This entry summarizes the structural characteristics and magnetic properties of trinuclear complexes containing the NiII-LnIII-NiII moiety. The ligands used are mainly polydentate Schiff base ligands and reduced Schiff base ligands and, in some cases, oximato, β-diketonato, pyridyl ketone ligands and others. The compounds reported are restricted to those containing one, two and three oxygen atoms as bridges between the metal ions; examples of carboxylato and oximato bridging are also included due to structural similarity. The magnetic properties of the complexes range from ferro- to antiferromagnetic depending on the nature of the lanthanide ion.
- heterometallic complexes
- trinuclear moiety
- nickel(II)
- lanthanide(III) ions
- crystal structures
- magnetic properties
1. Tripodal Polydentate Schiff Base Ligands and Reduced Schiff Base Ligands
A new phosphorus-supported ligand H3L1 = (S)P[M(Me)N=CH-C6H3-2-OH-3-OMe]3 (Scheme 1), prepared by the condensation of (S)P[M(Me)NH2]3 and o-vanillin, was used to synthesize the trinuclear isomorphous complexes [(NiL1)2Ln](ClO4) (Ln = La-Er, except Pm, 1–11)[1]. The cation consists of three nearly linear metal ions, two terminal NiII and one LnIII in the center (Figure 1). Each of the terminal NiII is bound to the three imino nitrogen and the three phenolato oxygen atoms of one (L1)3-, thus describing an in situ formed [NiL1]- metalloligand. Two such metalloligands are bound to the central LnIII ion through the phenolato and methoxy oxygen atoms of the two ligands, describing a distorted icosahedral. The Ln-O bond distances show a gradual reduction in accordance with lanthanide contraction. The Ni2Gd (7), Ni2Dy (9) and Ni2Er (11) complexes display ferromagnetic interactions between the metal ions. The magnetic susceptibility measurements of 7 were interpreted by using the spin Hamiltonian where SNi1 = SNi2 = 1 and SGd = 7/2. The best set of parameters obtained using this model is J/kB = +0.375 cm−1 and g = 2.04. The magnetization measurements of 7 as a function of the field show relatively rapid saturation of the magnetization at high fields and agree with an S = 11/2 spin ground state. Ac susceptibility measurements of the Ni2Dy complex (9) as a function of the temperature at different frequencies and also as a function of the frequency at different temperatures under zero and 3500 Oe dc field showed that 9 exhibits slow relaxation of the magnetization and observation of field induced single-molecule magnet behavior. The data were fitted to an Arrhenius law in order to estimate the energy gap of 10.8 K and pre-exponential factor τ0 = 2.3 × 10−5 s. The value of 10.8 K is in the range observed for similar complexes, however the value of τ0 is much larger than expected suggesting that the quantum pathway of relaxation is only partially suppressed by the applied field of 3500 Oe and hence that the energy gap of the thermally activated relaxation should be higher than 10.8 K. In any case, the magnetic study of 9 suggests SMM behavior generated by the high spin ground state of the complex and the magnetic anisotropy of the DyIII ion. All other complexes behave as simple paramagnetic systems. DFT calculations on the Ni2Gd (7) and Ni2La (1) complexes revealed good agreement between the experimental and computed J values, confirmed the ferro- and antiferromagnetic nature of the JNiGd and JNiNi interactions, respectively and gave information on the spin densities of the metal ions and bridging oxygen atoms[2].
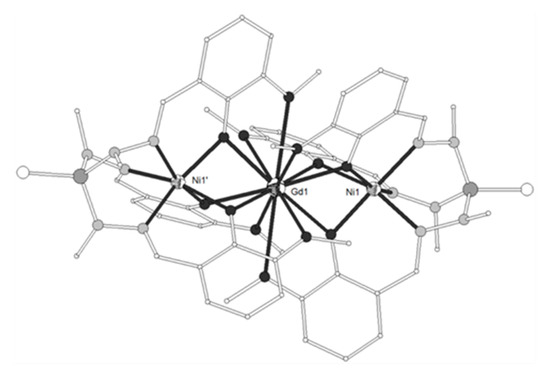
Figure 1. The molecular structure of the cation [(NiL1)2Gd]+ in complex 7. Primed atoms are generated by symmetry: (‘) = -x, y, 1-z. Color code: Gd large octant, Ni small octant, N light grey, O dark grey, C open small, P grey large, S open large[1].
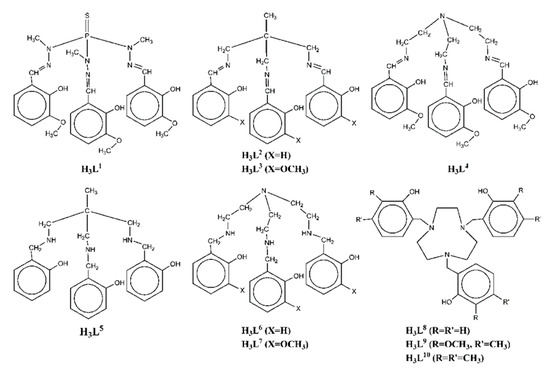
Scheme 1. The tripodal polydentate Schiff base and reduced Schiff base ligands used in the Ni2Ln complexes 1–70.
The tripodal hexadentate Schiff base-phenolate ligand H3L2 = 2,2’-((1E)-((2-(((E)-(2-hydroxybenzylidene)amino)methyl)-2-methylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylid ene))diphenol (Scheme 1) was used to synthesize the neutral trinuclear complexes [(NiL2)2Ln(NO3)] (LnIII = Gd, Eu, Tb, Dy, 12–15)[3][4] which show a Ni-Ln-Ni angle of ca. 140° (Figure 2). All four complexes have similar structures with the two terminal NiII ions coordinated to the three imino nitrogen and three phenolato oxygen atoms of one (L2)3−. The coordination geometry of each NiII ion is distorted from a regular octahedron toward a trigonal prism with trigonal twist angle f ~45° (trigonal prism = 0°, octahedron = 60°). The central LnIII ion is bound to the phenolato oxygen atoms of the ligands and to chelate nitrato ligand. All complexes exhibit 3D structures due to intermolecular π-π and CH-π interactions between neighboring molecules. The magnetic data for the Ni2Gd (12) complex are consistent with ferromagnetic coupling between the metal ions giving rise to a ground state with spin S = 11/2. The best-fit parameters to the experimental magnetic susceptibility data of 12 were g = 2.24, J(Ni-Gd) = +0.19 cm−1 and D = +2.1 cm−1. A ferromagnetic interaction is also suggested for the Ni2Tb (14) and Ni2Dy (15) complexes.
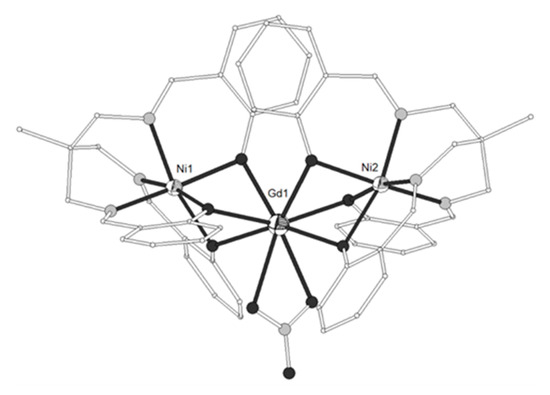
Figure 2. The molecular structure of complex [(NiL2)2Gd(NO3)] (12). Color code: Gd large octant, Ni small octant, N light grey, O dark grey, C open small[3].
The complex [(NiL3)2Gd](NO3) (16) (H3L3 = 6,6’-((1E)-((2-(((E)-(2-hydroxy-3-methoxybenzylidene)amino)methyl)-2-methylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylid ene))bis(2-methoxyphenol), Scheme 1) contains also two [NiL3]- metalloligands bound to a GdIII ion in an almost linear arrangement (~178°)[5]. The asymmetric unit contains two different cationic entities, the first one possesses two slightly different NiII environments, while the second one is symmetry-related through the GdIII ion. The coordination geometry around each NiII ion is distorted octahedral with N3O3 chromophore. The GdIII ion is coordinated to twelve oxygen atoms, deprotonated phenoxo oxygens and neutral methoxy oxygens. The magnetic susceptibility data were interpreted in terms of the spin Hamiltonian considering two equivalent Ni1-Gd and Ni2-Gd exchange interactions J and identical ZFS terms D. The best-fit parameters are JNiGd = 0.91 cm−1, g = 1.98 and D = 4.5 cm−1. The magnetization measurements at 2 K in the range 0–5 T were satisfactorily simulated with this set of parameters and confirmed an S = 11/2 ground state due to ferromagnetic coupling between the metal ions.
