The alarming increase in antimicrobial resistance, based on the built-in abilities of bacteria to nullify the activity of current antibiotics, leaves a growing number of bacterial infections untreatable. An appealing approach, advanced in recent decades, concerns the development of novel agents able to interact with the external layers of bacteria, causing irreparable damage. Regarding this, some natural cationic antimicrobial peptides (CAMPs) have been reconsidered, and synthetic cationic polymers, mimicking CAMPs and able to kill bacteria by non-specific detrimental interaction with the negative bacterial membranes, have been proposed as promising solutions. Lately, also dendrimers were considered suitable macromolecules for the preparation of more advanced cationic biomimetic nanoparticles, able to harmonize the typical properties of dendrimers, including nanosize, mono-dispersion, long-term stability, high functionality, and the non-specific mechanism of action of CAMPs. Although cationic dendrimers are extensively applied in nanomedicine for drug or gene delivery, their application as antimicrobial agents is still in its infancy. In this first part of our overview on the main types of cationic antibacterial dendrimers, the state of the art of the potential applications of PAMAM and PPI-based agents has therefore been reviewed here, with particular attention to the innovative case studies in reported in the literature
- antibiotic resistance
- novel antimicrobial agents
- cationic antimicrobial polymers
- non-specific membrane disruption
- biomimetic cationic dendrimer nanoparticles
- PAMAM and PPI antibacterial dendrimers
Introduction
The increasing growth of resistant bacterial strains, which represent a highly worrying trend that has characterized the last few years, has caused the appearance and the re-emergence of serious infections, in particular in nosocomial settings [1]. In this regard, pneumonia, bloodstream infections, wound or surgical site infections, and meningitis are often associated with the failure of antibiotic-based treatments or with the concomitant lack of new antimicrobial agents [2][3][4]. Gram-negative bacteria such as Klebsiella pneumoniae, Acinetobacter baumannii, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Burkholderia cepacia, and Escherichia coli pose a major threat to human health, since they are the most critically resistant and rapidly spreading bacteria [5][6][7]. Moreover, in addition to their intrinsic resistance mechanisms, these pathogens are rapidly becoming multidrug-or even pan-drug-resistant to most life-saving drugs. In particular, aerobic non-fermenting Gram-negative bacilli such as A. baumannii, P. aeruginosa, and S. maltophilia, are emerging as clinically relevant superbugs, contributing significantly, with their alarming resistance levels, to numerous therapeutic failures. Gram-negative bacteria, unlike Gram-positive bacteria, are characterized by high and similar resistance levels, both in Europe and in the United States.Given this situation, as well as being inspired by reports developed by a group of independent experts led by World Health Organization (WHO), the medical research community must develop new antimicrobial agents active on current resistant strains of Gram-positive and Gram-negative bacteria. Furthermore, because bacterial infections, especially if caused by biofilm production, hinder the durability, reliability, and performance of many medical devices and implants, antibiofilm strategies such as antibacterial coatings that repel bacteria and prevent biofilm formation are highly desirable.Natural cationic antimicrobial peptides (CAMPs) are a class of unconventional antimicrobial agents with a broad spectrum of action, active on a wide variety of Gram-positive and Gram-negative bacteria, fungi, protozoa and yeast. These molecules, without the need to enter the bacterium cell or to interfere with specific metabolic processes, basically act based on their positive charge. Their action is rapid and not specific and is based initially on electrostatic interactions with the bacterial surface, followed by the progressive damage of the bacterial outer and/or cytoplasmic membranes (OM and CM) that leads to the bacterial death. On the base of this mechanism of action, CAMPs kill pathogens simply through external contact, without the need to address the numerous resistance mechanisms due to genetic mutations that bacteria can develop. In other words, since these materials do not interfere with the vital processes for bacteria, which can eventually be modified—by genetic mutation—by resistant pathogens, they are generally indifferent to the multiple resistance mechanisms developed by the bacteria on which they act. Recently, cationic antimicrobial macromolecules, inspired by natural CAMPs, have gained increasing attention from the scientific community because, compared to small molecules of drug, they possess several advantages such as higher long-term activity, limited residual toxicity, chemical stability, non-volatility and the inability to permeate through the skin due to their macromolecular structure and high molecular weight (MW) [8]. Among polymers, dendrimers (Ds), a specific class of nanoscaled, hyperbranched, tree-like macromolecules, with a symmetric well-defined structure and a three-dimensional architecture [9][10][11][12], have recently shown to function as antibacterial agents and as antimicrobial surface coatings as well. The first synthesis of dendrimer materials, whose structure was conceptualized in the early 1970s, dates back to the mid-eighties. Although Ds, including positively charged ones, such as cationic poly(amidoamine) (PAMAM), polypropylenimine (PPI), dendritic polylysine, and peptide structures, have been actively investigated for a wide range of industrial and biomedical applications, their potential usages as antimicrobial agents mimicking CAMPs, both as drugs, as surface coating agents and drug-delivery systems, has been recognized only very recently.In this regard, based on the Scopus data, the scientific interest in cationic polymers as novel antimicrobials was limited until 2000, but has grown steadily and exponentially to date. On the contrary, the trend of scientific production and research in the field of antimicrobial dendrimers (ADs) over a period of 30 years definitively underscores how this was non-existent until 2007, then it started to increase, but not constantly, with the highest production in the last decade (Figure 1).
