and most of the research and scientific production belongs to the last decade.Among cationic Ds, PAMAM Ds were broadly studied[25], PPI Ds were principally considered when the interest in cationic Ds started, while a limited number of case studies concerning PEI-Ds were published[26][27][28]. To achieve a fair compromise between activity, biodegradability, selectivity for bacterial cells and limited hemolytic toxicity and cytotoxicity towards eukaryotic cells, poly (lysine) and peptide Ds were extensively prepared and evaluated[29][30]. Curiously, even though they were considered to be very attractive for biomedical applications as highly biodegradable and low cytotoxic, only very recently, polyester-based scaffolds, peripherally cationic for the presence of amino acids, have been taken into consideration as novel antimicrobial devices. These Ds, by presenting an uncharged hydrolysable matrix, capable of better balancing the density of charge on their surface, maintain a strong antibacterial activity associated with reduced toxicity.For each year of the last decade (x axis), Figure 3 shows the main classes of CADs that have been developed with the number of correlated studies (y axis), reflecting the researchers’ interest for the different dendrimer structures, over a 10 year period. It can be observed that although a high number of studies have been reported on cationic peptide Ds, dendrimers such as PAMAM Ds and peptide Ds have been developed throughout the decade. On the contrary, other structures, such as ammonium-terminated Ds (AT-Ds), including AT phosphorous and carbosilane Ds, attracted the interest of scientists only after 2015. Other Ds, such as PPI Ds, after initial consideration, were neglected, while PEI-Ds and organometallic Ds (OM-Ds), were taken into consideration only marginally. Polyester-based amino acid-modified Ds (PEAAM-Ds) have been considered and studied as antimicrobial devices only recently. However, all the cationic Ds reported in Figure 3 showed significant antimicrobial activity.
Figure 3. CADs developed in recent decades.
PAMAM and PPI Dendrimers (PAMAM Ds and PPI Ds)
Traditional and unmodified PAMAM Ds and PPI Ds possess a wide number of peripheral protonable primary amine groups and additional protonable tertiary amine groups in the inner matrix, which give them high antibacterial activity due to their high density of positive charge. Surprisingly, even though PAMAM Ds were one of the earliest to be synthesized and extensively studied for various biomedical applications, their activity as cationic antimicrobial agents has only recently been evaluated.Actually, the existence of a correlation between the generation number of PAMAM- or PPI Ds and their antibacterial activity is still under debate, and, according to some authors this correlation cannot be discerned, since both small and high-generation PAMAM Ds have shown considerable activity. As for PPI Ds, an incomplete correlation between these characteristics was found by Cooper et al. who assessed both the influence of the generation number and that of the length of a hydrophobic chain, introduced into the structure of the molecule and considered responsible for the antimicrobial activity against E. coli [23]. The effect of the generation number on antimicrobial activity showed that G5 > G4 > G1 > G2 > G3. This effect was attributed to the balance between the higher potency achieved with a greater number of quaternary amines and the decreased permeability of the larger dendrimer analogs. The effect of the hydrophobic chain length on antimicrobial activity showed that C10 > C8 > C12 > C14 ≈ C16. The authors rationally attributed this trend to the dual binding site theory, which suggests that on the bacterial cell surface dual sites exist binding with antibacterial agents, for which the relative binding activities of long and short hydrophobic ligands differ [23]. In this context, six poly(quaternary ammonium) chloride Ds were synthesized by functionalizing a PPI D with alkyl chains of various lengths obtaining a series of cationic Ds whose structures encompass both the amphiphilic character of natural CAMP and the multivalence of Ds [31]. In vitro antimicrobial activity was assessed by measuring the inhibition zone diameter by agar-well-diffusion method. Even though the authors indicated that the amphiphilic PPI-base Ds with shorter alkyl chains are potent antimicrobial agents with activity against Gram-positive and Gram-negative bacteria, including multidrug-resistant pathogens, such as the methicillin-resistant S. aureus, the assertion is questionable. As for reference data such as diameters of the inhibition zone of known antibiotics were not provided in the study, so that only a comparison between the synthetized Ds is possible. Furthermore, the concentrations of Ds used in that study (10 mg/mL) are extraordinarily high if compared to that of antibiotics (μg/mL). Finally, to assess the real applicability of these interesting Ds, investigations regarding their toxicity towards eukaryotic cells are lacking in the study, but mandatory.Moreover, also results obtained from comparison studies between