Exosomes are lipid bilayer particles released from cells into their surrounding environment. These vesicles are mediators of near and long-distance intercellular communication and affect various aspects of cell biology. In addition to their biological function, they play an increasingly important role both in diagnosis and as therapeutic agents.
- Exosomes
1. Introduction
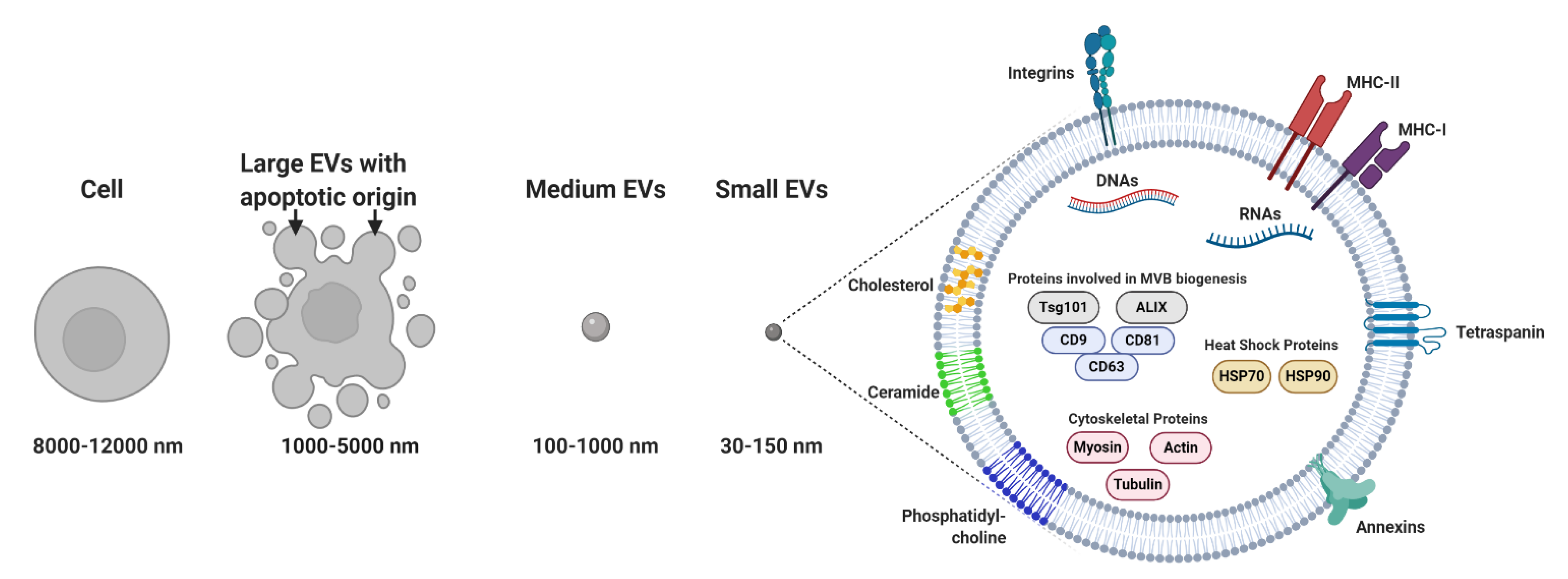
Membrane-bound and heterogeneous extracellular vesicles (EVs) were initially considered anecdotal examples of cell debris or apoptotic bodies released by the majority of cells [1]. EVs are now regarded as key diagnostic tools [2,3,4] and therapeutic agents [5]. EVs facilitate communication processes between near and distant cells. In addition, these vesicles can be grouped into two major categories: (a) microvesicles (MVs; 100–1000 nm), considered to be functional liposomes composed of molecules such as nucleic acids, proteins and functional lipids surrounded by a lipid bilayer and (b) exosomes (EXOs; 30–150 nm) (Figure 1) [6], which differ from MVs in their size, protein composition, buoyant density, release mechanism and potential physiological role [7,8,9,10].