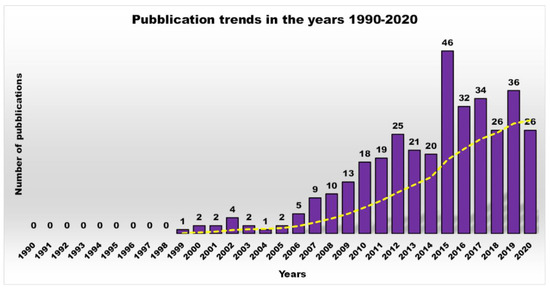
Figure 1. Number of publications as a function of time, obtained by typing the key words “antimicrobial dendrimers” in Scopus.
Furthermore, as shown in Figure 2, a more restricted investigation of the scientific output of the last decade concerning cationic antibacterial dendrimers (CADs) (yellow bars), the subject of this work, revealed that the actual number of studies is far lower than that reported in Figure 1 (purple bars), which generally depicts the number of publications on ADs.
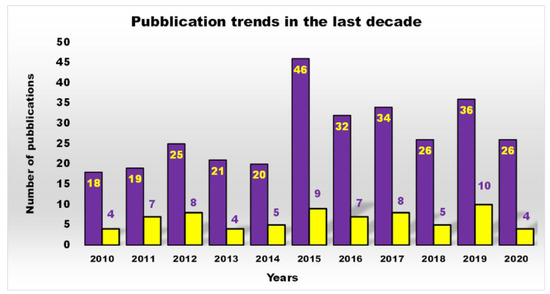
Figure 2. Number of publications, as a function of time, obtained by typing the key phrase “antimicrobial dendrimers” in Scopus (purple bars) and that obtained by typing “cationic antibacterial dendrimers” (yellow bars), topic of the review.
However, different types of Ds, including cationic ones, have been developed [13] for the treatment of infections sustained by multidrug-resistant bacteria, mainly during the last decade. In this context, some synthetized antimicrobial Ds also proved to have antibiofilm effects [14] or capabilities to act in synergism with commonly used antibiotics [15][16], and in some case studies in vivo evaluations were also performed [17]. Commercially available PAMAM and PPI dendrimers (PAMAM Ds and PPI Ds) are the most investigated, but despite their considerable broad-spectrum activity observed in vitro, if not opportunely modified to tone down several issues, such as low biodegradability, susceptibility to opsonization, toxicity to mammalian cells, including hemolytic toxicity, cytotoxicity, and hematological toxicity, as well as fast clearance, in their native form they are not suitable for clinical applications. To address these issues, as in other biomedical applications, uncharged dendrimer matrices, decorated with protonable residues, are worthy of consideration as a less toxic alternative. In this context, amino acid-modified, polyester-based dendrimer scaffolds should be the most attractive, also because of their good biodegradability [18][19]. In this first entry on cationic antibacterial dendrimers, after a short overview of the different classes of antimicrobial Ds developed up to 2010, the state of the art of PAMAM and PPI-based ones developed in the last decade, has been reviewed, highlighting the relationships structure/activity with particular attention to how dendrimer surface groups may affect the antimicrobial activity and their capability to inhibit the biofilm formation. Case studies have been also included and summarized.