antimicrobial Ds and hyperbranched polymers reveal conflicting outcomes. Concerning PPI Ds studied by Cooper and colleagues, when colonies of E. coli were exposed to 12 μg/mL of a PPI D and to the same amount of a hyperbranched polymer, with the same number of functional groups and similar MW, all bacteria were destroyed in the first case while in the second case only a reduction of 30–40% was observed. By contrast, the hyperbranched carbosilane polymers, investigated in another study and reported by Mintzer in 2012 , showed higher activity than the second- and third-generation carbosilane Ds with similar molecular weight and ζ potential, being the MIC values of 8–16 μg/mL vs. 16–64 μg/mL against E. coli and of 4 μg/mL vs. 4–8 μg/mL against S. aureus.Interestingly, in the earliest studies, PAMAM Ds were not evaluated as active antimicrobial agents per se, but rather as carriers for antimicrobial agents, with the purpose of improving their performance.In this context, Neelgund and colleagues reported the design of antimicrobial nanohybrid Ds, namely f-MWCNTs-CdS and f-MWCNTs-Ag2S, synthetized by covalent grafting of cationic hyperbranched dendritic PAMAMs onto multiwalled carbon nanotubes (MWCNTs) followed by deposition of CdS and Ag2S quantum dots (QDs), respectively[32]. The antibacterial activity of f-MWCNTs-CdS and f-MWCNTs-Ag2S hybrid Ds (20 μg/mL) was evaluated on E. coli, P. aeruginosa and S. aureus and it was found that the biocidal action of MWCNTs, already improved by grafting of PAMAM, was further enhanced by loading CdS and Ag2S QDs. In particular, the Ag2S Ds were more active than the CdS ones and both materials were more active against Gram-negative bacteria than towards the Gram-positive specie S. aureus. A little perplexity on the efficacy of f-MWCNTs-CdS and f-MWCNTs-Ag2S hybrid Ds prepared by Neelgund and colleagues arises from the fact that no reference data such as the growth inhibition activity of known antibiotics have been provided. In addition, to assess the real applicability of these interesting Ds, investigations regarding their toxicity towards eukaryotic cells are still lacking.Despite their established antimicrobial activity, various characteristics limit their potential use, such as the long synthesis process, necessary for high-generation compounds and their non-specific interaction with the negative charges of bacterial or eukaryotic membranes responsible for cytotoxicity and hemolytic toxicity. In addition, fast clearance from the blood circulation, as well as a high level of uptake in the reticuloendothelial system affect their activity. To address these issues, different types of structural modifications or synthetic combinations of PAMAM Ds with more hydrophobic Ds have been developed. In this regard, PAMAM-based dendrimers compounds, with tunable cationic and hydrophobic characters able to self-assemble, were prepared combining poly(aryl ether)-based dendrons with PAMAM-based dendrimer residues, which are different for generation and terminal groups. The obtained poly(aryl ether)-PAMAM-based amphiphilic Ds were assessed for their intrinsic antibacterial activity by determining MIC and MBC values and by time-killing experiments performed at 4 × MIC concentrations on S. aureus and E. coli strains. The best performing Ds were, as expected, the amine terminated ones but interestingly, the best between the two was that of the lower generation. Time-killing experiments established that AD-1 was the dendrimer able to cause the high reduction in the growth of both bacteria along 24 h of exposure, thus confirming its strongest bactericidal effects. The cytotoxicity essay performed on AD-1 highlighted that NIH/3T3 fibroblast cells showed significant damage only when they were exposed to AD-1 concentration four times higher than MIC or to an AD-1 dosage 2-times the MBC for both E. coli and S. aureus species.A highly cationic dendrimer, containing both a positively charged fourth-generation PAMAM architecture and a quaternary ammonium compound covalently linked, known as 1-hexadecyl-azoniabicylo [2.2.2] octane (C16-DABCO), and possessing antibacterial activity per se, was prepared. Additional mannoside end groups have also been incorporated into the antimicrobial dendrimer, to prevent the bacteria adhesion to the surface of eukaryotic cells by an intercepting mechanism. First, in the study of Vankoten and colleagues, the antibacterial activity of the obtained materials was tested against several Gram-positive and Gram-negative strains of pathogenic bacteria. With the exception of Streptococcus oralis, which showed MIC values > 20 μM, the remaining species were reported to be quite susceptible to the dendrimer, displaying MIC values in the range 0.13–2.0 μM. Unfortunately, the observed hemolytic toxicity (MHC = 0.6 μM) limits the clinical use of C16-DABCO dendrimer for the treatment of infections caused by S. aureus and B. cereus (MICs = 0.13 μM). A further study evaluating the tendency of the prepared dendrimer to select resistant strains within a specie was performed on E. coli and B. cereus. The results established that the multivalent antimicrobial dendrimer, due to