Cardiac masses are space occupying lesions within the cardiac cavities or adjacent to the pericardium. They include frequently diagnosed clinical entities such as clots and vegetations, common benign tumors such as myxomas and papillary fibroelastomas and uncommon benign or malignant primary or metastatic tumors. Given their diversity, there are no guidelines or consensus statements regarding the best diagnostic or therapeutic approach. In the past, diagnosis used to be made by the histological specimens after surgery or during the post-mortem examination. Nevertheless, evolution and increased availability of cardiovascular imaging modalities has enabled better characterization of the masses and the surrounding tissue. Transthoracic echocardiography using contrast agents can evaluate the location, the morphology and the perfusion of the mass as well as its hemodynamic effect. Transesophageal echocardiography has increased spatial and temporal resolution; hence it is superior in depicting small highly mobile masses. Cardiac magnetic resonance and cardiac computed tomography are complementary providing tissue characterization. The scope of this review is to present the role of cardiovascular imaging in the differential diagnosis of cardiac masses and to propose a step-wise diagnostic algorithm, taking into account the epidemiology and clinical presentation of the cardiac masses, as well as the availability and the incremental value of each imaging modality.
- cardiac tumors
- cardiac malignancies
- echocardiography
- transesophageal echocardiography
- contrast agents
- cardiac magnetic resonance
- cardiac computed tomography
1. Introduction
The diagnosis of space occupying lesions or masses in the heart can be done either in the context of investigation of a specific clinical symptomatology or incidentally, usually in the context of a cardiac imaging study performed for another reason. Cardiac masses can be classified as lesions that resemble tumors (clots, vegetations, calcifications or other rare lesions) and as benign or malignant, primary or metastatic, intracardial, or pericardial tumors [1][2][3]. Primary tumors are usually benign, with more than 50% chance of being a myxoma. Primary malignant tumors are rare, accounting for less than a quarter of primary heart tumors, in the majority of cases being sarcomas [4][5]. However, in autopsy studies a cardiac tumor most commonly represents a metastatic malignant tumor. The prevalence of primary cardiac tumors is 1:2000, while of metastatic 1:100 autopsies, reflecting a ratio of secondary to primary patients in the range of 20:1. The incidence of cardiac metastases varies among autopsy studies from 2.3% to 18.3% in patients with extracardiac malignant tumors [1][2][6].
Cardiac imaging modalities, including transthoracic (TTE) or transesophageal (TEE) echocardiography, cardiac magnetic resonance (CMR), cardiac computed tomography (CT), and 18Ffluorodeoxyglucose positron emission tomography (18F FDG-PET), have a complementary and reinforcing role for the evaluation of cardiac masses [7]. Given the diversity of cardiac masses, (from clots or vegetations to primary or metastatic malignant tumors), there are no guidelines or consensus statements regarding the best diagnostic or therapeutic approach. The scope of this review is to present the role of cardiovascular imaging in the differential diagnosis of cardiac masses and to propose a step-wise diagnostic algorithm, taking into account their epidemiology and clinical presentation, as well as the availability and the incremental value of each imaging modality.
2. Diagnostic Approach of Patients Presenting with Cardiac Masses
Knowledge of epidemiological data regarding frequency of each tumor, in the various age groups or between sexes and identification of accompanying symptoms and signs improves diagnostic accuracy of imaging tools.
The most frequent masses in the heart are clots or vegetations frequently accompanying a relevant clinical scenario such as mitral stenosis, atrial fibrillation, myocardial infarction, myocarditis with a very low ejection fraction, or infective endocarditis, respectively (Figure 1 and Figure 2).