The tripodal ligand H3L4 = 6,6’,6’’-((1E,1’E)-((nitrilotris(ethane-2,1-diyl))tris (azanylylidene)) tris(methanylylidene))tris(2-methoxyphenol) (Scheme 1) gave four heterometallic complexes, [(NiL4)2Ln](NO3) (LnIII = Gd, Tb, Dy, 17–19) and [(NiL4)2Dy](ClO4) (20) which contain linear Ni-Ln-Ni moieties (Figure 3)[6]. The GdIII ion exhibits rare seven-coordination to six bridging phenoxo oxygen atoms and one methoxy oxygen atom from one of the (L4)3- ligands and can be considered as an intermediate between the capped trigonal prism (CTPR-7, C2v) and the capped octahedron (CTPR-7, C3v). The LnIII ions in the remaining three complexes exhibit rare six-coordination which can be described as quasi trigonal antiprism (intermediate between octahedron OC-6, Oh and trigonal prism TPR-6 D3h). The magnetic susceptibility measurements of 17 were interpreted by using the spin Hamiltonian and gave the best-fit parameters g = 2.04, J = 0.64 cm−1 and zJ’ = 0.009 cm-1 (R = 6.99 × 10-3). The magnetization at 1.8 K increases upon increasing the magnetic field and reaches a value of 11.87 Nb at 7 T consistent with an S = 11/2 ground spin state. The magnetic study of 18–20 is consistent with ferromagnetic coupling between the metal ions. Ac susceptibility measurements of 18–20 between 1.8–10.0 K and frequencies 3-969 Hz under zero dc field showed temperature- and frequency-dependent out-of-phase peaks for 19 and 20 suggesting SMM behavior. The relaxation time derived from the χ’’ peaks follows the Arrhenius law τ = τ0exp(Δ/kBT) with effective energy barriers of 14.17 K (τ0 = 1.09 × 10-6 s) for 19 and 11.13 K (τ0 = 6.72 × 10−6 s) for 20 under zero dc field. Complexes 19 and 20 constitute rare examples of SMMs containing six-coordinate DyIII ions.
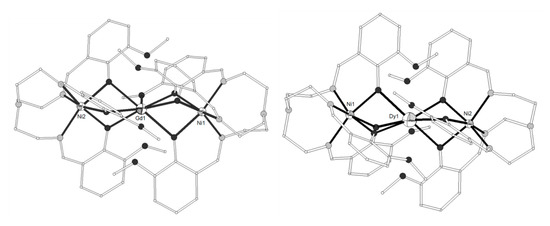
Figure 3. The molecular structures of the cations [(NiL4)2Gd]+ (17, left) and [(NiL4)2Dy]+ (20, right). Color code as in Figure 2[6].
The tripodal hexadentate amine phenol ligand H3L5 = 2,2’-(((2-(((2-hydroxybenzyl)amino)methyl)-2-methylpropane-1,3-diyl)bis(azanediyl))bis(methylen e))diphenol (Scheme 1), which is the reduced form of ligand H3L2, gave a series of isostructural complexes [(NiL5)2Ln(solv)x)](ClO4) (LnIII = La, Pr, Nd, Gd, Dy, Ho, Er, Yb; 21–28; x = 2, H2O/(MeOH)0.5/(EtOH)0.5 in Ni2La, 21; x = 2, H2O/MeOH in Ni2Dy, 25; x = 1, H2O in Ni2Yb, 28)[7]. The complexes consist of two [NiL5]- metalloligands bound around the LnIII ions with bent Ni-Ln-Ni moiety. The LaIII ion in 21 is eight-coordinate to two (L5)3- ligands which are tridentate with respect to the LaIII ion and hexadentate with respect to one NiII ion. Two solvate molecules complete the coordination of the LaIII ion which is described as a D4d square antiprism distorted toward a C2v bicapped octahedron. The DyIII ion is seven-coordinate to two [NiL5]- metalloligands, one bidentate and one tridentate and to two solvate molecules in a capped trigonal prismatic geometry. The YbIII ion is six-coordinate to two [NiL5]- metalloligands with a distorted octahedral geometry. The magnetic studies indicated that antiferromagnetic exchange coupling between the NiII and LnIII ions increases with decreasing size of LnIII.
The tripodal hexadentate amine phenol ligand H3L6 = 2,2’,2’’-(((nitrilotris(ethane-2,1-diyl))tris(azanediyl))tris(methylene))triphenol (Scheme 1) gave two series of isostructural complexes, [(NiL6)2Ln(MeOH)](NO3) (all LnIII except Ce and Pm, 29–41) and [(NiL6)2Ln(MeOH)](ClO4) (LnIII = La, Pr, Nd, Sm, Gd, Dy, Ho, Er, 42–49), which contain a bent Ni-Ln-Ni moiety with angles in the range ~139–144°[8][9]. In all structurally characterized complexes, the LnIII ion is seven-coordinate being bicapped by two tridentate [NiL6]- metalloligands and a methanol molecule in flattened pentagonal bipyramidal geometry. Each NiII ion is encapsulated by a full deprotonated ligand via four amine and two phenolato functions in approximately octahedral geometry. It is recognized that the coordination number of LnIII ions tends to decrease with increased atomic number, that is, as the ionic radius decreases. However, in the present case, the coordination number and geometry of the LnIII ions do not change along the entire Ln series plus LaIII. Magnetic studies indicated that ferromagnetic exchange occurs in the case of Ni2Ln with LnIII = Gd, Tb, Dy, Ho, Er.
The congener ligand H3L7 = 6,6’,6’’-(((nitrilotris(ethane-2,1-diyl))tris(azane diyl))tris(methyl ene))tris(2-methoxyphenol) (Scheme 1) gave two isomorphous complexes [(NiL7)2Ln)](ClO4) (LnIII = Gd, Dy, 50–51) which contain a bent Ni-Ln-Ni moiety with angle ~113°[10]. The three metal ions are arranged in an isosceles triangle manner (Figure 4). The two terminal NiII ions are coordinated to four amine and two phenolate groups, whereas the central LnIII ion is eight-coordinated to six bridging phenolato oxygen atoms and two methoxy oxygen atoms from the ligands presenting distorted dodecahedral geometry. The magnetic studies showed that both complexes exhibit ferromagnetic coupling between the metal ions. The magnetic susceptibility data of 50 were analyzed on the basis of the spin Hamiltonian (J’ is assumed to be zero due to large distance between the NiII ions) and gave J/k = 1.02 cm−1 and g = 2.01. At 2 K, the magnetization is 11.04 Nβ at 5 T which agrees with the saturation value of 11.94 Nβ expected for an S = 11/2 system, confirming the ferromagnetic interaction between the NiII and GdIII ions. The magnetization at 5 T for 51 is 12.65 Nβ and is larger than the theoretical value of 12 Nβ due to the presence of the anisotropic DyIII ion. The Ni2Dy complex 51 exhibited very weak field-induced slow relaxation of magnetization.
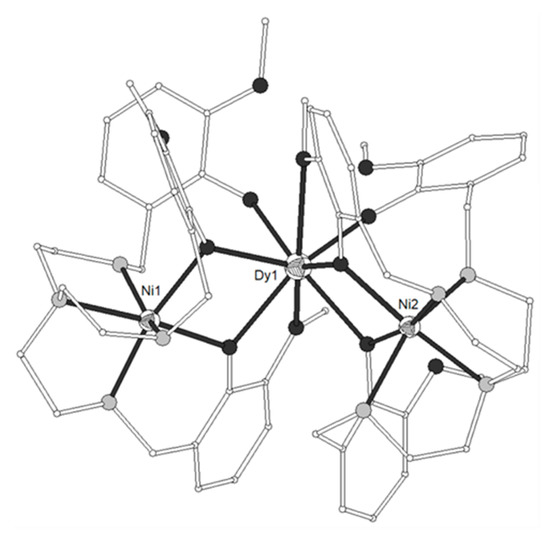
Figure 4. The molecular structure of the cation [(NiL7)2Dy)]+ in complex 51. Color code as in Figure 2[10].
The ligand H3L8 = 2,2’,2’’-((1,4,7-triazonane-1,4,7-triyl)tris(methylene))triphenol (Scheme 1) gave the trinuclear complexes [(NiL8)2Ln(MeCN)2](ClO4) (LnIII = La, Nd, Gd, Dy, Yb, 52–55) and [(NiL8)2Yb](ClO4) (56)[11]. The GdIII ion in 54 is eight-coordinate in square antiprism distorted to C2v bicapped trigonal prism geometry, bicapped by two deprotonated tridentate (NiL8)- metalloligands (Figure 5). Each NiII is distorted octahedral in N3O3 coordination. The Ni-Gd-Ni angle is ~142°. The YbIII ion in 56 is six-coordinated by six bridging phenolate oxygens (Figure 8). The decrease of the coordination number with increasing atomic number is common in LnIII complexes. The complex is linear with Ni-Yb-Ni angle ~176°. Magnetic studies indicated ferromagnetic interactions for LnIII = Gd, Dy, Yb and antiferromagnetic coupling for LnIII = Nd.
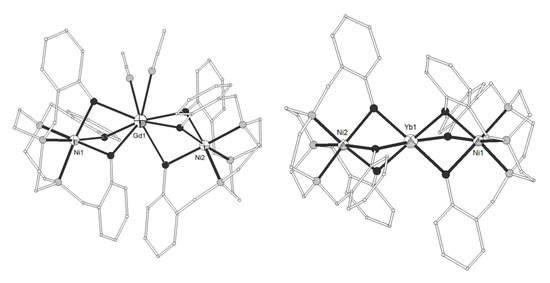
Figure 5. The molecular structures of the cations [(NiL8)2Gd(MeCN)2]+ 54, (left) and [(NiL8)2Yb]+ 56, (right). Color code as in Figure 2[11].