its ability to deliver a detrimental dose of positive charge to the bacteria, displayed a significantly reduced tendency to develop bacterial resistance. C16-DABCO dendrimer has also been tested for its ability to disrupt biofilm and/or inhibit biofilm formation. No inhibition or reduction of biofilm biomass was observed in any of the species tested. Interestingly, experiments aimed at evaluating the prevention of biofilm formation, proved that on membranes pretreated with a 1 mg/mL solution of C16-DABCO dendrimer (33.3 μM), and inoculated with S. aureus, no visible biofilms were present after three days of incubation.With the aim of reducing the incidence of implant-associated infections, severe post-surgery complications leading to patient disability and morbidity, and even death, Wang et al., (2011), for the first time, successfully coated three types of titanium-based substrates with PAMAM Ds modified with various percentages of poly(ethylene glycol) (PEG) to limit cytotoxicity [33]. Based on their inner and peripheral high content of amine groups, electrostatic interactions were more favored with bacterial membranes characterized by higher negative charge, causing their destruction. Consequently, the PAMAM coatings displayed greater bactericidal activity against Gram-negative P. aeruginosa, than against Gram-positive S. aureus, due to the higher negativity of their OM. The dendrimer adsorbed on titanium surfaces, proved durable bacteriophobic abilities, inhibited bacterial colonization even after 30 days and showed good stability and biocompatibility with human bone mesenchymal stem cells (hMSCs).In the same year, Navath and colleagues developed in situ forming PAMAM Ds-PEG biodegradable hydrogels, loaded with amoxicillin, and proposed these materials as novel agents for the delivery and sustained release of that specific antibiotic in the cervicovaginal region, in order to treat ascending genital infections. Briefly, the PAMAMs-based amoxicillin formulations were prepared by the cross-linking of [(NH2)49-G4-(NH-PDP)15] dendrimer and 8-arm-PEG via formation of disulfide bridges and were tested both in vitro and in vivo. Amoxicillin release from these hydrogels (3%, 6% and 10% w/v) was sustained for more than 240 h, while in vivo assessment of the hydrogels, carried out using a pregnant guinea pig model, showed excellent tolerability, lack of alterations in vaginal pH and/or erythema up to 72 h and slow degradation. The gels were retained in the maternal tissues without being transferred across the fetal membranes [17].Recently, another approach has been reported to obtain a synergistic action between cationic Ds and antibiotics. Instead of binding the antibiotic to the dendrimer backbone, thus making a device that possesses both the intrinsic antimicrobial activity of the carrier and that of the antibiotic, synthetic polycationic and polyanionic Ds were administered in combination with levofloxacin (LVFX), in order to reduce the development of resistance to the drug, decrease its dosage and toxicity, as well as the environmental pollution resulting from the wide spread use of fluoroquinolones. The germicidal activity of the combination made was investigated by treating E. coli ATCC 25922, Proteus hauseri ATCC 15442, and S. aureus ATCC 6538 strains for 24 h with fixed concentrations of LVFX and Ds. Satisfactory results, in terms of antimicrobial activity, were obtained only against the Gram-negative E. coli by the third-generation PPI-based cationic dendrimer, the only one of interest for this review, characterized by a dense maltose shell (PPI-G3-DS-Ma) and known to be endowed with a dose-dependent cytotoxicity. In fact, a combination of the dendrimer PPI-G3-DS-Ma (10 μM) and LVFX (0.01 μg/mL) reduced the growth of E. coli by 80%, while dendrimer or antibiotic alone reduced the same growth by only 6% or 4%, respectively. This strategy, even if limited to E. coli, has allowed to significantly reduce the LVFX dosage, using the cationic dendrimer at a dose which, for a similar dendrimer of higher generation previously reported, showed very limited cytotoxicity on several human cell lines (cell viability around 80–100%) [34].In this last study, in fact, the preparation and evaluation of the germicidal activity of an unmodified fourth-generation PPI D (PPI-G4) and of PPI Ds modified with 25% and 100% maltose attached to their surface (PPI-25%mG4 and PPI-100%mG4, respectively), was reported. The target bacteria were both Gram-positive (S. aureus ATCC 6538 and S. epidermidis ATCC 12228) and Gram-negative (E. coli ATCC 25922 and P. aeruginosa ATCC 15442) strains. In parallel, the cytotoxic effects of all tested Ds were checked on a Chinese hamster fibroblast cell line (B14), a human liver hepatocellular carcinoma cell line (HepG2), a mouse neuroblastoma cell line (N2a) and a rat liver cell line (BRL-3A). The obtained results established that Gram-negative bacteria were refractory to the Ds, also at very high concentrations (100 μM). The unmodified PPI-G4 was the most active dendrimer, killing 90% of S. epidermidis isolates at 30 μM and 60% of S. aureus strains at 30 μM. Unfortunately, due