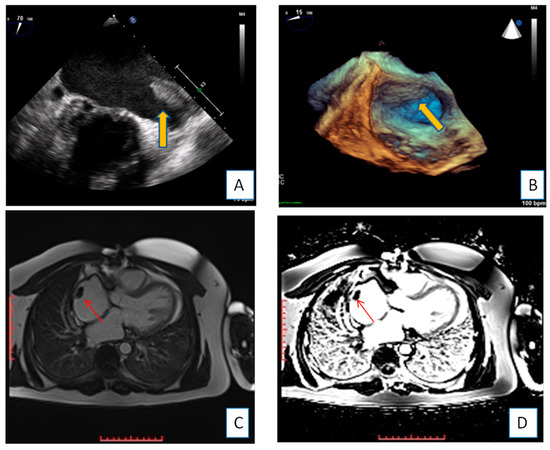
Figure 1. Transesophageal echocardiography depicting the left atrial appendage using 2D and 3D approach ((A,B), respectively). A huge thrombus (arrows) is demonstrated in the upper part of the “coumadin ridge” is an elderly woman with severe mitral stenosis. Clot in the RA (red arrows). Cine and EGE imaging ((C,D) respectively).
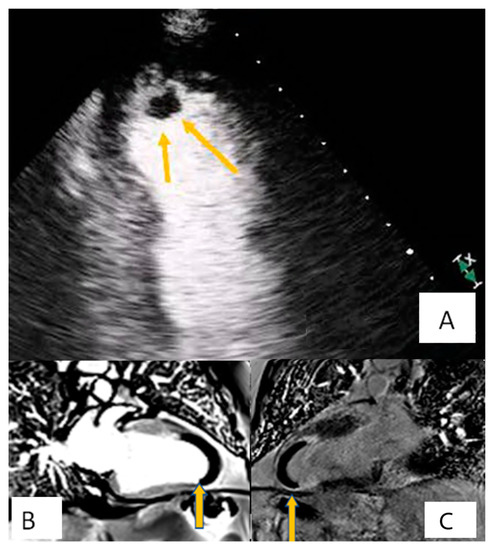
Figure 2. Patient presenting with anterior myocardial infarction the second day after primary percutaneous transluminar coronary angioplasty (PTCA). Apical 4-chamber view. (A) A lesion is depicted on cardiac apex (yellow arrows), mobile, without vasculature, findings compatible with clot. (B,C) Large laminar LV thrombus adjacent to an area of apical myocardial infarction. Two chambers EGE (image (B)) and LGE (image (C)) respectively.
Approximately 90% of the surgically-removed tumors are benign [4]. Most of these benign tumors are myxomas (80%), while the remaining ones are (in descending order) papillary fibroelastomas, fibromas, lipomas, and, even more rarely, calcified amorphous tumors, hemangiomas, teratomas, single developmental cysts, and rhabdomyomas [8,9,10,11]. Only about 10% of the primary cardiac tumors surgically removed are malignant: 90% of them identified as sarcomas or lymphomas [11].
On the other hand, secondary cancer metastases in myocardium and pericardium are more common. Secondary metastases can occur due to direct invasion of the primary tumor to the adjacent heart tissue (i.e., in case of breast or lung cancer) or hematogenous (arterial/venous) and lymphoid dispersion [12,13,14]. In certain tumor types, a genetic predisposition has been identified [15].
In infants and children, rhabdomyoma is the most frequent tumor type, followed by myxoma and fibroma [16,17]. Primary malignant tumors (usually rhabdomyosarcoma and teratoma) are very rare.
Clinical symptoms and signs usually depend on the location of the tumor rather than its histological type [18,19]. Primary benign tumors as well as secondary malignant tumors may cause myocardial or valve dysfunction and could be accompanied by heart failure symptoms (most commonly dyspnea), angina, syncope and electrical disturbances of the heart or even fatal arrhythmias [20,21]. Pericardial effusion with or without tamponade is characteristic of malignant tumors (Figure 3). Embolism due to tumor particles is a relatively frequent complication, occurring in a quarter of all cardiac tumor patients, and it is related to anatomical and histological characteristics of each tumor. Constitutional manifestations such as weight loss, malaise, and fatigue have also been described. However, most cardiac masses remain asymptomatic or present with mild and atypical symptoms [1,2,3]. Hence, they are usually recognized during outpatient care.
