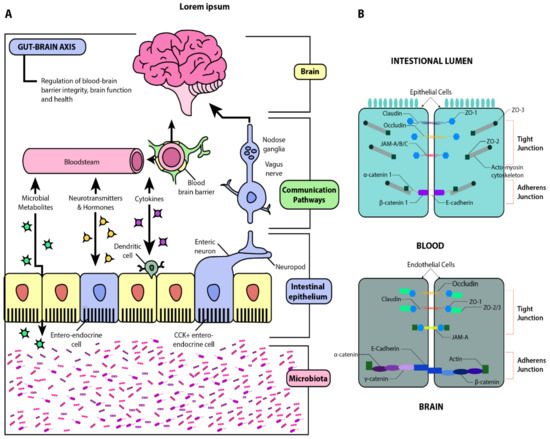
Gut microbiota plays a role in modulating complex signaling pathways, predominantly via the bidirectional gut-brain-axis (GBA).
1. Introduction
Autism spectrum disorders (ASDs) are characterized by a multifaceted range of neurobiological disorders with functional deficits in both social communication and cognitive domains as well as behavior abnormalities [1]. While numerous genetic and environmental factors are implicated in the complex pathophysiology of ASD and abnormal behavior manifestations, recent investigations have suggested that there may be a causal role of the gut microbiome [2,3].
The microbiome bi-directionally modulates the gut-brain-axis (GBA) through complex neuroendocrine, neuro-immune, and autonomic nervous signaling mechanisms [4] ().
Figure 1. Schematic illustration of the gut-brain axis (GBA). Panel (A): Beginning with the microbiota, this diagram illustrates the interactions between the gut and the brain via microbial metabolites, cytokines, neurotransmitters, and hormones. The gut-brain humoral connections which intersect at the blood-brain barrier and the neuronal connections by way of the enteric nervous system and vagus nerve are also illustrated. Through these pathways, dysbiosis and increased barrier permeability may affect neural development, maturation, and function. Panel (B): Structural similarities between the blood-brain barrier (BBB) and the intestinal epithelial barrier. Adapted from [5,6].
Furthermore, emerging studies have suggested an association between impaired gut microbiota and disrupted neurobiological development [4,5,6,7,8]. Clinical investigations of gut dysbiosis indicate a high coincidence of gastrointestinal (GI) symptoms and compositional changes within the gut microbiome in individuals with ASD [7,8]. Subsequent studies suggest that the degree of GI symptomatology, including constipation and diarrhea, may correlate with the severity of ASD [9,10].
Gut dysbiosis is also implicated in initiating systemic inflammation and neurological dysfunction [11,12]. Clinically, this bidirectional inflammation has been linked to dysregulation of neurobiology and neuroimmunology, which parallels the pathogenesis of GI symptomology in patients with ASD [11,12,13,14]. Recent studies have indicated microbiome-induced inflammation’s potential to alter the blood-brain barrier (BBB) permeability [5,15,16,17,18]. This review will discuss the predominant mediators of blood-brain barrier integrity and the signaling mechanisms by which they influence neurobiological development. A clear understanding of the neurobiochemical underpinnings will enable researchers to better understand aberrant neurodevelopmental mechanisms, such as in ASD. These mechanisms have significant implications for the development of novel therapies targeting specific dysfunctions in the GBA. Such therapeutics, if administered during critical periods of neurodevelopment, may have strong potential to minimize, prevent, and possibly reverse the neurocognitive deficits observed in ASD patients.
"Gut-induced Inflammation'' refers to a systemic inflammatory condition that was triggered by an imbalanced gut microbiota, referred to as gut dysbiosis. The proposed mechanism is that an imbalance in the gut microbiome composition will cause the epithelial barrier of the gut lining to become compromised and give rise to microbial translocation from the gut lumen into the bloodstream which then sets off an inflammatory process."
2. Gut-Brain-Axis
The gut-brain axis (GBA), also known as the microbiome-gut-brain axis, describes the complex bidirectional communication between the central nervous system (CNS) and enteric nervous system (ENS), which is highly modulated by gut microbiota (). The multifaceted interactions of the GBA include linkage of peripheral intestinal functions with emotional and cognitive brain regions such as the hypothalamus, limbic system, and prefrontal cortex [
19,
20]. Physiologically, the GBA helps maintain gastrointestinal homeostasis and mediates neuro-immune-endocrine communication—notably, immune activation, intestinal permeability, entero-endocrine signaling, and enteric reflexes [
21]. Recent advances in our understanding of the GBA have also demonstrated the contributory role of gut microbiota on motivation and higher cognitive functioning [
11].