to its IC50 values in the range of 3.18–6.91 μM against the eukaryotic cells analyzed, its use for clinical applications must be excluded. PPI-100%mG4 was practically inactive against all the tested pathogens, while PPI-25%mG4 proved good activity against S. aureus, at concentrations that allowed high viability maintenance of all eukaryotic cell lines essayed.Ds, due to a high density of exterior functional groups available for nitric oxide (NO) loading, were particularly attractive for preparing (NO)-releasing Ds characterized by large NO payloads and antibacterial activities against a wide range of pathogenic bacteria, including P. aeruginosa and S. aureus. The antibacterial activity of a library of (NO)-releasing PPI Ds was evaluated against Gram-positive and Gram-negative isolates, including methicillin-resistant S. aureus (MRSA) by Sun et al. (2012)[35] and it was compared with that of analogous PPI Ds, non-NO-releasing, taken as control. From the results, the NO-releasing Ds displayed both enhanced biocidal action directly proportional to Ds size (in terms of MW) and reduced toxicity against mammalian fibroblast cells. In addition, minimal toxicity against fibroblasts and the strongest biocidal activity (≥99.999% killing) was exerted by NO-releasing PPI Ds modified with styrene oxide (SO). The MBC values for each bacterial strain were determined in a non-conventional way, i.e., as the concentration of Ds that caused either a 3 or 5 log reduction in cell viability (compared to untreated cells for a particular bacterial strain) after 2 h. Concerning the cytotoxic effects against L929 fibroblasts cell lines, a very low cell viability (<20%) was observed when cells were exposed to concentrations of all G5-PPI Ds necessary to kill bacteria. On the other hand, both G2-PPI-PO and G2-PPI-NH2 exhibited minimal toxicity to fibroblast cells when tested at bactericidal concentrations. When administered at concentrations 2× MBCs, G2-PEG- and SO-modified Ds (namely 3 and 5) still inhibited fibroblast proliferation by approximately 76% and 64% respectively, therefore showing considerable cytotoxicity. Significantly, the toxicity of NO-releasing Ds against L929 fibroblasts was minimal (≥60% viability) when the devices were administered at both the minimum and twice the minimum concentration required to elicit 3-log killing against all tested bacterial strains. In particular, both G2 and G5 NO-releasing PPI-SO Ds, and their precursors, proved to be non-toxic to the fibroblast cells (>80% viability) even at concentrations necessary to induce 5-log killing against all species studied (10 and 1 μM).A library of amphiphilic NO-releasing PAMAM Ds were synthesized by Lu et al. (2013), through a ring-opening reaction between terminal primary amines present on the dendrimer and propylene oxide (PO), 1,2-epoxy-9-decene (ED) or different ratios of the two, followed by reaction with NO, in order to achieve the NO-releasing derivatives [36]. The hydrophobicity of non-NO-releasing and of NO-releasing Ds was tuned by varying the ratio of the external functionalization PO/ED. The bactericidal efficacy of the NO-releasing vehicles obtained was evaluated against planktonic Gram-negative P. aeruginosa strains and established P. aeruginosa biofilm. The results were correlated with the Ds exterior hydrophobicity (i.e., ratio of PO/ED), their size (i.e., generation), NO release and was compared to that one of non-NO-releasing compounds with analogous composition, taken as controls. It was observed that both the size and the exterior functionalization of Ds were pivotal factors influencing several parameters affecting the antibacterial activity such as dendrimer-bacteria electrostatic interactions and the capability to disrupt bacterial membranes, the efficacy to deliver NO, their migration within the biofilm, and their toxicity against mammalian cells. All the NO-releasing Ds analyzed displayed higher antimicrobial activity than the non-NO-releasing Ds. In more details, the first-generation (G1) PO-Ds were ineffective both against planktonic cells and biofilm cells of P. aeruginosa, while the G1 ED-Ds proved to have a potent antimicrobial activity against the same planktonic cells (MBC = 5 μg/mL), associated with low toxicity against L929 mouse fibroblast cells, and good activity against the biofilm cells (MBC = 15 μg/mL), unfortunately associated with considerable toxicity to L929 mouse fibroblast cells (cell viability < 40%). The optimal PO to ED ratios for biofilm eradication with minimal toxicity against L929 mouse fibroblast cells were 5:5 (MBC = 20 μg/mL, cell viability in the range 90–100%). The study by Lu et al. demonstrated the importance of both the D size and its external functionalization (PO/ED) in determining the efficacy of the devices against established biofilms without compromising their biocompatibility with mammalian cells. In the field of NO-releasing Ds, Worley et al. (2014) [37] described the synthesis of NO-releasing quaternary ammonium (QA)-functionalized generation 1 (G1) and generation 4 (G4) PAMAM Ds, where QA groups were supported by different alkyl chain lengths (i.e., methyl, butyl, octyl, dodecyl) via