The ligand H3L9 = 6,6’,6’’-((1,4,7-triazonane-1,4,7-triyl)tris(methylene))tris(2-methoxy-3-methylphenol) (Scheme 1) gave a series of 13 linear trinuclear complexes [(NiL9)2Ln(solv)x](ClO4) (LnIII = Y, La, Ce-Lu except Pr, Pm, Yb; x = 1, H2O in Ni2Sm, Ni2Eu, Ni2Tb; x = 0 in all other complexes, 57–69)[12]. For complexes with LnIII = Sm, Eu, Tb, the central lanthanide is seven-coordinate showing monocapped trigonal prismatic geometry (Figure S4). For the rest of the complexes, the central lanthanide as well as the terminal NiII ions are six-coordinate with coordination geometry between trigonal antiprism and trigonal prism and distorted octahedral, respectively. The Ni-Ln-Ni angles are in the range 165–179°. For 57, 58 and 69 the χMT at 300 K is 2.48 cm3Kmol−1, 2.33 cm3Kmol−1 and 2.76 cm3Kmol-1, higher than the theoretically expected value of 2.0 cm3Kmol-1 because the diamagnetic LnIII ions may induce small geometric variations and different ligand fields at the NiII ions, thus affecting the magnetic properties of the three complexes. The reduced magnetization for 57 and 69 shows a splitting of the isofield lines, which indicates a zero-field splitting. The best fit leads to JNi-Ni = -0.294 cm−1, gNi = 2.09, DNi = 1.933 cm−1 and E/D = 0.154 for 57 and JNi-Ni = -0.129 cm−1, gNi = 2.18, DNi = 2.838 cm−1 and E/D = 0.468 for 69. The fit of the magnetic data of the Ni2Gd complex 63 gave JNi-Ni = -0.377 cm−1, JGd-Ni = -0.009 cm−1, gNi = 2.102, gGd = 1.974 and χTIP = 0.003 cm3Kmol-1. Paramagnetic nuclear magnetic resonance (NMR) spectroscopy showed that in solution all complexes are isostructural showing the expected D3 symmetry for all metal ions being six-coordinate. These complexes have small magnetic anisotropies and the NMR data agree with the solid state SQUID measurements.
The ligand H3L10 = 6,6’,6’’-((1,4,7-triazonane-1,4,7-triyl)tris(methylene))tris(2,3-dimethylphenol) (Scheme 1) was used to prepare the linear complex [(NiL10)2Tb](ClO4) (70)[13]. Each NiII ion is coordinated by three nitrogen and three phenolate oxygen atoms in octahedral geometry and the TbIII ion is bound to six phenolate oxygen atoms in octahedral geometry. The magnetic studies revealed ferromagnetic interactions between the adjacent NiII and TbIII ions.
2. Other Schiff Base Ligands
The Schiff base ligand HL11 = (Z)-2-methoxy-6-((phenylimino)methyl)phenol (Scheme 2) derived from the condensation of o-vanillin with aniline, gave the linear trinuclear complexes [(NiL113)2Ln](NO3) (LnIII = La, Pr, Gd, Tb, 71–74)[14][15] which contain two terminal NiII ions in octahedral N3O3 coordination and a central LnIII ion bound to six phenolato and six methoxy oxygen atoms from six (L11)- ligands. The central LnIII ion sits on inversion center and displays distorted icosahedron geometry (Figure 6). The Ni2Gd complex 73 displays ferromagnetic coupling giving a ground spin state value of S = 11/2. Simultaneous fitting to χMT(T) and isothermal M(H) plots by using the spin Hamiltonian and considering a single unique Ni-Gd interaction J1, gave J1 = +0.54 cm−1 with fixed g = 2.01. Q-band EPR spectra of polycrystalline 73 at 5 K can be reproduced by a model with S = 11/2 and ZFS parameters D = -0.135 cm−1, E/D = 0.004 with g = 2.05. The magnetocaloric efficiency of the Ni2Gd cluster 73 was studied for the first time for a linear Ni2Gd cluster, via heat capacity and isothermal magnetization measurements which revealed a value of 13.74 Jkg−1K−1 for the magnetic entropy change at 4 K and ΔH = 7 T. Weak ferromagnetic exchange between the NiII ions is found in the Ni2La complex 71. The experimental data were fitted by considering the spin Hamiltonian considering the zero-field splitting parameter D and gave J = +0.46 cm−1, g = 2.245, D = +4.91 cm−1. Dc magnetic susceptibility measurements revealed that the NiII ions are coupled ferromagnetically with the TbIII ion in 74 and antiferromagnetically with the PrIII ion in 72. Ac susceptibility measurements performed on the Ni2Tb complex 74 under zero dc field and under H = 0.5 T revealed the onset of frequency dependent χ’’M signals indicating the possibility of SMM behavior. The absence of clear maxima in the χ’’M(T) plots down to 2 K indicates fast magnetic relaxation or fast QTM or perhaps both.
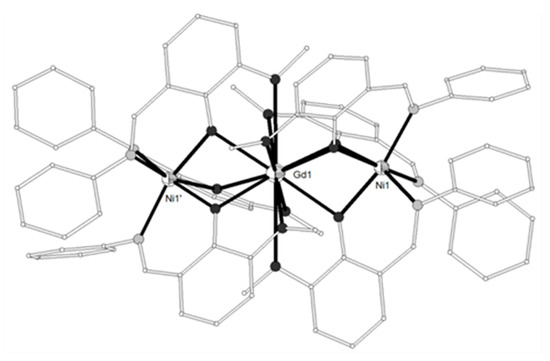
Figure 6. The molecular structure of the cation [(NiL113)2Gd]+ in complex 73. Primed atoms are generated by symmetry (‘) 1-x, y, 0.5-z. Color code as in Figure 2[14].
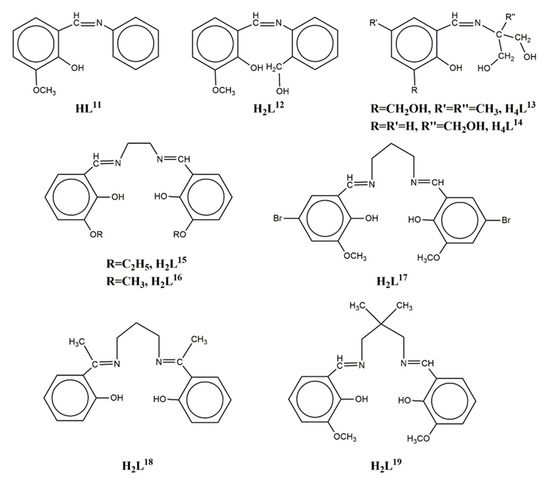
Scheme 2. The Schiff base ligands used in Ni2Ln complexes 71–93.
The Schiff base ligand H2L12 = (Z)-2-(((2-(hydroxymethyl)phenyl)imino)methyl)-6-methoxyphenol (Scheme 2) gave, among others, the linear trinuclear complex [{Ni(HL12)2}2La(NO3)](NO3)2 (75) which contains two NiII ions in distorted octahedral N2O4 environment and a central LaIII ion bound to four phenolato and four methoxy oxygen atoms from four (HL12)- ligands and two oxygen atoms from a chelate nitrate[16]. The coordination geometry around the LaIII ion is best described as sphenocorona JSPC-10 (CShM = 3.37915). Weak antiferromagnetic exchange between the NiII ions is found in the complex via the closed shell LaIII ion. The fit of the χMT data from 150 K to 2 K using a HDVV Hamiltonian yielded parameters J = -0.978 cm−1, g = 2.177, D = 3.133 cm−1.
The pentadentate Schiff base ligand H4L13 = (Z)-2-((2-hydroxy-3-(hydroxymethyl)-5-methylbenzylidene)amino)-2-methylpropane-1,3-diol (Scheme 2) was used to prepare a family of isostructural complexes [{Ni(H3L13)2}2Ln](NO3)3 (LnIII = Gd, Tb, Dy, Ho, 76–79) with linear metal arrangement (Figure 7)[17]. Each of the terminal NiII ions is distorted octahedral in N2O4 environment; the four oxygen atoms are derived from two phenolates and two pendant -CH2OH arms of the two different (H3L13)− ligands. The two imino nitrogen atoms around Ni are trans with respect to each other. The central LnIII ion is coordinated to eight oxygen atoms (four benzyl alcohol groups and four phenolato O-atoms) in square antiprismatic geometry. Each Ni2Ln complex interacts with four neighboring molecules through the -CH2OH groups of the ligands and the NO3- counteranions, leading to 2D H-bonded network along the ab plane. The static magnetic properties of all four complexes showed a predominant ferromagnetic interaction between the metal ions and only the Ni2Dy complex exhibited frequency dependent tails in the χ’’M vs T plots under zero dc field. The magnetic properties of complex 76 were analyzed by using the spin Hamiltonian assuming two equivalent Ni(O)2Gd bridging halves. The best-fit parameters are Jex = +0.67 cm−1, g = 2.117, D = 4.92 cm−1 (R = 7 × 10−7). The magnetocaloric properties of the Ni2Gd were estimated from the experimental isothermal field-dependent magnetization data yielding −ΔSm = 11.85 Jkg−1K−1 at 4 K and ΔH = 5 T.