These bidirectional interactions occur via a complex integrated signaling pathway between the CNS, enteric nervous system (ENS), autonomic nervous system (ANS), and hypothalamic-pituitary-adrenal (HPA) axis [
12]. The ANS, which is comprised of sympathetic and parasympathetic branches, regulates both afferent and efferent communication between the CNS and the intestinal tract [
22]. The gut microbiome has been found to actively interact with the sympathetic nervous system extrinsic to the gut [
23]. Recent experiments have shown that microbial depletion in the gut leads to increased expression of cFos, a marker of neuronal activity and circuits; colonization of germ-free (GF) mice with gut microflora suppressed this pathway via short-chain fatty acid (SCFA) action in the gut-associated sympathetic ganglia [
24]. Moreover, it is also hypothesized that there is a more direct, bidirectional communication between the gut and the brain via vagal nerves. Retrograde neuronal tracing has identified links from the intestinal wall to specific brainstem nuclei, which are activated after microbial depletion, and has identified efferent links from sympathetic glutamatergic neurons in the brain which regulate GI function [
24]. These pathways provide evidence for a functionally relevant bi-directional link between the gut and the brain. The brainstem nuclei are the nuclei in the brainstem that include cranial nerve nuclei, red nucleus, and substantia nigra.
The HPA axis predominantly oversees the body’s response to emotional or physiological stress [
25]. Moreover, as an integral component of the limbic system, the HPA axis ultimately regulates the release of cortisol from the adrenal glands. Cortisol, the body’s primary stress hormone, plays a pivotal role in the systemic effects of the body’s adaptive response to stress, including effects on the gut microbiome [
13]. Although the precise mechanism is not fully understood, cortisol is a potent regulator of immune function and has been demonstrated to induce pathologic shifts in gut microbiome composition and increase intestinal permeability [
26,
27]. Through parallel signaling pathways like these, reciprocal hormonal signaling between the CNS and ENS allows both the brain and endogenous gut microbiota to modulate enteric function [
11].
3. Blood-Brain Barrier
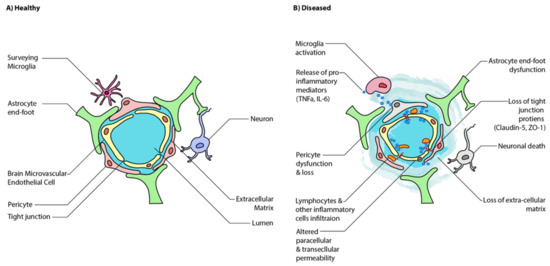
While the neuronal interplay between GBA is increasingly established, the gut may also affect the BBB’s integrity and alter functioning in the CNS itself. The BBB is formed by the tightly packed endothelial lining of the capillaries that supply the brain (). It is designed to protect the brain from pathogens and to maintain homeostasis by preventing substances from diffusing freely into the brain. Passage across this barrier is determined by solubility across the lipid bilayer and recognition by specific transport molecules. The proteins sealing the gaps between endothelial cells are critical to this strict regulation, namely claudin-5,-11,-12,-25, and zonulin-1 () [
28,
29]. Interestingly, these same molecules are found in the intestinal epithelial barrier, where they mediate intestinal permeability [
30]. Under disease state, BBB has increased translocation of inflammatory mediators, immune cells, and microglial activation, which further increases BBB permeability (). These findings have been observed in human ASD brain samples where there is increased neuroinflammation coupled with an alteration in the expression of genes associated with BBB integrity and a corresponding decrease in gut barrier integrity [
31].
Figure 2. Schematic illustration of healthy and disease state blood-brain barrier (BBB) epithelium. (
A) A healthy BBB is tightly sealed by the tight junctions of endothelial cells and astrocytes. (
B) A disease state BBB has increased translocation of inflammatory mediators, immune cells, and microglial activation, which further increases BBB permeability. Ultimately, this results in neuroinflammation and neuronal cell death. Adapted from [
5].