a ring-opening reaction. The secondary amines resulting from these reactions were further modified with N-diazeniumdiolate NO donors to achieve the NO-releasing QA-modified PAMAM Ds, capable of spontaneous NO release. Differently from non-NO-releasing QA-modified PAMAM Ds, the NO-releasing ones can exert an antibacterial dual action. In detail, the highly cationic structure of the macromolecules can inactivate the pathogens by disrupting their membranes, furthermore the release of NO can disarm bacteria, by causing oxidative and nitrosative stresses, by promoting the production of reactive NO byproducts [(N2O3), peroxynitrite (ONOO−)], by causing membrane destruction via peroxynitrite-induced lipid peroxidation and by triggering protein S-nitrosation and DNA deamination. In this regard, the antibacterial activity of the NO-releasing QA-modified PAMAM Ds was evaluated against the Gram-positive S. aureus and the Gram-negative P. aeruginosa species after four hours of exposure. Overall, the bactericidal activity of the devices was found to be influenced by dendrimer generation, QA alkyl chain length, and bacterial Gram class. The presence of shorter alkyl chains conferred an increased bactericidal activity, particularly against P. aeruginosa, for both generations, with NO-releasing Ds resulting markedly more potent in killing bacteria. The toxicity of non-NO-releasing Ds and of NO-releasing Ds on L929 fibroblasts was minimal (about 60–100% and 80–110% viability respectively) when administered at the MBC concentrations, but a lower toxicity was observed for NO-releasing Ds, if compared to the non-NO-releasing ones.NO-releasing scaffolds, including Ds, were developed by Backlund et al. (2014) and were evaluated as alternatives to current treatments for periodontitis (e.g., scaling/root planning and chlorhexidine), which are affected by limited efficacy, since they fail to suppress microbial biofilms satisfactorily over time, and since the use of adjunctive antimicrobials, in those conditions, can promote the emergence of antibiotic-resistant organisms [38]. The engineered NO-releasing G1-PAMAM-PO-Ds demonstrated a 3-log reduction in the growth of periodontal pathogenic bacteria such as Actinomycetemcomitans and Porphyromonas gingivalis (MBC = 2000 and 1000 μg/mL). Streptococcus mutans and Streptococcus sanguinis, typical caries-associated organisms, were, on the contrary, substantially less sensitive to NO treatment (MBC = 48,000 μg/mL). NO-releasing Ds showed less toxicity against human gingival fibroblasts at concentrations needed to eradicate periodontal pathogens than clinical chlorhexidine concentrations. Although the authors considered these results promising and suggested NO-release dendrimer scaffolds as novel platforms for the development of periodontal disease therapeutics, in our opinion, the MBC values observed are too high to make these structures usable in clinical practice.Later, Backlund and colleagues prepared generation 1 (G1) propyl-, butyl-, hexyl-, octyl-, and dodecyl-functionalized PAMAM-D core scaffolds, subsequently converted to N-diazeniumdiolate NO donors. The killing effect of hydrophobic G1 NO-releasing Ds on S. mutans and their capability to disperse its biofilm were examined at pH 7.4 and 6.4, the latter being a value promoting the dental caries process [39]. The bactericidal action of the NO-releasing Ds against both planktonic and biofilm-producing strains of S. mutans was shown to be greater by increasing the alkyl chain length and lowering the pH (pH = 6.4). The authors hypothesized that the improvement in bactericidal efficacy at pH 6.4 was attributed to both increased surface positive charge of the scaffold, responsible for a better dendrimer-bacteria interaction, resulting in membrane damage, and to a faster NO-release kinetics from proton-labile N-diazeniumdiolate NO donors. Specifically, only octyl- and dodecyl-modified PAMAM Ds were actually effective in eradicating planktonic S. mutans cells (MBC values in the range of 12–25 μg/mL), but were ineffective in eradicating S. mutans biofilm cells (MBC values in the range of 1000–2000 μg/mL). In addition, even if NO-release Ds showed mitigated cytotoxicity on HGF-1 human gingival fibroblasts, compared with non-NO-releasing Ds, the viability of the cells at the concentrations required for biofilm eradication was in the range of 15–20%. These findings established that octyl- and dodecyl-modified PAMAM Ds can be taken into consideration for clinical application with the aim of killing planktonic forms of S. mutans strains, but too toxic to counteract its biofilm structure. The following year, alkyl-modified NO-releasing PAMAM Ds, from first to fourth generation, comprising butyl and hexyl chains, and non-NO-releasing analogs, similar to the PAMAM Ds developed by Backlund et al. (2014), were prepared by Worley et al.