Antibacterial Dendrimers (Ds)
Starting from 1978 a series of differently structured dendrimer scaffolds, endowed with different physicochemical features, have been prepared and applied in several industrial and medical areas. The increase in mortality rates, related to the emergency of non-curable bacterial infectious diseases, underlines the incessant development of resistance strategies to drugs activated by bacteria through the transmission or the acquisition of new genetic material, not only between strains of the same species, but even between strains of different species. To reach this goal to counteract bacterial resistance and to replace ineffective antibiotics, various classes of Ds that can inhibit microbial pathogens (even by killing them), have been designed. Their development, nevertheless, is still limited (Figure 1 and Figure 2) and their application in therapy is still in the exploratory phase. Interestingly, such dendrimer devices can act either as antimicrobial agents, drug-delivery devices, or bacteriophobic coatings.
Principal Types of Antimicrobial Dendrimers Developed in the Previous Decade
Although the aim and main purposes of this document is to review the state of the art of cationic antimicrobial Ds, in order to have a more complete view of all the types of Ds that possess the suitable structural characteristics to function as antimicrobial and/or antibiofilm compounds, this section quickly introduces those developed in the previous decade and already reviewed by Castonguay et al. (2012) [13] and by Mintzer et al. (2012)[3]. These include glyco Ds, cationic Ds, anionic Ds, and peptide Ds, which in some cases can also be either surface-adsorbed or metal-conjugated. Interestingly, cationic PAMAM Ds, before being considered to be per se active antimicrobial Ds, were mainly investigated as drug carriers to solubilize and deliver conventional antibiotics with synergistic intents.In their relevant paper, Mintzer et al. focused on the mechanisms of action of the different types of Ds, concentrating in particular on the effects of multivalence deriving from the tree-like and generational structure of the Ds, and also on the influence of the abundance of the active functions on the antibacterial potency of the developed dendrimers.
Cationic Antibacterial Dendrimers (CADs)
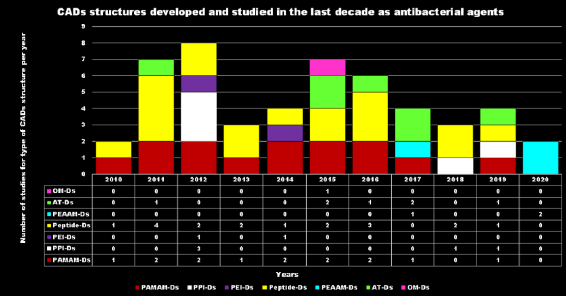
After the promising results of cationic polymers as antimicrobial agents capable of mimicking the destructive and non-specific action of CAMPs, in recent years cationic dendrimer nanoparticles have emerged as promising new antibiotic agents[20].The antibacterial activity of cationic material such as CAMPs and of their mimics depends on the electrostatic interaction between the positive charges of the device and the anionic bacteria cell surface, the progressive permeabilization of the bacterial membranes, the disruption of the lipid bilayer, the lack of cytoplasmic content, vital ions and cell death due to the disruption of the lipid bilayer. Thus, similarly to other ADs, in CADs, the multivalence in terms of positive charges plays a key role in their antimicrobial activity, and high-generation cationic Ds proved to be biocides with high activity, capability of bring localized in specific organs reduced systemic toxicity and increased duration of action[21]. Research on these agents, in fact, focuses both on their intrinsic antimicrobial activity and on the possibility they offer as drug release systems, since they are suitable for encapsulating or covalently connecting biologically active agents. This latter strategy improves the solubility of poorly soluble antibiotics, protects them from fast metabolism, increases their residence time in the circle, targets the drugs transported to specific sites of action and achieves synergistic cooperation between the cationic dendrimer carrier and the antibiotic, thus allowing a reduced dosage[22]. According to Scopus, although some sporadic case studies were reported before[23][24], the interest in cationic Ds started in 2005 and most of the research and scientific production belongs to the last decade.Among cationic Ds, PAMAM Ds were broadly studied[25], PPI Ds were principally considered when the interest in cationic Ds started, while a limited number of case studies concerning PEI-Ds were published[26][27][28]. To achieve a fair compromise between activity, biodegradability, selectivity for bacterial cells and limited hemolytic toxicity and cytotoxicity towards eukaryotic cells, poly (lysine) and peptide Ds were extensively prepared and evaluated[29][30]. Curiously, even though they were considered to be very attractive for biomedical applications as highly biodegradable and low cytotoxic, only very recently, polyester-based scaffolds, peripherally cationic for the presence of amino acids, have been taken into consideration as novel antimicrobial devices. These Ds, by presenting an uncharged hydrolysable matrix, capable of better balancing the density of charge on their surface, maintain a strong antibacterial activity associated with reduced toxicity.For each year of the last decade (x axis), Figure 3 shows the main classes of CADs that have been developed with the number of correlated studies (y axis), reflecting the researchers’ interest for the different dendrimer structures, over a 10 year period. It can be observed that although a high number of studies have been reported on cationic peptide Ds, dendrimers such as PAMAM Ds and peptide Ds have been developed throughout the decade. On the contrary, other structures, such as ammonium-terminated Ds (AT-Ds), including AT phosphorous and carbosilane Ds, attracted the interest of scientists only after 2015. Other Ds, such as PPI Ds, after initial consideration, were neglected, while PEI-Ds and organometallic Ds (OM-Ds), were taken into consideration only marginally. Polyester-based amino acid-modified Ds (PEAAM-Ds) have been considered and studied as antimicrobial devices only recently. However, all the cationic Ds reported in Figure 3 showed significant antimicrobial activity.