The body’s immune system is critical for the proper development of the BBB. For example, the maternal gut microbiota plays a role in regulating the fetal BBB in the womb by upregulating the expression of proteins like claudin-5 [
15]. This is an especially interesting finding considering many population-based studies have found an increased risk associated with maternal antibiotic use in the third trimester, particularly with penicillin [
32,
33]. This is supported by a murine study that found that low-dose penicillin in late pregnancy and early postnatal life increased long-term adverse effects in the offspring of the mice by increasing cytokine expression in the frontal cortex, modifying the blood-brain barrier integrity, and altering behavior [
34].
Viral infections, often implicated in the pathogenesis of ASD, have also been associated with altering the BBB permeability. For example, HSV, HTLV-1, Rabies virus, West Nile virus, and Lymphocytic choriomeningitis virus (LCMV) are known to gain access to the brain. However, for many viruses, the precise molecular mechanisms underlying their entry and the extent of BBB disruption are unknown. Nevertheless, the principles behind viral entry and disruption of the BBB may be similar. The most studied virus in this regard is HIV-1. HIV-1 induces pathways that increase reactive oxygen species (ROS), upregulate inflammatory cytokines, proteasomal degradation of tight junction proteins within cells, and increases expression of matrix metallopeptidases (MMPs), which are key mediators of BBB dysfunction that act by degrading the extracellular matrix (ECM) and other protein-based molecules [
35]. Furthermore, many viruses disrupt the actin cytoskeleton, which is essential for tight junction and BBB function [
36]. In addition to disruption mediated directly in the barrier cells, viruses like rabies and LCMV induce CD8
+ and CD4
+ T cell dependent permeability [
37,
38]. Indeed, Epstein-Barr virus and Herpes Simplex virus 2, viruses identified as risk factors for ASD, can directly infect human brain endothelial cells [
39,
40]. Finally, it is worth mentioning that besides viruses and bacteria, many well-known parasites breach the BBB through similar mechanisms, including
Toxoplasma gondii and
Plasmodium spp., although associations have not been found between
Toxoplasma gondii antibodies and ASD [
35,
39].
Regardless of the virus and its mechanisms, as the virus promotes inflammation and disrupts tight junctions, it allows cytokines, leukocytes like monocytes, macrophages, and T cells to enter the brain and accelerate disease progression [
28]. Even if the viruses do not enter the brain, they can still activate an immune response from the microglia that may initiate neuroinflammation or encephalitis [
41,
42,
43].
Additionally, it is interesting to note that endothelial and epithelial tight junctions have many similar features and share many of the same core claudins and occludins with varying degrees of permeability and are subject to the same mechanisms of disruption. This deepens the gut-brain connection by broaching the concept that common viral gastroenterophathies might also induce subclinical BBB permeability.
4. Gut-Brain Connection
The cognitive effects of ASD can also be understood via the gut-brain axis association with the autonomic nervous system (ANS). A recent study by Kong et al. found that measures of autonomic function, gut microbiome markers, and autism behaviors, assessed by the Autism Treatment Evaluation Checklist (ATEC), were significantly associated with each other [47]. Specifically, alpha diversity was negatively correlated with ATEC total score, as well as the Sensory/Cognitive Awareness and Speech/Language subsections (higher ATEC scores indicate more severe symptoms). Additionally, other autonomic function markers such as body temperature and blood volume pulse (BVP) were significantly associated with ATEC scores. Moreover, these same autonomic indices correlated with changes in the gut microbiome, for example, a positive correlation between BVP and firmicutes/bacteroidetes ratio in all subjects. Importantly, although this study attempted to develop predictive models using these indices and ATEC scores, their models were not statistically significant [47].
Many specific diets have also been frequently hypothesized to play a beneficial role in improving cognition in individuals with ASD. For example, iron deficiency has been implicated in ASD’s adverse cognitive effects, given that it is frequently deficient in those with ASD and its importance in motor, behavioral, and cognitive development [48]. Moreover, it has been seen that ketogenic diets, gluten-free and casein-free diets, as well as increased intake of folic acid and vitamin D are beneficial in improving social, communicative, cognitive, and motor skills in children with ASD. However, it is vital to note that some of these studies contradict each other and refute their findings [49]. Moreover, it does not appear that any of these interventions are decisive factors in the development or progression of ASD. Rather, they positively contribute to beneficial trends within disease development, progression, and therapy. Future studies should make active efforts to design larger, randomized, blinded, and controlled trials to delineate the precise mechanisms of these diet’s effect [49].
This entry is adapted from the peer-reviewed paper 10.3390/jcm10010027