[40]. They were evaluated for their bactericidal activity within 24 h against biofilms produced by Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains, assuming that the antibiofilm abilities of the alkyl chain modified NO-releasing and non-NO-releasing Ds could be improved by increasing the size of the Ds and the density of the functional groups. The results showed that the antibiofilm action of the Ds was dependent on their generation, the bacterial species, and the length of the alkyl chain of the devices. In particular, the more effective eradication of the biofilm was associated with the greater infiltration capacity of the Ds into the biofilm biomass. In this regard, the introduction of the ability to release NO has significantly improved the antibiofilm activity of those Ds incapable of effective biofilm penetration. In particular, G1 butyl Ds were practically ineffective against planktonic cells of P. aeruginosa, S. aureus and against MRSA strains, while G1/G4-butyl/hexyl non-NO-releasing Ds and the analogous NO-releasing devices showed, against P. aeruginosa, MBC values in the range of 10–50 μg/mL and of 25–50 μg/mL respectively. The G3-hexyl non-NO-releasing D was recorded as the most active one (MBC value = 10 μg/mL). The same non-NO-releasing and NO-releasing Ds that showed activity against P. aeruginosa were practically ineffective against S. aureus and MRSA strains, with the most active one being the G3-hexyl non-NO-releasing D, which showed an MBC value of 50 μg/mL, against both S. aureus and MRSA strains. Minimum biofilm eradication concentrations after 24 h of exposure (MBEC24h) against biofilm produced by P. aeruginosa, S. aureus, and MRSA isolates were very high for all the tested Ds and, although in general the NO-releasing Ds were better than the non-NO-releasing ones, the most active Ds were the G3 hexyl (MBEC24h = 200, 100, 100 μg/mL against the three species respectively) and the G3 hexyl/NO ones (MBEC24h = 100, 100, 100 μg/mL against the three species respectively). Interestingly, the best performant G3 hexyl Ds, against both planktonic cells and biofilm, showed in in vitro cytotoxicity essay on L929 mouse fibroblasts, an IC50 of 450 μg/mL, either for non-NO-releasing and for NO-releasing compounds, thus establishing a low level of cytotoxicity at both MBC and MBEC24h concentrations.In the search for new alternatives to overcome bacterial drug resistance, an appealing option could be the application of bacteriophage enzymes, such as endolysins, which are able to degrade bacterial peptidoglycan (PG), and to lead to bacterial cell lysis. Starting from this approach, and in order to help endolysins to gain access to PG, Ciepluch et al. (2019) recently combined the endolysins produced by phage KP27 with unmodified and peripheral modified 20% maltose containing PPI Ds, known for their capacity to destabilize the bacterial OM [41]. The antibacterial activities of mixtures containing the modified PPI Ds and the endolysins (12 μM) in a molar ratio of 1/1 and ¼, were essayed against P. aeruginosa PAO1 (ATCC 15692) wild-type and mutant strains with reduced antigen O in LPS. The results were compared to those obtained by the administration of endolysins 12 μM or PPI Ds (12 μM and 50 μM), administered separately. The findings showed that the co-administration of endolysins and maltose-modified PPI D in ratio of 1/1 (the combination used contained a concentration of the dendrimer not toxic to eukaryotic cells), significantly enhanced the antibacterial activity of the enzyme (40–50%) against P. aeruginosa PAO1 and WAAL strains.By divergent growth method, Gholami et al. (2017) synthesized a high MW (116,493 g/mol) G7 PAMAM-D owing 512 positively charged amine groups, thus making the most potent broad-spectrum CAD synthesized to date, endowed with low levels of cytotoxicity on two types of eukaryotic cell lines. Its antibacterial behavior was evaluated on several species such as P. aeruginosa (n = 15), E. coli (n = 15), A. baumannii (n = 15), S. dysenteriae (n = 15), K. pneumoniae (n = 10), P. mirabilis (n = 15), S. aureus (n = 15) and B. subtilis (n = 10). Additionally, representative standard strains for each species were included. G7-PAMAM-D inhibited the growth of both Gram-positive and Gram-negative pathogens, displaying MIC50 and MIC90 values in the range of 2–4 μg/mL and 4–8 μg/mL, respectively, corresponding to very low micromolar concentrations (0.017–0.034 and 0.034–0.068 μM, respectively). MBC50 and MBC90 values were found to be 64–256 μg/mL and 128–256 μg/mL respectively, corresponding to micromolar concentrations of 0.55–2.2 and 1.1–2.2 μM, respectively. The cytotoxicity of G7-PAMAM was evaluated by MTT assay on human intestinal cancer cell lines HCT116 and NIH 3 T3, observing a reduction in viability of 44.6% and 43% respectively at the highest concentration tested (0.85 μM), after 72 h of exposition. It should be kept in mind that cytotoxicity data do not provide information concerning hemolytic toxicity, deriving from a non-specific disruptive action on RBC membranes, which is one of the biggest concerns associated with the possible clinical use of highly cationic and typically membrane-active antimicrobial agents. Based on such considerations, further investigations on the hemolytic toxicity of G7-PAMAM are mandatory to consider it as a promising antimicrobial alternative to conventional antibiotics.To achieve antimicrobial coatings able