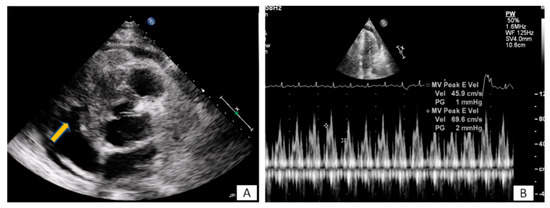
Figure 3. (A) A 65-year-old patient with fever and dyspnea for 15 days. Transthoracic echo (modified short axis view) demonstrated an amorphous mass (arrow) infiltrating the tricuspid annulus, right ventricular free wall and pericardium, causing a moderate in size pericardial effusion. (B) The pericardial fluid led to tamponade (B). Biopsy revealed a lymphoma.
3. Information Extracted by the Use of the Imaging Modalities
Certain cardiac tumors tend to appear in specific locations and structures [8]. Myxomas usually develop within the left atrium [9] (Figure 4). Most sarcomas also develop within the left atrium and can be mistakenly confused pre-operatively with myxomas. Angiosarcomas, on the other hand, are more often found within the right atrium [10]. Rhabdomyomas and fibromas are located in the ventricles, while papillary fibroelastomas are located on the valves [11] (Figure 5). Finally, metastatic tumors can be located anywhere in the heart, depending on the way cancer has spread [12]. In most cases masses spread through inferior vena cava to the right atrium (Figure 6).
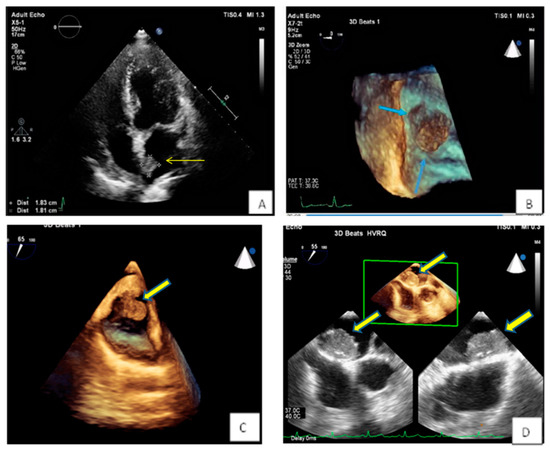
Figure 4. (A,B) Spherical lesion 1.8 × 1.8 cm attached through a wide base close to fossa ovalis was depicted at TTE (A) (yellow arrow) and subsequently TEE (B) (blue arrows—3D zoom). The location of the tumor in the left atrium as well as its morphological characteristics are compatible with a myxoma. (C) 3D TEE showing a polypoid (yellow arrow), with a smooth or mildly lobar surface located at the entrance of the inferior vena cava. Echocardiographic findings are consistent with a myxoma in a rare position. (D) 3D TEE depicting a large mass with spot calcific areas located at the fossa ovalis with a wide base, findings consistent with a left atrium myxoma. Please note the areas with higher echogenicity within the mass (yellow arrows).