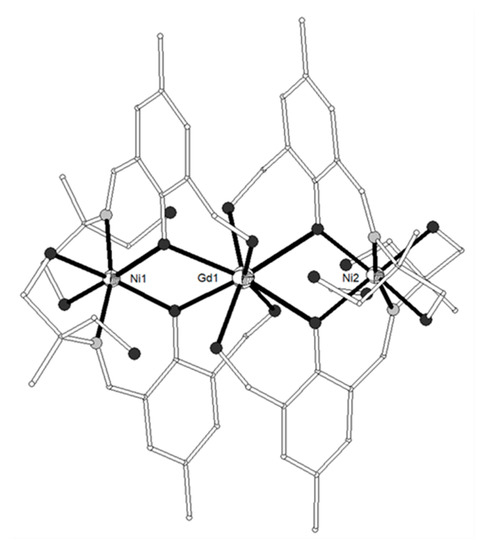
Figure 7. The molecular structure of the cation [{Ni(H3L13)2}2Ln]3+ in complex 76. Color code as in Figure 2[17].
The Schiff base ligand H4L14 = (Z)-2-((2-hydroxybenzylidene)amino)-2-(hydroxymethyl)propane-1,3-diol (Scheme 2) afforded four isostructural complexes with formula [{Ni(H3L14)2}2Ln(O2CMe)2](NO3)3 (LnIII = Sm, Eu, Gd, Tb, 80–83) with strictly linear metal arrangement (Figure 8)[18]. The two NiII ions display N2O4 distorted octahedral coordination and the central LnIII ion is coordinated to four phenolato oxygen atoms from four (H3L14)- ligands and four carboxylato oxygen atoms from two chelate acetates describing square antiprismatic geometry. The magnetic susceptibility data of 80 revealed weak antiferromagnetic coupling between the two NiII ions with best fit parameters J = -0.37 cm−1, g = 1.97 and TIP = 0.001 cm3mol−1. The χMT product of 81 at 300 K is higher than expected and can be explained by assuming that the first excited states for the EuIII ion are populated at r.t. because they are very close to the ground state. The magnetic susceptibility data of 82 revealed dominant ferromagnetic interactions between the NiII and GdIII ions and were fitted by using the spin Hamiltonian yielding JNiGd = +0.42 cm−1, D = +2.95 cm−1 (gNi = gGd = 1.98), resulting in S = 11/2 spin ground state. The magnetocaloric effect of 82 was determined by isothermal magnetization measurements in the temperature range 2–12.5 K under applied magnetic fields up to 5 T with −ΔSm = 14.2 Jkg−1K−1 at 2 K. The magnetic susceptibility data for 83 are consistent with dominant antiferromagnetic interactions between the metal ions and/or thermal depopulation of the TbIII excited states.
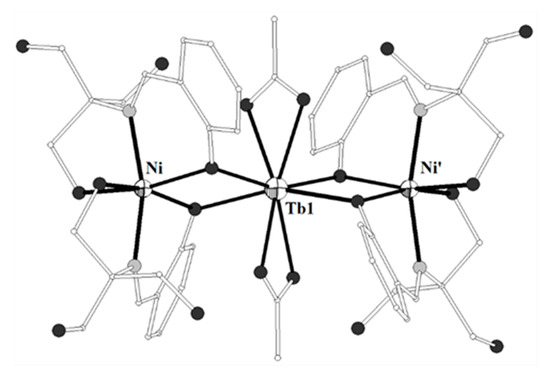
Figure 8. The molecular structure of the cation [{Ni(H3L14)2}2Ln(O2CMe)2]+ in complex 83. Primed atoms are generated by symmetry: (‘) 1.5-x, 0.5-y, z. Color code as in Figure 2[18].
The hexadentate Schiff base ligand H2L15 = 6,6’-((1E,1’E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-ethoxyphenol) (Scheme 2) gave two isostructural trinuclear complexes [(NiL15)2Ln(NO3)2](NO3) (LnIII = La, Ce, 84–85) with bent Ni-Ln-Ni arrangement[19]. The central LnIII ion is bound to four phenolato and four ethoxy oxygen atoms as well as to two chelate NO3- anions in distorted icosahedron geometry. Each of the terminal NiII ions is coordinated to the two imino nitrogen and two phenolato oxygen atoms of the ligand in square planar geometry. The LnIII ion is bridged to each of the NiII ions via two phenolato and two ethoxy oxygen atoms. Both complexes showed antimicrobial activity on cultures of E.coli, S.aureus and CA. The congener ligand H2L16 (Scheme 2) gave a similar Ni2Ce complex with Ni-Ce-Ni angle of ~62°[20].
The ligand H2L17 = 6,6’-((1E,1’E)-(propane-1,3-diylbis(azanylylidene))bis (methanylylidene))bis(4-bromo-2-methoxyphenol) (Scheme 2) gave four isostructural trinuclear complexes [(NiL17)2Ln(O2CMe)2(MeOH)2](NO3) (LnIII = La, Nd, Ce, Pr, 87–90)[21][22] which contain a central LnIII ion in an inversion center bound to four phenolato and four methoxy oxygen atoms as well as to two acetato oxygen atoms from two carboxylato ligands in pentagonal antiprismatic geometry (Figure 9). Each NiII ion occupies the N2O2 compartment of the ligand and has distorted octahedral geometry with MeOH and bridging acetato oxygen atoms in the apical positions. The LnIII ion is bridged to each of the NiII ions via the two phenolato oxygen atoms and the acetato group. Weak antiferromagnetic coupling between the NiII ions through the diamagnetic LaIII ion was found in the Ni2La complex 87. The magnetic susceptibility data were interpreted based on the isotropic Heisenberg model ( and the best least-squares fit yielded J = -0.75 cm−1, g = 2.18. The susceptibility data of the Ni2Nd, Ni2Ce and Ni2Pr complexes 88, 89, 90 respectively obey the Curie-Weiss law with the Curie constant of C = 3.71 cm3 K mol−1 and the Weiss constant of θ = -7.4 K for 88, C = 3.23 cm3 K mol−1 and θ = -9.9 K for 89 and C = 4.05 cm3 K mol−1 and θ= -25.5 K for 90. The negative values of Weiss constant confirm the antiferromagnetic exchange coupling between the metal ions. For complexes 89 and 90, the crystal field parameters for the CeIII/PrIII ions and the exchange coupling constant were estimated by using the generalized van Vleck formalism. The best fit yielded JNiCe = -1.1(4) cm−1, gNi = 2.23(3), D = 6.3(4) cm−1, A02<r2> = -265(10) cm−1, A04<r4> = 291(6) cm−1 for 89 and JNiPr = -1.3(8) cm−1, gNi = 2.15(2), D = 7.1(4) cm−1, A02<r2> = -310(9) cm−1, A04<r4> = 2335(11) cm−1, A06<r6> = 80(8) cm−1 for 90.
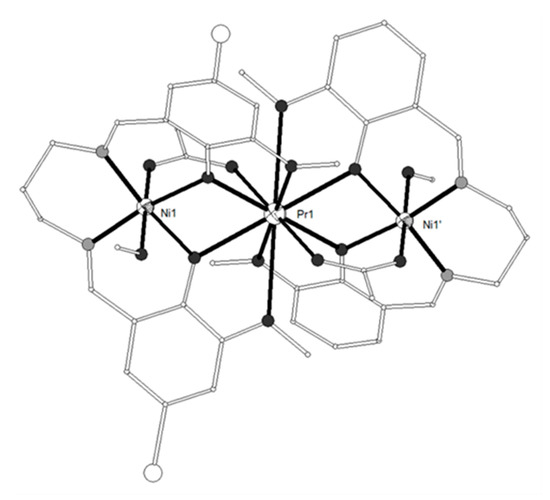
Figure 9. The molecular structure of the cation [(NiL17)2Pr(O2CMe)2(MeOH)2]+ in complex 90. Primed atoms are generated by symmetry: (‘) 1-x, 1-y, 1-z. Color code: Gd large octant, Ni small octant, N light grey, O dark grey, C open small, Br open large[22].
The tetradentate Schiff base ligand H2L18 = 2,2’-((1E,1’E)-(propane-1,3-diylbis (azanylylidene))bis(ethan-1-yl-1-ylidene))diphenol (Scheme 2) gave a trinuclear complex [(NiL18)2Ce(NO3)3] (91) with a central CeIII ion bound to two terminal [Ni(L18)] metalloligands in a transoid orientation to the central lanthanide ion[23]. The CeIII ion is ten-coordinate to four phenolato oxygen atoms and to three chelate nitrates, the coordination polyhedron can be described as distorted tetradecahedron. Each NiII ion is in N2O2 square planar geometry. The Ni-Ce-Ni moiety is bent with angle ~122.5°.
The ligand H2L19 = 6,6’-((1E,1’E)-((2,2-dimethylpropane-1,3-diyl)bis(azanyl ylidene))bis(methanyl ylidene))bis(2-methoxyphenol) (Scheme 2) gave the trinuclear complexes [{NiL19(H2O)}2Ln(H2O)](trif)3 (LnIII = Gd, Eu, 92–93; trif = triflate anion) which contain a nine-coordinate central LnIII ion bound to four phenolato and four methoxy oxygen atoms from two (L19)2− ligands and a water molecule (Figure 10)[5][24]. Each of the terminal NiII ions is five-coordinate, linked to the N2O2 site of the ligand in the equatorial plane and to a water molecule in the apical position. The magnetic susceptibility data of 92 were fitted considering two different J parameters for the Ni-Gd magnetic exchange and an equivalent D term for both nickel ions, according to the spin Hamiltonian . The best fit yielded JNiGd = 4.8(3) cm−1, jNiGd = 0.05(2) cm−1, g = 2.03(1) and D = 0.03(1) cm−1 (R = 1 × 10−5). A very similar J value (0.5 cm−1) yielded when the data were fitted without j and D parameters. The observed magnetization of 9.3 Nβ at 5 T was explained considering a ferromagnetic Ni-Gd dinuclear unit plus a mononuclear pentacoordinate Ni ion having a large magnetic anisotropy due to zero field splitting. The magnetization curve was fitted with J = 5 cm−1, j = 0, D = 12.4 cm−1, gNi = 2.16 and gGd = 2.00. The magnetic susceptibility data of 93 is governed by at least two magnetic phenomena that is, the depopulation of the Stark levels of the EuIII ion and the zero-field splitting of the NiII ions at very low temperatures.