to prevent bacterial adhesion, the Zhan’s group (2015) successfully prepared hyaluronic acid/PAMAM dendrimer (HA/PAMAM-D) multilayers on a poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-4HB)] substrate by a layer-by-layer self-assembly method[42]. By using QCM-D, the authors showed that both the HA outer layer and the PAMAM-D outer layer revealed anti-adhesion activity against E. coli. Otherwise, by using a live/dead assay, it was observed that while the PAMAM-D outer layer could also exhibit bactericidal activity against E. coli, the outer layer of HA had no such activity. HA/PAMAM-D coatings were able to maintain the antibacterial and anti-adhesion activity after storage in phosphate-buffered saline for up to 14 days. Moreover, the in vitro MTT assay showed that the multilayers were not cytotoxic against L929 cells, and that HA molecules in the multilayers could also improve the biocompatibility of the film.In previous studies, with the aim of obtaining a bacteriophobic coating to be applied on cotton fabric, cationic PAMAM-chitosan (CTS) dendrimers (PAMAM-CTS-Ds) were prepared by Klaykruayat et al. (2010) [43]. Briefly, a quaternary ammonium hyperbranched dendritic PAMAM of generation 2.5, customized by post-synthesis methylation of a methyl ester terminated PAMAM-D, was employed to modify flake CTS, and the cationic PAMAM-CTS-D obtained was applied to cotton fabric at 1% w/w, using a padding method. The antimicrobial performance of the newly modified fabric was assessed against S. aureus and compared to a similar fabric obtained by applying unmodified CTS. Furthermore, the antimicrobial activity of not neutralized CTS films and of cationic PAMAM-CTS-D films was also evaluated. The antimicrobial evaluations were preliminarily carried out in terms of visual detection and reduction of the percentage of microbial growth, using a single application dose. The 1% w/w CTS-impregnated fabric, treated with NaNO2, according to a necessary procedure, showed no antimicrobial activity, thus indicating that CTS itself was not sufficient to inhibit the growth of S. aureus, when applied on fabric. Nonetheless, native CTS film (0.33 g) showed an excellent antimicrobial activity (100% reduction of the growth of S. aureus), due to the positively charged CTS amine groups. The cationic PAMAM-CTS-D film (0.25 g), also exhibited a strong antimicrobial activity against S. aureus (99.99% reduction), comparable to that of the native CTS film. Significantly, in contrast to cotton fabric coated with CTS alone, cotton fabric treated with cationic PAMAM-CTS-D (1% w/w) displayed an excellent antimicrobial activity (98.75% reduction of the growth of the pathogen). Although cationic PAMAM Ds could potentially enhance the performance of CTS as an antimicrobial coating for cotton fabric, probably due to their high cationic character, the preliminary findings highlighted by Klaykruayat et al. require further characterization and verification on other bacterial species. Similarly, more investigations are needed regarding the dose-dependence of the effect observed within the fabric, including its resistance to multiple wash cycles.
References
- Schito, A.M.; Alfei, S. Antibacterial activity of non-cytotoxic, amino acid-modified polycationic dendrimers against Pseudomonas aeruginosa and other non-fermenting Gram-negative bacteria. Polymers 2020, 12, 1818.
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting Dendrimer Multivalency To Combat Emerging and Re-Emerging Infectious Diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hooper, D.C. Hospital-Acquired Infections Due to Gram-Negative Bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- WHO. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Antibacterial Agents in Preclinical Development: An Open Access Database; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Alfei, S.; Signorello, M.G.; Schito, A.; Catena, S.; Turrini, F. Reshaped as polyester-based nanoparticles, gallic acid inhibits platelet aggregation, reactive oxygen species production and multi-resistant Gram-positive bacteria with an efficiency never obtained. Nanoscale Adv. 2019, 1, 4148–4157. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress.”. Drug Deliv. Transl. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Castonguay, A.; Ladd, E.; van de Ven, T.G.M.; Kakkar, A. Dendrimers as bactericides. New J. Chem. 2012, 36, 199–204. [Google Scholar] [CrossRef]
- Bahar, A.A.; Liu, Z.; Totsingan, F.; Buitrago, C.; Kallenbach, N.; Ren, D. Synthetic dendrimeric peptide active against biofilm and persister cells of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2015, 99, 8125–8135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qu, H.; Ma, M.; Xu, Z.; Xu, P.; Fang, Y.; Xu, T. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur. J. Med. Chem. 2007, 42, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Wronska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic effects of anionic/cationic dendrimers and levofloxacin on antibacterial activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef] [PubMed]