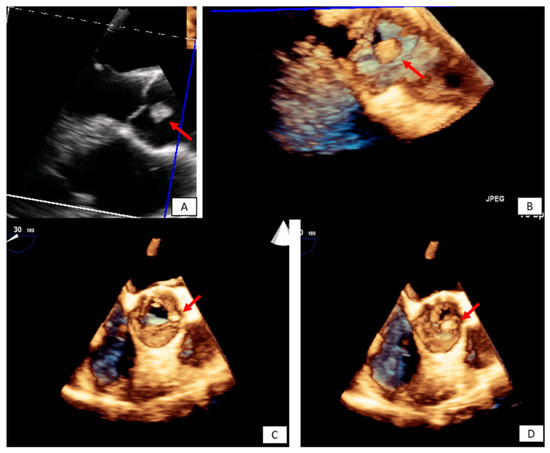
Figure 5. Incidental finding on TTE in an asymptomatic patient followed by TEE. The transesophageal image revealed a papillary fibroelastoma attached to the left cusp of the aortic valve (red arrows) 3D TEE with cropping in long axis (A,B), and short axis view ((C,D) during systole and diastole, respectively). Please note that the pedunculated lesion is attached on the tip of the aortic surface of the aortic valve, is not protruding in the LVOT during systole and doesn’t lead to valve destruction or insufficiency.
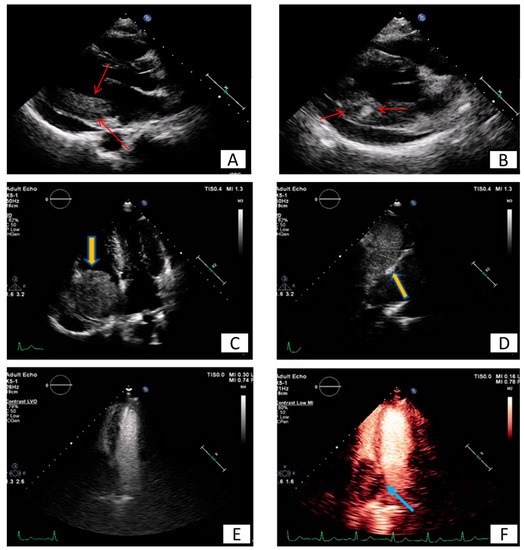
Figure 6. Long (A) and modified short axis (B) view of transthoracic echocardiography in a young female patient with metastatic carcinoma. Note the presence of pericardial effusion and the increased echogenicity and inhomogeneity of the basal posterior myocardial wall finding consistent with malignancy (red arrows). (C–F) Patient presenting with dyspnea on exertion and right heart failure symptoms during last months. A large mass (5.2 × 4.8 cm) is depicted inside the right atrium (C), originating from inferior vena cava (D). Utilizing echo contrast agents, the increased vasculature of the mass is revealed, a finding supportive of malignancy (E,F).
4. Conclusions
The differential diagnosis of the cardiac masses is critical for the clinical management of the patient. Patient history and clinical examination are evaluated in combination with cardiovascular imaging data. Experience when performing TTE or TEE and application of newer echocardiographic techniques such as contrast echocardiography, usually set the initial diagnosis and guide further work-up with the use of CT, CMR, and 18F FDG PET-CT. The latter offer additional incremental information regarding diagnosis, therapeutic management and prognosis (Figure 7).
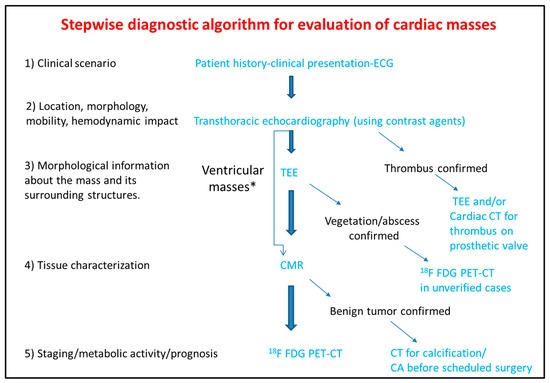
Figure 7. Step-by-step diagnostic algorithm for cardiac masses using cardiovascular imaging. Patient’s history, clinical data and ECG should be acquired and evaluated before extensive imaging work-up. With data extracted by the clinical evaluation and the TTE, a physician may initiate differential diagnosis and plan further work-up approach. When the mass is proved to be a thrombus (consider using ultrasound enhancing agents) no further work-up is needed. When thrombus is found on a prosthetic valve, TEE and/or CT can also be used. The next step is TEE especially for atrial masses. When the mass is vegetation or abscess then usually no-further work up is needed (consider PET scan as next best step when diagnosis is still under question). For other masses CMR is the next best step and guides further work-up which could include CT, or 18F FDG * For ventricular masses which are not adjacent to the cardiac valves and are not highly mobile, CMR can be alternatively used after the transthoracic echocardiogram without the need of TEE.