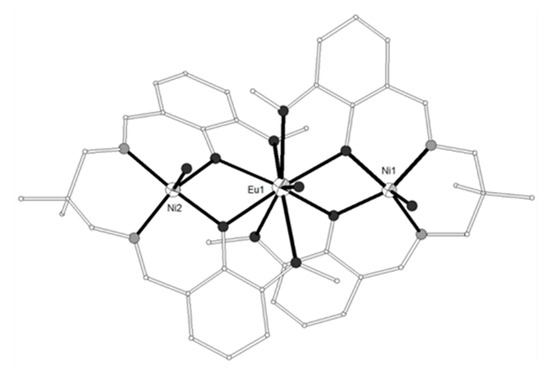
Figure 10. The molecular structure of the cation [{NiL19(H2O)}2Eu(H2O)]3+ in complex 93. Color code as in Figure 2[24].
3. Miscellaneous Ligands
The dipodal Schiff base ligand H4L20 = 3,3’-((1E,1’E)-((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene)) bis(2-hydroxybenzoic acid) (Scheme 3) gave a family of isomorphous V-shaped trinuclear complexes, [{Ni(H2L20)(tren)2}2Ln](NO3)3 [LnIII = Gd, Dy, Er, Lu, 94–97; tren = tris(2-aminoethyl)-amine)[25]. The ligand was prepared in situ and this fact justifies the complexation of tren around the NiII ions (Figure 11). The LnIII ions are eight-coordinate by four phenolato and four carboxylato oxygen atoms from two different ligands in distorted square antiprismatic geometry. Each of the terminal NiII ions is bound to four nitrogen atoms from the tren ligand, one amino group and one carboxylato oxygen atom from the Schiff base ligand in distorted octahedral geometry. Magnetic studies on all four complexes suggest the presence of weak antiferromagnetic interactions between neighboring ions. The magnetic susceptibility data of the Ni2Gd complex 94 were interpreted considering the spin Hamiltonian . The best set of parameters obtained using this model is J/kB = -0.083 cm−1 and g = 2.03. In complex 97, the two NiII ions are linked by the diamagnetic LuIII ion and the magnetic susceptibility data were interpreted using the spin Hamiltonian considering the axial single-ion zero-field splitting of the two NiII ions. The data were correctly fitted with D = 3.2(0) K and g = 2.19(2). The fit of the magnetic susceptibility data for 95 and 96 in the range 50–300 K to the Curie-Weiss law gave Curie constant C of 16.19 and 14.42 cm3 K mol−1 and Weiss temperature θ of -4.2 and -7.9 K for 95 and 96 respectively. The negative θ value indicates the presence of antiferromagnetic interactions within the NiII-LnIII-NiII moiety.
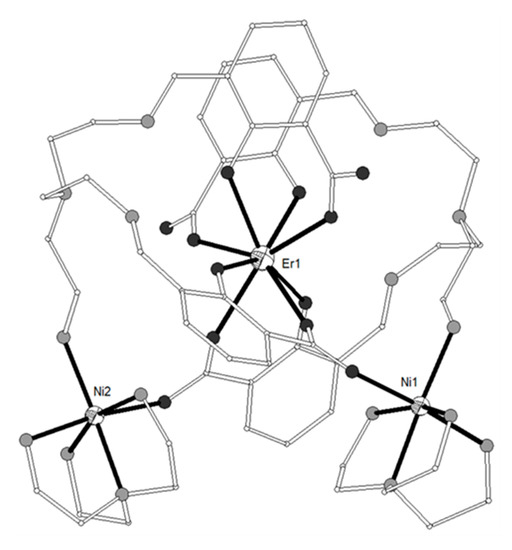
Figure 11. The molecular structure of the cation [{Ni(H2L20)(tren)2}2Er]3+ in complex 96. Color code as in Figure 2[25].
Scheme 3. The ligands used in the Ni2Ln complexes 94–145.
The ligand 8-hydroxyquinoline, HL21 (Scheme 3) gave the trinuclear complex [{Ni(L21)3}2La(L21)], 98[26]. The two NiII ions are six-coordinate with distorted fac-octahedral coordination geometry derived from three chelating (L21)− ligands (O,N). The LaIII ion is eight-coordinate with distorted square antiprismatic geometry bound to six bridging oxygen atoms of six (L21)− ligands and to a chelate (L21)- ligand through the nitrogen and oxygen atoms. The three metal ions form an angle of ~130°.
The ligand H2L22 = 7,7’-(ethane-1,1-diyl)bis(quinolin-8-ol) (Scheme 3) was formed in situ under the solvo(hydro)thermal conditions used to prepare complexes [{Ni(L22)1.5}2Ln(OH)] (LnIII = Eu, Tb, Gd, 99–101) from 8-hydroxyquinoline as proligand[27]. All complexes are isomorphous and crystallize in the hexagonal space group P63/m. The LnIII ion has position occupation 0.16667 and presents tricapped trigon-prismatic geometry comprised six oxygen atoms from three ligands and three terminal OH- groups with 0.16667 position occupation. The one crystallographically independent NiII ion has position occupation 0.33333 and is coordinated to three oxygen and three nitrogen atoms from three ligands creating a regular trigon-antiprismatic geometry. Supramolecular C-H∙∙∙π interactions between neighboring molecules result in an overall 3D net. The dc susceptibility studies of the Ni2Tb complex 100 displayed paramagnetic behavior in the temperature range 300–12.5 K and below that temperature a slow decrease in the χMT product mainly due to the thermal depopulation of crystal field effect.
The ligand HL23 (Scheme 3) was formed in situ via transition metal promoted nucleophilic addition of methanol to a nitrile group of dicyanonitrosomethanide (dcnm) and gave two families of trinuclear complexes, (Me4N)[{(Ni(L23)3}2Ln(L23)2] (LnIII = La, Ce, Pr, Nd, Sm, 102–106) and (Et4N)2[{(Ni(L23)3}2Ln(dcnm)2](ClO4) (LnIII = La, Ce, 107–108)[28]. The two NiII octahedral metal sites are each coordinated by three (L23)- ligands which chelate through the nitrogen atoms of the nitroso and imine groups to form a [Ni(L23)3]- metalloligand. The central LnIII ion is bound to two [Ni(L23)3]- metalloligands and presents ten-coordination completed by two (L23)- ligands or two unreacted dcnm- ligands, all of them bound via the N,O atoms of the nitroso group (Figure 12). The coordination geometry around the LnIII ions is best described as sphenocorona JSPC-10. The Ni2Ln moiety is bent in both type of complexes with Ni-Ln-Ni angle of ~142 and ~133° for (Me4N)[{(Ni(L23)3}2Ln(L23)2] and (Et4N)2[{(Ni(L23)3}2Ln(dcnm)2](ClO4) complexes, respectively. Variable temperature magnetic susceptibilities on these complexes revealed practically zero Ni-Ni exchange coupling in the Ni2La complexes 102 and 107, possible weak antiferromagnetic coupling in Ni2Pr 104 and possible weak ferromagnetic coupling in the Ni2Ce complexes 103 and 108 but thermal depopulation and ligand-filed effects on the central LnIII ion, particularly for SmIII, make the unambiguous assignment of ferro- versus antiferromagnetic coupling rather difficult. In any case the magnetic behavior of 102–108 is similar to those of congener complexes.
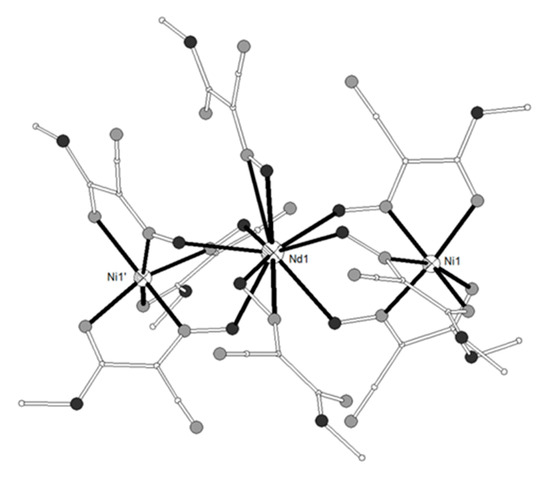
Figure 12. The molecular structure of the anion [{(Ni(L23)3}2Nd(L23)2]- in complex 105. Primed atoms are generated by symmetry: (‘) 1/3 + y, -1/3 + x, 1/6-z. Color code as in Figure 2 [28].