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM Dendrimer–PEG Hydrogels for the Treatment of Genital Infections: Formulation and in Vitro and in Vivo Evaluation. Mol. Pharm. 2011, 8, 1209–1223. [Google Scholar] [CrossRef]
- Stenström, P.; Hjorth, E.; Zhang, Y.; Andrén, O.C.J.; Guette-Marquet, S.; Schultzberg, M.; Malkoch, M. Synthesis and in Vitro Evaluation of Monodisperse Amino-Functional Polyester Dendrimers with Rapid Degradability and Antibacterial Properties. Biomacromolecules 2017, 18, 4323–4330. [Google Scholar] [CrossRef]
- Quadir, M.A.; Haag, R. Biofunctional nanosystems based on dendritic polymers. J. Control. Release 2012, 161, 484–495. [Google Scholar] [CrossRef]
- Xue, X.; Chen, X.; Mao, X.; Hou, Z.; Zhou, Y.; Bai, H.; Meng, J.; Da, F.; Sang, G.; Wang, Y.; et al. Amino-Terminated Generation 2 Poly(amidoamine) Dendrimer as a Potential Broad-Spectrum, Nonresistance-Inducing Antibacterial Agent. AAPS J. 2013, 15, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Akbari Dourbash, F.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, 395. [Google Scholar] [CrossRef]
- Yang, H.; Lopina, S.T. Penicillin V-conjugated PEG-PAMAM star polymers. J. Biomater. Sci. Polym. Ed. 2003, 14, 1043–1056. [Google Scholar] [CrossRef]
- Chen, C.Z.; Tan, N.C.B.; Cooper, S.L. Incorporation of dimethyldodecylammonium chloride functionalities onto poly(propylene imine) dendrimers significantly enhances their antimicrobial properties. Chem. Commun. 1999, 1585–1586. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Kannan, R.; Prabakaran, P.; Basu, R.; Pindi, C.; Senapati, S.; Muthuvijayan, V.; Prasad, E. Mechanistic Study on the Antibacterial Activity of Self-Assembled Poly(aryl ether)-Based Amphiphilic Dendrimers. ACS Appl. Bio Mater. 2019, 2, 3212–3224. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kumar, P.V.; Koushik, O.S. Dendrimeric Biocides—A Tool for Effective Antimicrobial Therapy. J. Nanomed. Nanotechnol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Avval, M.M.; Murthy, S.V.; Shashikanth, S. Synthesis and antimicrobial activity evaluation of poly ethylene imine (PEI) dendrimer modified with 1,2,4-oxadiazole derivatives. Int. J. Chem. Pharm. Sci. 2014, 2, 678–683. [Google Scholar]
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Young, A.W.; Liu, Z.; Zhou, C.; Totsingan, F.; Jiwrajka, N.; Shi, Z.; Kallenbach, N.R. Structure and antimicrobial properties of multivalent short peptides. Med. Chem. Comm. 2011, 2, 308–314. [Google Scholar] [CrossRef]
- Pakrudheen, I.; Banu, A.N.; Murugan, E. Cationic amphiphilic dendrimers with tunable hydrophobicity show in vitro activity. Environ. Chem. Lett. 2018, 16, 1513–1519. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 100, 215–221. [Google Scholar] [CrossRef]
- Wang, L.; Erasquin, U.J.; Zhao, M.; Ren, L.; Zhang, M.Y.; Cheng, G.J.; Wang, Y.; Cai, C. Stability, antimicrobial activity, and cytotoxicity of poly (amidoamine) dendrimers on titanium substrates. ACS Appl. Mat. Interfaces 2011, 3, 2885–2894. [Google Scholar] [CrossRef]
- Felczak, A.; Wronska, N.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Appelhans, D.; Voit, B.; Rozalska, S.; Lisowska, K. Antimicrobial activity of poly(propylene imine) dendrimers. New J. Chem. 2012, 36, 2215–2222. [Google Scholar] [CrossRef]
- Sun, B.; Slomberg, D.L.; Chudasama, S.L.; Lu, Y.; Schoenfisch, M.H. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules 2012, 13, 3343–3354. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Shah, A.; Schoenfisch, M.H. Nitric oxide-releasing amphiphilic poly (amidoamine) (PAMAM) dendrimers as antibacterial agents. Biomacromolecules 2013, 14, 3589–3598. [Google Scholar] [CrossRef]
- Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing quaternary ammonium-modified poly (amidoamine) dendrimers as dual action antibacterial agents. Bioconj. Chem. 2014, 25, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Sergesketter, A.R.; Offenbacher, S.; Schoenfisch, M.H. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J. Dent. Res. 2014, 93, 1089–1094. [Google Scholar] [CrossRef]
- Backlund, C.J.; Worley, B.V.; Schoenfisch, M.H. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly-(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.V.; Schilly, K.M.; Schoenfisch, M.H. Anti-biofilm efficacy of dual-action nitric oxide-releasing alkyl chain modified poly (amidoamine) dendrimers. Mol. Pharm. 2015, 12, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Ciepluch, K.; Maciejewska, B.; Gałczyńska, K.; Kuc-Ciepluch, D.; Bryszewska, M.; Appelhans, D.; Drulis-Kawa, Z.; Arabski, M. The influence of cationic dendrimers on antibacterial activity of phage endolysin against P. aeruginosa cells. Biorgan. Chem. 2019, 91, 103121. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, L.; Liu, S.; Chen, J.; Ren, L.; Wang, Y. Antimicrobial Hyaluronic Acid/Poly(amidoamine) Dendrimer Multilayer on Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Prepared by a Layer-by-Layer Self-Assembly Method. ACS Appl. Mater. Interfaces 2015, 7, 13876–13881. [Google Scholar] [CrossRef]
- Klaykruayat, B.; Siralertmukul, K.; Srikulkit, K. Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr. Polym. 2010, 80, 197–207. [Google Scholar] [CrossRef]