PET-CT.ECG: Electrocardiogram; TEE: Transesophageal echocardiogram; CMR: Cardiac magnetic resonance; CT: Computed tomography; 18F FDG PET: Fluorodeoxyglycose positron emission tomography; CA: Coronary angiography.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics10121088
References
- Basso, C.; Rizzo, S.; Valente, M.; Thiene, G. Cardiac masses and tumours. Heart 2016, 102, 1230–1245.
- Butany, J.; Nair, V.; Naseemuddin, A.; Nair, G.M.; Catton, C.; Yau, T. Cardiac tumours: Diagnosis and management. Lancet Oncol. 2005, 6, 219–228.
- Capotosto, L.; Elena, G.; Massoni, F.; De Sio, S.; Carnevale, A.; Ricci, S.; Vitarelli, A. Cardiac tumors: Echocardiographic diagnosis and forensic correlations. Am. J. Forensic Med. Pathol. 2016, 37, 306–316.
- Rahouma, M.; Arisha, M.J.; Elmously, A.; El-Sayed Ahmed, M.M.; Spadaccio, C.; Mehta, K.; Baudo, M.; Kamel, M.; Mansor, E.; Ruan, Y.; et al. Cardiac tumors prevalence and mortality: A systematic review and meta-analysis. Int. J. Surg. 2020, 76, 178–189.
- Taguchi, S. Comprehensive review of the epidemiology and treatments for malignant adult cardiac tumors. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 257–262.
- Butany, J.; Leong, S.W.; Carmichael, K.; Komeda, M. A 30-year analysis of cardiac neoplasms at autopsy. Can. J. Cardiol. 2005, 21, 675–680.
- Lemasle, M.; Lavie Badie, Y.; Cariou, E.; Fournier, P.; Porterie, J.; Rousseau, H.; Petermann, A.; Hitzel, A.; Carrié, D.; Galinier, M.; et al. Contribution and performance of multimodal imaging in the diagnosis and management of cardiac masses. Int. J. Cardiovasc. Imaging 2020, 36, 971–981.
- Burke, A.; Tavora, F. The 2015 WHO Classification of tumors of the heart and pericardium. J. Thorac. Oncol. 2016, 11, 441–452.
- Colin, G.C.; Gerber, B.L.; Amzulescu, M.; Bogaert, J. Cardiac myxoma: A contemporary multimodality imaging review. Int. J. Cardiovasc. Imaging 2018, 34, 1789–1808.
- Neuville, A.; Collin, F.; Bruneval, P.; Parrens, M.; Thivolet, F.; Gomez-Brouchet, A.; Terrier, P.; De Montpreville, V.T.; Le Gall, F.; Hostein, I.; et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: Clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am. J. Surg. Pathol. 2014, 38, 461–469.
- Tsugu, T.; Nagatomo, Y.; Endo, J.; Kawakami, T.; Murata, M.; Yamazaki, M.; Shimizu, H.; Fukuda, K.; Mitamura, H.; Lancellotti, P. Multiple papillary fibroelastomas attached to left ventricular side and aortic side of the aortic valve: A report of new case and literature review. Echocardiography 2019, 36, 1194–1199.
- Yanagawa, B.; Mazine, A.; Chan, E.Y.; Barker, C.M.; Gritti, M.; Reul, R.M.; Ravi, V.; Ibarra, S.; Shapira, O.M.; Cusimano, R.J.; et al. Surgery for Tumors of the Heart. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 385–397.