Figure 3. CADs developed in recent decades.
References
- Schito, A.M.; Alfei, S. Antibacterial activity of non-cytotoxic, amino acid-modified polycationic dendrimers against Pseudomonas aeruginosa and other non-fermenting Gram-negative bacteria. Polymers 2020, 12, 1818.
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting Dendrimer Multivalency To Combat Emerging and Re-Emerging Infectious Diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hooper, D.C. Hospital-Acquired Infections Due to Gram-Negative Bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- WHO. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Antibacterial Agents in Preclinical Development: An Open Access Database; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Alfei, S.; Signorello, M.G.; Schito, A.; Catena, S.; Turrini, F. Reshaped as polyester-based nanoparticles, gallic acid inhibits platelet aggregation, reactive oxygen species production and multi-resistant Gram-positive bacteria with an efficiency never obtained. Nanoscale Adv. 2019, 1, 4148–4157. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress.”. Drug Deliv. Transl. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Castonguay, A.; Ladd, E.; van de Ven, T.G.M.; Kakkar, A. Dendrimers as bactericides. New J. Chem. 2012, 36, 199–204. [Google Scholar] [CrossRef]
- Bahar, A.A.; Liu, Z.; Totsingan, F.; Buitrago, C.; Kallenbach, N.; Ren, D. Synthetic dendrimeric peptide active against biofilm and persister cells of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2015, 99, 8125–8135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qu, H.; Ma, M.; Xu, Z.; Xu, P.; Fang, Y.; Xu, T. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur. J. Med. Chem. 2007, 42, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Wronska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic effects of anionic/cationic dendrimers and levofloxacin on antibacterial activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef] [PubMed]
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM Dendrimer–PEG Hydrogels for the Treatment of Genital Infections: Formulation and in Vitro and in Vivo Evaluation. Mol. Pharm. 2011, 8, 1209–1223. [Google Scholar] [CrossRef]
- Stenström, P.; Hjorth, E.; Zhang, Y.; Andrén, O.C.J.; Guette-Marquet, S.; Schultzberg, M.; Malkoch, M. Synthesis and in Vitro Evaluation of Monodisperse Amino-Functional Polyester Dendrimers with Rapid Degradability and Antibacterial Properties. Biomacromolecules 2017, 18, 4323–4330. [Google Scholar] [CrossRef]
- Quadir, M.A.; Haag, R. Biofunctional nanosystems based on dendritic polymers. J. Control. Release 2012, 161, 484–495. [Google Scholar] [CrossRef]
- Xue, X.; Chen, X.; Mao, X.; Hou, Z.; Zhou, Y.; Bai, H.; Meng, J.; Da, F.; Sang, G.; Wang, Y.; et al. Amino-Terminated Generation 2 Poly(amidoamine) Dendrimer as a Potential Broad-Spectrum, Nonresistance-Inducing Antibacterial Agent. AAPS J. 2013, 15, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Akbari Dourbash, F.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, 395. [Google Scholar] [CrossRef]
- Yang, H.; Lopina, S.T. Penicillin V-conjugated PEG-PAMAM star polymers. J. Biomater. Sci. Polym. Ed. 2003, 14, 1043–1056. [Google Scholar] [CrossRef]
- Chen, C.Z.; Tan, N.C.B.; Cooper, S.L. Incorporation of dimethyldodecylammonium chloride functionalities onto poly(propylene imine) dendrimers significantly enhances their antimicrobial properties. Chem. Commun. 1999, 1585–1586. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Kannan, R.; Prabakaran, P.; Basu, R.; Pindi, C.; Senapati, S.; Muthuvijayan, V.; Prasad, E. Mechanistic Study on the Antibacterial Activity of Self-Assembled Poly(aryl ether)-Based Amphiphilic Dendrimers. ACS Appl. Bio Mater. 2019, 2, 3212–3224. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kumar, P.V.; Koushik, O.S. Dendrimeric Biocides—A Tool for Effective Antimicrobial Therapy. J. Nanomed. Nanotechnol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Avval, M.M.; Murthy, S.V.; Shashikanth, S. Synthesis and antimicrobial activity evaluation of poly ethylene imine (PEI) dendrimer modified with 1,2,4-oxadiazole derivatives. Int. J. Chem. Pharm. Sci. 2014, 2, 678–683. [Google Scholar]
