The eyes are the window to the world and the key to communication, but they are vulnerable to multitudes of ailments. More serious than is thought, corneal infection by herpes simplex viruses (HSVs) is a prevalent yet silent cause of blindness in both the paediatric and adult population, especially if immunodeficient. Globally, there are 1.5 million new cases and forty thousand visual impairment cases reported yearly. The Herpetic Eye Disease Study recommends topical antiviral as the front-line therapy for HSV keratitis. Ironically, topical eye solutions undergo rapid nasolacrimal clearance, which necessitates oral drugs but there is a catch of systemic toxicity. The hurdle of antiviral penetration to reach an effective concentration is further complicated by drugs’ poor permeability and complex layers of ocular barriers.
- ocular drug delivery
- herpes simplex virus keratit
1. Introduction
Eye infection is a prevalent problem in primary care and remains a crucial healthcare concern. According to the American Academy of Ophthalmology (AAO), herpes simplex virus (HSV) keratitis (HSK) is the leading cause of infectious blindness worldwide [1]. HSK is defined as a corneal inflammatory condition caused by the HSV infection [1][2]. The global incidence of herpetic keratitis is estimated at 1.5 million per year, resulting in 40,000 new cases of severe visual impairment associated with corneal scarring and opacification [3][4]. HSV type I (HSV-1) is by far the most predominant causative pathogen of eye infections (Figure 1) [1]. HSV-1 is also known for causing orolabial herpes, HSV folliculitis, herpes gladiatorum, herpetic whitlow, and eczema herpeticum [4][5][6]. HSV can be transferred to the eye by touching an active lesion and then the eye [1]. The National Health and Nutrition Evaluation revealed a seroprevalence of HSV-1 in 53.9% of 14–49 year olds, and 90% of adults 50 years or older [7][8], indicating that the majority of the population has been exposed to this virus thus are at risk of developing HSK.
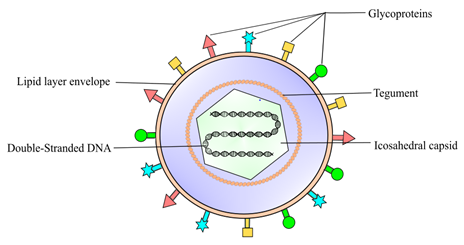
Figure 1. Herpes simplex virus 1 (HSV-1) is classified into the Alphaherpesvirinae family, a sub-family of Herpesviridae. It is an enveloped DNA virus consisting of a linear double-strand genome protected inside an icosahedral capsid. The inner tegument is made up of a layer of mRNA and proteins, whereas the outer lipid bilayer envelope contains glycoproteins. These glycoproteins are responsible for the invasion of the virus into the host cell.
The HSK manifestations can be categorised according to epithelial keratitis, stromal keratitis, and endotheliitis [5]. Most of the initial infection cases (80%) usually manifest as acute epithelial keratitis [9]. Patients may report symptoms like sudden eye pain and redness, watery discharge, photophobia, and blurry vision [1][2]. Under slit-lamp microbioscopy examination, the patterns of epithelial lesions can be differentiated into punctate, dendritic, or geographic ulcers [5]. Punctate lesions appear like granular vesicles which can rapidly coalesce into a linear branching dendritic pattern [10]. Further dendritic extensions at the edges can cause geographic ulcers. These lesions contain highly contagious and active replicating viruses, which results in the desquamation of the corneal structure. Without appropriate and prompt treatment, patients may suffer prolonged infection, making them susceptible to recurrent keratitis [9][10][11]. Although the symptoms typically affect the unilateral eye, bilateral infection is common in younger age groups and immunocompromised patients [12]. Compared to adults, children have overall worse visual outcomes and hence they are at greater risk of permanent vision loss from amblyopia [12]. Furthermore, the impact of the disease in developing nations with limited access to treatment and immunosuppression perhaps contributes to a significantly higher visual morbidity than currently known [12].
The Herpes Eye Disease Study (HEDS) recommends topical formulations containing antiviral agents as the front-line treatment for herpes epithelial keratitis. Undeniably, topical formulations are the most popular choices for ocular treatment as they are convenient to use. Nonetheless, there are multiple drawbacks associated with the present topical antiviral agents. Limited ocular bioavailability and a lack of controlled drug release profile are factors that hinder the effectiveness of topical antiviral formulations. Oral antiviral drugs may be the alternative treatment, but when given at high doses, the drugs can cause untoward systemic toxicities [13]. Hence, the need the leverage pharmaceutical technology to fabricate a more efficient, sustained release, and affordable antiviral topical formulations to halt disease progression and further elucidate their prospective roles in HSK prevention.
2. Pathophysiology of Herpes Simplex Virus Keratitis Condition
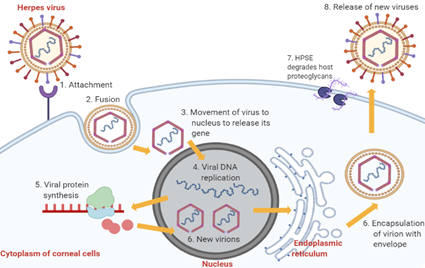
The pathophysiology of HSK involves complex sequential processes. Primary active infection begins with HSV-1 entry into corneal epithelial cells (Figure 2). The attachment of HSV-1 glycoproteins to the receptors on the epithelial cell surface enables the viral envelope to fuse with the cytoplasmic membrane [14]. After that, the viral tegument and nucleocapsid proteins are released into the host cytoplasm [14]. The capsid then moves towards the nucleus to begin viral DNA replication using host DNA polymerase while the virus mRNA is being transcribed and translated into new proteins [14]. The proteins and viral genes are assembled in the nucleus to form new virions, which travel to the endoplasmic reticulum and Golgi apparatus to acquire new envelopes [14]. In the end, HSV-1 results in cytolysis of infected epithelial cells while the new viral progenies are released with the help of heparanase (HPSE) and, subsequently, they infect other neighbouring cells [14].