The ligand 2,6-di(acetoacetyl)pyridine, H2L24 (Scheme 3) gave 18 trinuclear NiII2LnIII complexes with LnIII = La-Lu except for Pm, which crystallize in four different types: (A) [(NiL24)2Ln(NO3)2(MeOH)4](NO3) (LnIII = La, Ce, Pr, Nd, Sm, Eu, Gd, 109–115), (B) [(NiL24)2Ln(NO3)2(H2O)2(MeOH)2](NO3) (LnIII = Sm, Eu, Gd, 116–118), (C) [(NiL24)2Ln(NO3)3(MeOH)4] (LnIII = Gd, Tb, Dy, 119–121) and (D) [(NiL24)2Ln(NO3)2(H2O)(MeOH)3](NO3) (LnIII = Ho, Er, Tm, Yb, Lu, 122–126)[29]. All types of complexes are linear with Ni-Ln-Ni angles 180° (A), ~178° (B), ~173° (C) and ~179° (D). The two terminal NiII ions present O6 distorted octahedral geometry bound to the 1,3-diketonate sites from two (L24)2- ligands together with MeOH and H2O molecules. The central LnIII ion is coordinated to the 2,6-diacylpyridine site from two (L24)2- ligands and to two or three nitrate ions in an overall ten-coordinate environment (Figure 13). The coordination geometry around the LnIII ions is best described as hexadecahedron HD-10 in 109–115 and tetradecahedron TD-10 in 116–126. The magnetic studies revealed that the Ni-Ln interaction is weakly antiferromagnetic for Ln = Ce, Pr, Nd and ferromagnetic for Ln = Gd, Tb, Dy, Ho, Er. The magnetic susceptibility data for 109 and 126 which contain the diamagnetic LaIII and LuIII ions, respectively, were interpreted based on the isotropic Heisenberg model . The best-fit parameters are J = -0.63 cm−1, g = 2.22 for 109 and J = -0.65 cm−1, g = 2.17 for 126. For the Ni2Gd complex, the spin Hamiltonian (J is the exchange integral between the adjacent NiII and GdIII ions and J’ is the exchange integral between the terminal NiII ions) was used to fit the susceptibility data and considering gNi = 2.20 and J’ = -0.64 cm−1 (the mean values for 109 and 126) the best-fit parameters are J = +0.79 cm−1 gGd = 2.02. The magnetic data of the remaining complexes were evaluated by adopting an empirical method taking into account the behavior of the congener Zn2Ln and Ni2La complexes by using the equation , for ferromagnetic and for antiferromagnetic interaction.
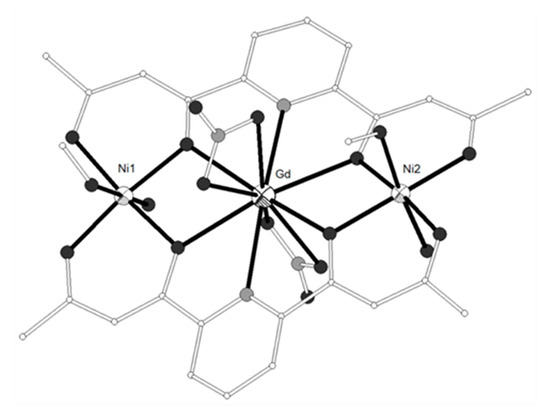
Figure 13. The molecular structure of the cation [(NiL24)2Gd(NO3)2(H2O)2(MeOH)2]+ in complex 118. Color code as in Figure 2[27].
The congener ligand 2,6-bis(acetobenzoyl)pyridine H2L25 (Scheme 3) gave the trinuclear complexes [(NiL25)2Ln(O2CMe)3(MeOH)x] (LnIII = Gd, Ce; x = 2 or 3, 127–128) and [(NiL25)2Ln(O2CPh)3(solv)x] (LnIII = Gd, solv = MeOH, x = 2, 129; Ce, solv = MeOH/H2O x = 2, 130)[30]. Each (L25)2− ligand is bound to the NiII sites through the 1,3-diketonate sites and to the central LnIII ion through the 2,6-diacylpyridine site. Two carboxylato ligands act as bidentate bridging between the NiII and the LnIII ions and the third act as chelate bidentate around the central lanthanide ion. The six-coordination around the NiII ions is completed by solvate molecules. The coordination number around the CeIII ion is 10, whereas for the GdIII is 9 or 10. The N2O7 nine-coordination around GdIII in 127 is described as HH-9 (hula-hoop) and the N2O8 ten-coordination around the LnIII ions in 128–130 is described as staggered dodecahedron SDD-10.
The ligand 2,6-dipicolinoylbis(N,N-diethylthiourea) H2L26 (Scheme 3) gave the trinuclear complex [(NiL26)2Pr(O2CMe)3(MeOH)2], 131[31] [47]. The two NiII ions are bound to the S,O atoms from two (L26)2− ligands, one oxygen atom from the bridging acetato ligand and one methanol in distorted octahedral geometry. The PrIII ion is ten-coordinate and is bound to the O,N,O group of the 2,6-diacylpyridine site of each (L26)2− ligand, two bridging and one chelate acetato groups. The polyhedron around the PrIII ion is described as double-capped square antiprism.
The neutral trinuclear complexes [{Ni(piv)3(bpy)}2Ln(NO3)].MeCN (Hpiv = pivalic acid, bpy = 2,2’-bipyridine, LnIII = Gd (132), Sm (133)) are isomorphous and contain a bent Ni-Ln-Ni moiety with angles ~153°[32]. The central LnIII ion is bound to each of the terminal NiII ions through two syn,syn pivalato groups and one μ-O carboxylato oxygen which belongs to a chelate-monodentate bridging pivalato group. The distorted octahedral coordination around each NiII ion consists of four carboxylato oxygen atoms and two nitrogen atoms of the bpy. The magnetic susceptibility data of 132 were interpreted by using the spin Hamiltonian . The best fit yielded JNiGd = 0.105(5) cm−1, JNiNi = -0.70(5) cm−1, gNi = 2.015(1), gGd = 2.00 (fixed), tip = 0.0001 (R2 = 1.28 × 10−5). The neutral trinuclear complexes [{Ni(piv)3(Hpiv)(MeCN)}2Ln(NO3)] (LnIII = La, Pr, Sm, Eu, Gd, 134–138)[32] are isomorphous and contain a Ni-Ln-Ni moiety with angles ~144°. The central LnIII ion is bound to each of the terminal NiII ions in similar fashion as in 134–138. The coordination of each NiII ion is completed by a monodentate Hpiv and MeCN molecules and is distorted octahedral. The coordination of the central LnIII consists of six carboxylato oxygen atoms and one chelate nitrato group and is described as single-capped pentagonal bipyramid. The magnetic susceptibility data of 138 were interpreted by using the spin Hamiltonian . The best fit yielded JNiGd = 0.44(2) cm−1, JNiNi = -2.25(5) cm−1, gNi = gGd = 2.00 (fixed), molar content of the S = 1 impurity is 5.5% (R2 = 1.5 × 10−4). The magnetic susceptibility data of 134 can be interpreted by using either the exchange Hamiltonian which yields Jex = -1.0(3) cm−1, g = 2.24(1) and zJ’ = +0.9(1) cm−1, tip = 2.4(9) × 10−4 (R2 = 2.6 × 10−4) or by considering the zero-field splitting of the NiII ions which yields D = 6.0(5) cm−1, g = 2.227(1), zJ’ = +0.05(1) cm−1, tip = 3.6(5) × 10−4 (R2 = 2.5 × 10−3). The magnetic susceptibility data for 135–137 which contain PrIII, SmIII and EuIII ions can be interpreted by assuming that the exchange interactions between NiII and LnIII ions are absent, whereas the weak antiferromagnetic interactions between the NiII ions ‘through the lanthanide’ do exist.
The ligands HL27 = (Z)-1-(pyridine-2-yl)ethenone oxime and HL28 = (E)-N’-hydroxypyrimidine-2-carboximidamide (Scheme 3) gave the trinuclear complexes [{Ni(L27)3}2Tb](NO3) (139)[33][34] and [(Ni(HL28)3}2Tb](NO3) (140)[35], respectively. Both complexes contain strictly linear Ni-Tb-Ni moiety (Figures 14). Each of the terminal NiII ions in 139–140 is coordinated to three pyridine and three oximato nitrogen atoms from three (HL27)− or (HL28)− ligands in distorted octahedral geometry. The central TbIII ion is coordinated to six oximato oxygen atoms from the three (HL27)− or (HL28)− ligands (Scheme 3) in distorted octahedral geometry. The χMT values of 139 at 300 and 2 K are 13.54 and 2.80 cm3 K mol−1, respectively. The field dependence of the magnetization at 2 K is 6.41 NμB at 2 K. The χMT value of 140 at 300 K is 11.74 cm3 K mol−1 which is slightly low with respect to the expected value for two NiII (S = 1) and one TbIII (S = 3, L = 3, g = 3/2) non-interacting ions. The χMT value decreases on lowering the temperature and reaches the value of 5.41 cm3 K mol−1 at 5 K. The decrease of the χMT value as the temperature decreases for 139 and 140 is governed by the thermal depopulation of the ground-state sublevels as result of the spin-orbit coupling and the low symmetry crystal field.