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Young, A.W.; Liu, Z.; Zhou, C.; Totsingan, F.; Jiwrajka, N.; Shi, Z.; Kallenbach, N.R. Structure and antimicrobial properties of multivalent short peptides. Med. Chem. Comm. 2011, 2, 308–314. [Google Scholar] [CrossRef]
- Pakrudheen, I.; Banu, A.N.; Murugan, E. Cationic amphiphilic dendrimers with tunable hydrophobicity show in vitro activity. Environ. Chem. Lett. 2018, 16, 1513–1519. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 100, 215–221. [Google Scholar] [CrossRef]
- Wang, L.; Erasquin, U.J.; Zhao, M.; Ren, L.; Zhang, M.Y.; Cheng, G.J.; Wang, Y.; Cai, C. Stability, antimicrobial activity, and cytotoxicity of poly (amidoamine) dendrimers on titanium substrates. ACS Appl. Mat. Interfaces 2011, 3, 2885–2894. [Google Scholar] [CrossRef]
- Felczak, A.; Wronska, N.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Appelhans, D.; Voit, B.; Rozalska, S.; Lisowska, K. Antimicrobial activity of poly(propylene imine) dendrimers. New J. Chem. 2012, 36, 2215–2222. [Google Scholar] [CrossRef]
- Sun, B.; Slomberg, D.L.; Chudasama, S.L.; Lu, Y.; Schoenfisch, M.H. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules 2012, 13, 3343–3354. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Shah, A.; Schoenfisch, M.H. Nitric oxide-releasing amphiphilic poly (amidoamine) (PAMAM) dendrimers as antibacterial agents. Biomacromolecules 2013, 14, 3589–3598. [Google Scholar] [CrossRef]
- Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing quaternary ammonium-modified poly (amidoamine) dendrimers as dual action antibacterial agents. Bioconj. Chem. 2014, 25, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Sergesketter, A.R.; Offenbacher, S.; Schoenfisch, M.H. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J. Dent. Res. 2014, 93, 1089–1094. [Google Scholar] [CrossRef]
- Backlund, C.J.; Worley, B.V.; Schoenfisch, M.H. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly-(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Alfei, S. Antibacterial activity of non-cytotoxic, amino acid-modified polycationic dendrimers against Pseudomonas aeruginosa and other non-fermenting Gram-negative bacteria. Polymers 2020, 12, 1818. [Google Scholar] [CrossRef] [PubMed]
- Ciepluch, K.; Maciejewska, B.; Gałczyńska, K.; Kuc-Ciepluch, D.; Bryszewska, M.; Appelhans, D.; Drulis-Kawa, Z.; Arabski, M. The influence of cationic dendrimers on antibacterial activity of phage endolysin against P. aeruginosa cells. Biorgan. Chem. 2019, 91, 103121. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, L.; Liu, S.; Chen, J.; Ren, L.; Wang, Y. Antimicrobial Hyaluronic Acid/Poly(amidoamine) Dendrimer Multilayer on Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Prepared by a Layer-by-Layer Self-Assembly Method. ACS Appl. Mater. Interfaces 2015, 7, 13876–13881. [Google Scholar] [CrossRef]
- Klaykruayat, B.; Siralertmukul, K.; Srikulkit, K. Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr. Polym. 2010, 80, 197–207. [Google Scholar] [CrossRef]
- Worley, B.V.; Schilly, K.M.; Schoenfisch, M.H. Anti-biofilm efficacy of dual-action nitric oxide-releasing alkyl chain modified poly (amidoamine) dendrimers. Mol. Pharm. 2015, 12, 1573–1583. [Google Scholar] [CrossRef] [PubMed]