2. Applications of Exosomes in Biomedicine
2.1. Exosomes as Biomarkers
Exosomes are now regarded as new players in regenerative medicine due to their therapeutic capacity and their potential as noninvasive biomarkers for early diagnosis; the evaluation of treatment efficacy and monitoring of the progression of cancer, neurodegenerative, metabolic and infectious diseases [5,305]. They offer a simple method for the molecular analysis of biofluids that reduces invasive surgery and promotes more precise medical interventions. Several clinical trials have been launched for both early screening and accurate diagnosis to reduce mortality rates and to increase recovery rates. The molecular content of exosomes reflects the origin and pathophysiological conditions of releasing cells, suggesting that the analysis of exosomal markers is a highly specific and sensitive method that could potentially replace invasive biopsies. In addition, their small volume, specific biological information, strong permeability through body tissue barriers, abundance and long half-lives in all biological fluids make these biomarkers highly attractive targets for clinical diagnostic applications. In addition to nucleic acids, exosomal proteins have been found to be potential biomarkers for a variety of pathologies, including cancer, as well as a number of noncancer diseases in different organs, such the central nervous system [195,197], the kidneys [306,307], liver [308] and lungs [309].
2.2. Use of Exosomes as Therapeutic Agents
In many studies, exosomes have been used as delivery vectors for small-molecule therapeutic agents, as they are capable of traveling from one cell to another and of conveying their cargo in a biologically active form, thus acting as attractive gene and drug delivery vehicles [360]. Cancer cells internalize a significantly larger percentage of exosomes as compared to normal cells. HEK293 and MSC exosomes were therefore effectively used as delivery vectors to transport PLK-1 small interfering RNA (siRNA) to bladder cancer cells in vitro, resulting in the selective gene silencing of PLK1 [361]. In addition, the internalization of exosomes in tumor cells is ten times greater than that of liposomes of comparable size due to their lipid composition and surface proteins, indicating the superior specificity of exosomes for cancer targeting [362]. Furthermore, exosomes offer several advantages over standard delivery vehicles. For example, exosomes are able to cross biological barriers, such as the blood–brain barrier (BBB), have poor immunogenicity and can be cell-specific [363]. Therefore, exosomes could be next-generation nontoxic delivery tools that combine nanoparticle sizes with high capacity levels, making them powerful vectors for the treatment of a variety of pathologies [364].
Doxorubicin-loaded exosomes are transported to tumor tissues and reduce tumor growth in mice without any adverse effects observed from this equipotent free drug [365]. Tian and coworkers used mouse immature dendritic cells (imDCs) for exosome production due to their lack of immunostimulatory markers. Purified imDC-derived exosomes were gently mixed with doxorubicin (DOX) in an electroporation buffer and then examined under a transmission electron microscope to verify the recovery of their plasma membrane. After loading the therapeutic cargo, these vesicles successfully delivered DOX to the targeted cell nucleus, leading to the inhibition of tumor growth without overt toxicity [366]. In another study, exosomes derived from a brain endothelial cell line, bEND.3, were loaded with DOX and used to deliver the anticancer drug across the blood–brain barrier (BBB) for the treatment of brain cancer in a zebrafish model [367]. The membrane vesicles mediated the autonomous intercellular migration of anticancer agents through multiple cancer cell layers and enabled hydrophobic and hydrophilic compounds to significantly penetrate both spheroids and in vivo tumors, thereby enhancing their therapeutic efficacy [368]. Interestingly, chemotherapeutic agents epirubicin and paclitaxel increased miR-503 levels in exosomes released from human umbilical vein endothelial cells (HUVECs) as compared to control conditions and were demonstrated to induce antitumor responses during breast cancer chemotherapy [369].
Exosomes also have the potential to deliver oligonucleotides, such as mRNA, miRNA and various noncoding RNAs, as well as mitochondrial and genomic DNA, to other cells, thus offering considerable advantages as ideal delivery systems for gene therapy [370]. As with the incorporation of genetic material into living cells, Alvarez-Erviti and colleagues used electroporation to deliver short interfering siRNA analogs to the brain in mice via exosomes [363]. In addition, Wahlgren and coworkers used plasma exosomes as gene delivery vectors to transport exogenous siRNA to human blood cells. The vesicles successfully delivered the administered siRNA to monocytes and lymphocytes, leading to robust gene silencing of mitogen-activated protein kinase 1, thus suggesting exosomes as a new generation of drug carriers that enable the development of safe and effective gene therapies [371]. Similarly, Kamerkar et al. demonstrated a technique for the direct and specific targeting of oncogenic KRAS in tumors using electroporated MSC-derived exosomes with siRNA. This treatment suppressed cancer in multiple mouse models of pancreatic cancer and significantly increased overall survival rates [372]. The same method was used to load exosomes with miRNA to the epidermal growth factor receptor (EGFR) expressed in breast cancer cells, indicating that exosomes can be used therapeutically to target EGFR-expressing cancerous tissues with nucleic acid drugs [373]. Finally, endothelial cells treated with chemotherapeutic agents are reported to release more exosomes that contain miRNA-503. Given that miRNA-503 is downregulated in exosomes released from endothelial cells cultured under tumoral conditions, the introduction of miRNA-503 into breast cancer cells altered their proliferative and metastatic capacities by inhibiting both CCND2 and CCND3 [369].