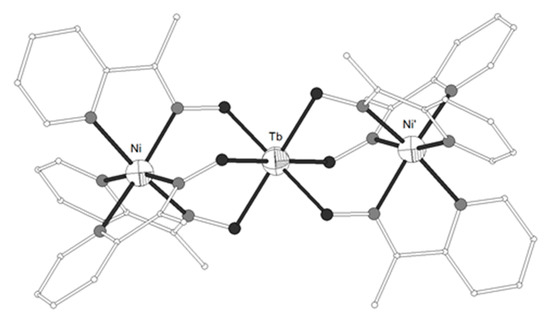
Figure 14. The molecular structure of the cation complex [{Ni(L27)3}2Tb]+ 139. Primed atoms are generated by symmetry: (‘) -x+y, -x, z. Color code: Tb large octant, Ni small octant, N light grey, O dark grey, C open small, S open large[34].
It is well known that ligand L29 = di(pyridine-2-yl)methanone or di-2-pyridyl ketone (Scheme 3) can undergo nucleophilic addition on the carbonyl carbon atom to form the hemiketal and/or the gem-diol ligands L29’ = ethoxydi(pyridine-2-yl)methanol and L29’’ = di(pyridin-2-yl)methanediol, respectively. Both L29’ and L29’’ are formed in situ in the presence of metal ions. Complexes [Ni2(L29’)3(L29’’)Ln(NO3)(H2O)](ClO4)2 (LnIII = Gd 141, Tb 142) and [Ni2(L29’)4Ln(NO3)(H2O)][Ln(NO3)5](ClO4)2 (LnIII = Tb 143, Dy 144, Y 145) consist of dications which contain triangular Ni-Ln-Ni moieties with angles ~54° (Figure 15)[36][37]. The metal ions are linked through one μ3-O atom and three μ2-O atoms from the ligands (Scheme 3). Each NiII ion is coordinated to three oxygen and two nitrogen atoms from three respective ligands in distorted octahedron. Each LnIII ion is coordinated to N2O6 chromophore which consists of three carbonyl O-atoms, three pyridine N-atoms, one chelate nitrato and one terminal aqua ligands. The magnetic susceptibility data of 141 revealed χMT values of 12.61 and 18.63 cm3 K mol−1 at 300 and 2 K respectively. The data were interpreted by using the spin Hamiltonian and the best fit yielded J = +1.03(8) cm−1, J’ = +0.9(2) cm−1, g = 2.246(1). The magnetization measurement at 2 K revealed saturation under 5 T at 12.9 μβ, indicative of an S = 11/2 ground state with a g value larger than 2.0. The magnetic data of 143 revealed ferromagnetic exchange interactions between the metal ions. The magnetic susceptibility data of 145 which contains the diamagnetic YIII ion allowed the determination of the magnetic exchange interaction between the NiII ions according to the above spin Hamiltonian by considering J = 0. The best fit gave J’ = +8.0(2) cm−1 and g = 2.15(1).
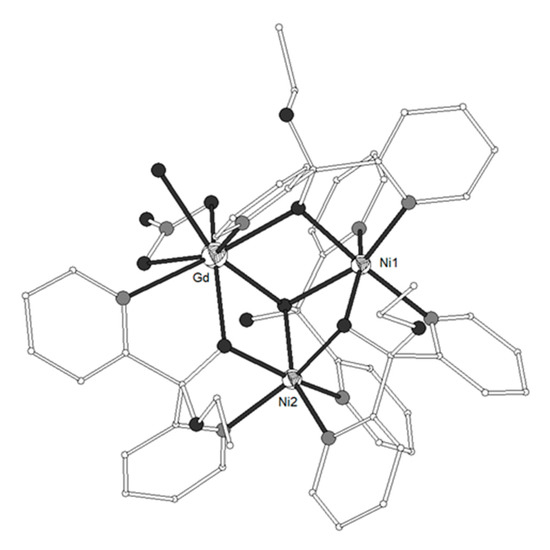
Figure 15. The molecular structure of the dication complex [Ni2(L29’)3(L29’’)Gd(NO3)(H2O)]2+ 141. Color code: Gd large octant, Ni small octant, N light grey, O dark grey, C open small, S open large[36][37].
The coordination geometry around the NiII ions in complexes 1–145 is distorted octahedral; the only exceptions are complexes 12–15 which can be more acutely described as trigonal prismatic. The bridging modes and the coordination around the NiII ions observed in complexes 1–145 is depicted in Scheme 4. The NiII ions is complexes 1–28, 52–74, 98–101 and 141–145 are coordinated to three nitrogen and three oxygen atoms. The configuration around the NiII ions consisting of N3O3 coordination sphere is chiral with either a Δ or a Λ configuration due to the screw coordination arrangement of the achiral tripodal ligands around the metal ion. When two chiral molecules associate, both homochiral (Δ-Δ or Λ-Λ) and heterochiral (Δ-Λ) pairs are possible. Since the above complexes crystallize in centrosymmetric space groups, molecules with Δ-Δ and Λ-Λ pairs coexist in the crystals to form racemic crystals. The NiII ions in complexes 29–51, 75–83, 91–93 and 109–131 are coordinated to four nitrogen and two oxygen atoms. The NiII ions in 89–90 and 132–138 are coordinated to two nitrogen and four oxygen atoms and in 94–97 are coordinated to five nitrogen and one oxygen atoms. The NiII ions in 102–108 and 139–140 show N6 coordination sphere. On the other hand, the coordination around the LnIII ions in 1–145 displays a variety of geometries from the rare octahedral and quasi trigonal prism for O6 coordination, capped trigonal prism and capped octahedron for O7 coordination, square antiprism and bicapped octahedron for O8 and NO7 coordination, tricapped trigonal prism for O9 coordination and distorted icosahedron for O12 coordination.
This entry is adapted from the peer-reviewed paper 10.3390/cryst10121117
References
- Chandrasekhar, V.; Pandian, B.M.; Boomishankar, R.; Steiner, A.; Vittal, J.J.; Houri, A.; Clérac, R. Trinuclear heterobimetallic Ni2Ln complexes [L2Ni2Ln](ClO4) (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, and Er; LH3 = (S)P[N(Me)N=CH-C6H3-2-OH_3-OMe]3): From simple paramagnetic complexes to single-molecule magnet behavior. Inorg. Chem. 2008, 47, 4918–4929.
- Singh, S.K.; Rajeshkumar, T.; Chandrasekhar, V.; Rajaraman, G. Theoretical studies on {3d-Gd} and {3d-Gd-3d} complexes: Effect of metal substitution on the effective exchange interaction. Polyhedron 2013, 66, 81–86.
- Yamaguchi, T.; Sunatsuki, Y.; Kojima, M.; Akashi, H.; Tsuchimoto, M.; Re, N.; Osa, S.; Matsumoto, N. Ferromagnetic NiII-GdIII interactions in complexes with NiGd, NiGdNi, and NiGdGdNi cores supported by tripodal ligands. Chem. Commun. 2004, 1048–1049.
- Yamaguchi, T.; Sunatsuki, Y.; Ishida, H.; Kajima, M.; Akashi, H.; Re, N.; Matsumoto, N.; Pochaba, A.; Mroziński, J. Synthesis, structures, and magnetic properties of double face-sharing heterotrinuclear NiII-LnIII-NiII (Ln = Eu, Gd, Tb, and Dy) complexes. Bull. Chem. Soc. Jpn. 2008, 81, 598–605.
- Jean-Pierre Costes; Tomoka Yamaguchi; Masaaki Kojima; Laure Vendier; Experimental Evidence for the Participation of 5d GdIIIOrbitals in the Magnetic Interaction in Ni−Gd Complexes. Inorganic Chemistry 2009, 48, 5555-5561, 10.1021/ic900142h.
- Min-Xia Yao; Zhao-Xia Zhu; Xing-Yun Lu; Xiao-Wei Deng; Su Jing; Rare single-molecule magnets with six-coordinate Ln III ions exhibiting a trigonal antiprism configuration. Dalton Transactions 2016, 45, 10689-10695, 10.1039/C6DT01606E.
- Zhiqiang Xu; Paul W. Read; David E. Hibbs; Michael B. Hursthouse; K. M. Abdul Malik; Brian O. Patrick; Steven J. Rettig; Mehran Seid; David A. Summers; Maren Pink; et al. Coaggregation of paramagnetic d- and f-block metal ions with a podand-framework amine phenol ligand.. Inorganic Chemistry 2000, 39, 508-516, 10.1021/ic991171b.
- Bayly, S.R.; Xu, Z.; Patrick, B.O.; Rettig, S.J.; Pink, M.; Thompson, R.C.; Orvig, C. d/f complexes with uniform coordination geometry: Structural and magnetic properties of an LnNi2 core supported by a heptadentate amine phenol ligand. Inorg. Chem. 2003, 42, 1576–1583.
- Mustapha, A.; Reglinski, J.; Kennedy, A.R. The use of hydrogenated Schiff base ligands in the synthesis of milti-metallic compounds. Inorg. Chim. Acta 2009, 362, 1267–1274.
- He-Rui Wen; Piao-Ping Dong; Sui-Jun Liu; Jin-Sheng Liao; Fu-Yong Liang; Cai‐Ming Liu; 3d–4f heterometallic trinuclear complexes derived from amine-phenol tripodal ligands exhibiting magnetic and luminescent properties. Dalton Transactions 2017, 46, 1153-1162, 10.1039/c6dt04027f.
- Cheri A. Barta; Simon R. Bayly; Paul W. Read; Brian O. Patrick; Robert C. Thompson; Chris Orvig; Molecular Architectures for Trimetallic d/f/d Complexes: Structural and Magnetic Properties of a LnNi2Core. Inorganic Chemistry 2008, 47, 2280-2293, 10.1021/ic701612e.
- Peter Comba; Markus Enders; Michael Großhauser; Markus Hiller; Dennis Müller; Hubert Wadepohl; Solution and solid state structures and magnetism of a series of linear trinuclear compounds with a hexacoordinate Ln III and two terminal Ni II centers. Dalton Transactions 2017, 46, 138-149, 10.1039/C6DT03488H.
- He-Rui Wen; Jia-Li Zhang; Fu-Yong Liang; Kai Yang; Sui-Jun Liu; Jinsheng Liao; Cai‐Ming Liu; TbIII/3d–TbIII clusters derived from a 1,4,7-triazacyclononane-based hexadentate ligand with field-induced slow magnetic relaxation and oxygen-sensitive luminescence. New Journal of Chemistry 2019, 43, 4067-4074, 10.1039/c8nj05777j.
- Upadhyay, A.; Komatireddy, N.; Ghirri, A.; Tuna, F.; Langley, S.K.; Srivastava, A.K.; Sañudo, E.C.; Moubaraki, B.; Murray, K.S.; McInnes, E.J.L.; et al. Synthesis and magnetothermal properties of a ferromagnetically coupled NiII-GdIII-NiII cluster. Dalton Trans. 2014, 43, 259–266.
- Upadhyay, A.; Das, C.; Langley, S.K.; Murray, K.S.; Srivastava, A.K.; Shanmugam, M. Heteronuclear Ni(II)-Ln(III) (Ln = La, Pr, Tb, Dy) complexes: Synthesis and single-molecule magnet behavior. Dalton Trans. 2016, 45, 3616–3626.
- Ahmed, N.; Das, C.; Vaidya, S.; Kumar Srivastava, A.; Langley, S.K.; Murray, K.S.; Shanmugam, M. Probing the magnetic and magnetothermal properties of M(II)-Ln(III) complexes (where M(II) = Ni or Zn; Ln(III) = La or Pr or Gd). Dalton Trans. 2014, 43, 17375–17384.
- Das, S.; Dey, A.; Kundu, S.; Biswas, S.; Mota, A.J.; Colacio, E.; Chandrasekhar, V. Linear {NiII-LnIII-NiII} complexes containing twisted planar Ni(μ-phenolate)2Ln fragments: Synthesis, structure, and magnetothermal properties. Chem. Asian. J. 2014, 9, 1876–1887.
- Anastasia N. Georgopoulou; M. Pissas; Vassilis Psycharis; Yiannis Sanakis; Catherine P. Raptopoulou; Trinuclear NiII-LnIII-NiII Complexes with Schiff Base Ligands: Synthesis, Structure, and Magnetic Properties. Molecules 2020, 25, 2280, 10.3390/molecules25102280.
- Sui, Y.; Hu, R.-H.; Liu, D.-S.; Wu, Q. Adjustment of the structures and biological activities by the ratio of NiL to RE for two sets of Schiff base complexes [(NiL)nRE] (n = 1 or 2; RE = La or Ce). Inorg. Chem. Comm. 2011, 14, 396–398.
- Ali Güngör, S.; Kose, M. Synthesis, crystal structure, photoluminescence and electrochemical properties of a sandwiched Ni2Ce complex. J. Mol. Struct. 2017, 1150, 274–278.
- Cristovao, B.; Kłak, J.; Miroslaw, B. Synthesis, crystal structures and magnetic behavior of NiII-4f-NiII compounds. Polyhedron 2012, 43, 47–54.
- Cristóvão, B.; Kłak, J.; Pełka, R.; Miroslaw, B.; Hnatejko, Z. Heterometallic trinuclear 3d-4f-3d compounds based on a hexadentate Schiff base ligand. Polyhedron 2014, 68, 180–190.
- Ghosh, S.; Ghosh, A. Coordination of metalloligand [NiL] (H2L = salen type N2O2 Schiff base ligand) to the f-block elements: Structural elucidation and spectrophotometric investigation. Inorg. Chim. Acta 2016, 442, 64–69.
- Costes, J.-P.; Donnadieu, B.; Gheorghe, R.; Novitchi, G.; Tuchagues, J.-P.; Vendier, L. Di- or trinuclear 3d-4f Schiff base complexes: The role of anions. Eur. J. Inorg. Chem. 2008, 5235–5244.
- Bhunia, A.; Yadav, M.; Lan, Y.; Powell, A.K.; Menges, F.; Riehn, C.; Niedner-Schatteburg, G.; Jana, P.P.; Riedel, R.; Harms, K.; et al. Trinuclear nickel-lanthanide compounds. Dalron Trans. 2013, 42, 2445–2450.
- Deacon, G.B.; Forsyth, C.M.; Junk, P.C.; Leary, S.G. A rare earth alloy as a synthetic reagent: Contrasting homometallic rare earth and heterobimetallic outcomes. New J. Chem. 2006, 30, 592–596.
- Zhu, Y.; Luo, F.; Song, Y.-M.; Feng, X.-F.; Luo, M.-B.; Liao, Z.-W.; Sun, G.-M.; Tian, X.-Z.; Yuan, Z.-J. The first one-pot synthesis of multinuclear 3d-4f metal-organic compounds involving a polytopic N,O-donor ligand formed in situ. Cryst. Growth Des. 2012, 12, 2158–2161.
- Chesman, A.S.R.; Turner, D.R.; Moubaraki, B.; Murray, K.S.; Deacon, G.B.; Batten, S.R. Synthesis and magnetic properties of a series of 3d/4f/3d heterometallic trinuclear complexes incorporating in situ ligand formation. Inorg. Chim. Acta 2012, 389, 99–106.
- Shiga, T.; Ito, N.; Hidaka, A.; Okawa, H.; Kitagawa, S.; Ohba, M. A series of trinuclear CuIILnIIICuII complexes derived from 2,6-di(acetoacetyl)pyridine: Synthesis, structure, and magnetism. Inorg. Chem. 2007, 46, 3492–3501.
- Trieu, T.N.; Nguyen, M.H.; Abram, U.; Nguyen, H.H. Syntheses and structures of new trinuclear MIILnMII (M = Ni, Co; Ln = Gd, Ce) complexes with 2,6-bis(acetobenzoyl)pyridine. Z. Anorg. Allg. Chem. 2015, 641, 863–870.
- Nguyen, H.H.; Jegathesh, J.J.; Takiden, A.; Hauenstein, D.; Pham, C.T.; Le, C.D.; Abram, U. 2,6-dipicolinoylbis(N,N-dialkylthioureas) as versatile building blocks for oligo- and polynuclear architectures. Dalton Trans. 2016, 45, 10771–10779.
- Burkovskaya, N.P.; Orlova, E.V.; Kiskin, M.A.; Efimov, N.N.; Bogomyakov, A.S.; Fedin, M.V.; Kolotilov, S.V.; Minin, V.V.; Aleksandrov, G.G.; Sidorov, A.A.; et al. Synhtesis, structure, and magnetic properties of heterometallic trinuclear complexes {MII-LnIII-MII} (MII = Ni, Cu; LnIII = La, Pr, Sm, Eu, Gd). Russ. Chem. Bull Int. Ed. 2011, 60, 2490–2503.
- Christina D. Polyzou; Constantinos G. Efthymiou; Albert Escuer; Luís Cunha-Silva; Constantina Papatriantafyllopoulou; Spyros P. Perlepes; In search of 3d/4f-metal single-molecule magnets: Nickel(II)/lanthanide(III) coordination clusters. Pure and Applied Chemistry 2013, 85, 315-327, 10.1351/pac-con-12-09-08.
- Constantina Papatriantafyllopoulou; Marta Estrader; Constantinos G. Efthymiou; Despina Dermitzaki; Konstantinos Gkotsis; Aris Terzis; Carmen Díaz; Spyros P. Perlepes; In search for mixed transition metal/lanthanide single-molecule magnets: Synthetic routes to NiII/TbIII and NiII/DyIII clusters featuring a 2-pyridyl oximate ligand. Polyhedron 2009, 28, 1652-1655, 10.1016/j.poly.2008.10.024.
- Kalogridis, C.; Palacios, M.A.; Rodríguez-Diéguez, A.; Mota, A.J.; Choquesillo-Lazarte, D.; Brechin, E.K.; Colacio, E. Heterometallic oximato-bridged linear trinuclear NiII-MIII-NiII (MIII = Mn, Fe, Tb) complexes constructed with the fac-O3 [Ni(HL)3]− metalloligand (H2L = pyrimidine-2-carboxamide oxime): A theoretical and experimental magneto-structural study. Eur. J. Inorg. Chem. 2011, 5225–5232.
- Efthymiou, C.G.; Georgopoulou, A.N.; Papatriantafyllopoulou, C.; Terzis, A.; Raptopoulou, C.P.; Escuer, A.; Perlepes, S.P. Initial employment of di-2-pyridyl ketone as a route to nickel(II)/lanthanide(III) clusters: Triangular Ni2Ln complexes. Dalton Trans. 2010, 29, 8603–8605.
- Georgopoulou, A.N.; Efthymiou, C.G.; Papatriantafyllopoulou, C.; Psycharis, V.; Raptopoulou, C.P.; Manos, M.; Tasiopoulos, A.J.; Escuer, A.; Perlepes, S.P. Triangular NiII2LnIII and NiII2YIII complexes derived from di-2-pyridyl ketone: Synthesis, structures and magnetic properties. Polyhedron 2011, 30, 2978–2986.