Lee and colleagues demonstrated that exosomes derived from MSCs deliver specific miRNA mimics (miRNA-124 and miRNA-145) and decrease glioma cell migration and the stem cell properties of cancer cells, providing an efficient route of therapeutic miRNA delivery in vivo [374]. In addition, the intratumoral injection of exosomes derived from miRNA-146-expressing MSCs results in a considerable reduction in glioma xenograft development in a rat brain tumor model and decreases cell growth and invasion, suggesting that the export of specific therapeutic miRNA into MSC exosomes represents an effective treatment strategy for malignant glioma [375]. O’Brien and coworkers engineered EVs loaded with miRNA-134, which is substantially downregulated in breast cancer tissue as compared to healthy tissue. It has been demonstrated that miRNA-134-enriched EVs reduce STAT5B and Hsp90 levels in target breast cancer cells, as well as cellular migration and invasion, and enhance the sensitivity of these cancer cells to anti-Hsp90 drugs [376]. Similarly, MSC-derived exosomes encapsulated with miRNA-379 were administered in breast cancer therapy in vivo. The results of this study show that miRNA-379-enriched EVs are potent tumor suppressors with an exciting potential as an innovative therapy for metastatic breast cancer [377]. Bovy et al. identified miRNA-503, whose expression levels are downregulated in exosomes released from endothelial cells cultured under tumoral conditions. Endothelial cells are able to transfer miRNA-503 via exosomes to breast cancer cells, thus impairing their growth and altering their proliferative capacity [369]. Breast cancer cells prime MSCs to secrete exosomes containing distinct miRNA contents, which promotes quiescence in a subset of cancer cells and confers drug resistance. According to this study, a novel therapeutic approach to target dormant breast cancer cells based on the systemic administration of MSCs loaded with antagomiRNA-222/223 resulted in the chemosensitization of cancer cells and increased survival rates [148].
Shtam et al. introduced two different anti-RAD51 and -RAD52 siRNAs into Henrietta Lacks (HeLa) cell-derived exosomes. These exosomes effectively delivered siRNA into the recipient cancer cells and caused strong RAD51 knockdown, providing additional evidence of the ability to use human exosomes as vectors in cancer therapy [378]. In a similar study, Shimbo and coworkers found that the transfer of miRNA-143 by means of MSC-derived exosomes decreases in the in vitro migration of osteosarcoma cells [379]. In addition, miRNA-122-transfected adipose tissue-derived MSCs (AMSCs) can effectively generate miRNA-122-encapsulated exosomes, which can mediate miRNA-122 communication between AMSCs and hepatocellular carcinoma (HCC) cells, thereby elevating tumor cell sensitivity to chemotherapeutic agents through the alteration of miRNA-122 target gene expression in HCC cells [380]. Usman and colleagues have described a strategy for generating large-scale amounts of exosomes for the delivery of RNA drugs, including antisense oligonucleotides (ASOs). They chose human red blood cells (RBCs), which are devoid of DNA, for EV production. RBC EVs were demonstrated to deliver therapeutic ASOs in order to effectively antagonize oncogenic micro-RNAs (oncomiRNAs) and to suppress tumorigenesis [381]. Exosomes could potentially deliver therapeutic proteins to recipient cells, with a recent study reporting the feasibility of using exosomes as biocompatible vectors that could improve the targeting and delivery of therapeutic proteins to specific cells in diseased tissues [382]. In addition, Haney et al. used a new method to treat Parkinson’s disease (PD). In fact, catalase-loaded exosomes produce a potent neuroprotective effect on both in vitro and in mouse brains following intranasal administration. This result demonstrates the capacity of exosomes to load fully functional proteins and to treat specific disorders [383]. Several approaches have envisaged the utilization of specific conserved domains in order to enhance the loading of proteins. For instance, Sterzenbach and colleagues exploited late-domain (L-Domain) proteins and ESCRT machinery pathways to load Cre recombinase into exosomes. This protein was successfully delivered to neurons through a nasal route, a well-characterized noninvasive method to deliver exogenous proteins to the brain via exosomes [384]. Human ubiquitin was also used as a sorting sequence to deliver diverse proteins into exosomes such as EGFP and nHER2. Interestingly, C-terminal–ubiquitin fusion may act as an efficient signal sequence of antigenic proteins into exosomes, which could support the use of exosomes as vaccines [385].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13010084