Following acute epithelial infection, cytokines facilitate the infiltration of polymorphonuclear leucocytes, natural killer cells, macrophages, and Langerhans cells into the corneal stroma and endothelium layers [14][15]. Type I interferon (IFN-a/b), HSV-specific IgG, and IgA may help prevent virus spread . However, uncontrolled viral multiplication may happen specifically among patients who are immunocompromised. Subsequently, it may result in corneal basement membrane rupture and initiation of stromal disease . As HSV is neurotropic, some may stay dormant in optic nerves or trigeminal nerves before reactivation [15]. Virus reactivation is likely to happen due to a combination of host, virus, and environmental factors, which can be stress, hormonal changes, ultraviolet light exposure, and laser treatment.
Stromal and endothelial keratitis results primarily from the host’s immune response towards the virus spread from epithelial infection or viral reactivation. It is postulated that the recruited pro-inflammatory cytokines and growth factors initially assist in virus removal but later cause tissue destruction, scarring, and neovascularisation. Connective tissue in corneal scars is organised abnormally, leading to increased light scatter and corneal opacity. Some other complications of HSK are neurotrophic keratopathy and superinfection. Moreover, patients had reported significant impairment in quality of life associated with ocular pain, especially those who encounter multiple relapses [16]. Therefore, antiviral treatment is essential in suppressing antiviral spread to prevent the severe sequelae of HSK.
Figure 2. Stages of HSV-1 replication in corneal epithelial cells. Abbreviation: HPSE, heparanase, an enzyme which cleaves heparan sulfate proteoglycans to prevent virions from being trapped by the proteoglycans, thus promoting virion egress.
3. Available Treatments for Herpes Simplex Virus Keratitis and Associated Limitations
According to the AAO HSK Treatment Guideline 2014, the treatment approach varies depending on the classification and the severity of the ocular infection. The goal of treatment is to minimise corneal scarring, delay progression of stromal damage and improve the patient’s quality of life. In general, the therapeutic interventions for HSK include antiviral agents, immunosuppressive agents, debridement, and surgical transplantation. There are advantages and challenges associated with each treatment option. The AAO HSK Treatment Guideline 2014 and HEDS recommend topical antiviral agents as first-line pharmacotherapy for epithelial HSK. The early generation antivirals are idoxuridine, iododesoxycytidine, vidarabine, and trifluridine [17][18]. Except for trifluridine, the rest of them have been discontinued due to reported harmful ocular side effects. Trifluridine is a synthetic pyrimidine nucleoside that inhibits thymidine incorporation into replicating DNA, thereby preventing the production of functional viral proteins and new virions. Trifluridine 1% ophthalmic solution was the United States Food and Drug Administration (FDA)-approved treatment for treating epithelial HSK and keratoconjunctivitis. It is used up to nine times daily for one week; the dose is then tapered down after one week. Due to the non-selective inhibition of DNA synthesis in both virus-infected and uninfected cells, trifluridine can cause local toxicities with prolonged use, i.e., ulceration, dysplasia, and canalicular stenosis. Hence, it should not be continuously used for more than 21 days due to a high risk of ocular toxicity. These ocular toxicities have led to the decline of trifluridine use compared to new topical antivirals.
Acyclovir and ganciclovir are the new generation of antiviral agents. Acyclovir is a purine nucleoside with a better antiviral selectivity compared to trifluridine, thus having fewer ocular side effects. Acyclovir comes in the form of ointment due to its lipophilicity characteristic and it is formulated with a polyethylene glycol (PEG) base . Acyclovir ointment is widely used in other countries outside of the U.S., such as in Europe and Australia [19]. It is prescribed to patients with epithelial HSK with three to five times daily administration for one to three weeks. Furthermore, ganciclovir is a synthetic purine nucleoside shown to be as effective as acyclovir in achieving a cornea cure rate [20]. Ganciclovir was marketed as FDA-approved 0.15% gel in 2009. Ganciclovir provides a greater antiviral spectrum, including human herpes virus, varicella-zoster virus, cytomegalovirus, Epstein–Barr virus, and adenovirus. The ganciclovir molecule is relatively more potent than acyclovir in causing rapid apoptosis of HSV-infected cells. Thus, it is effective at a lower concentration (0.15% gel) compared to acyclovir (3% ointment) [21]. It can be given to patients with epithelial HSK with five times daily administration until healing of the corneal ulcer; the dose frequency is then tapered down to three times daily for a week. Under some circumstances, the oral antiviral therapy has been used in place of topical agents in treating epithelial HSK. For example, when the patient is more susceptible to ocular surface toxicity (due to pre-existing eye disease) and paediatric patients refractory to topical antiviral .
At the same time, oral antiviral agents are indicated to treat HSV stromal and endothelial keratitis. The oral antiviral agent aims to decrease viral load and reduce the magnitude of the inflammatory response in conjunction with a topical corticosteroid. Examples of oral antiviral agents are acyclovir, valacyclovir, and famciclovir. Valacyclovir has good systemic bioavailability and safety profile compared to acyclovir. Sozen et al. reported that oral valacyclovir was more efficient in terms of epithelial healing rate and lower photophobia score [22]. Famciclovir is a prodrug of penciclovir proven to have clinical benefits in treating herpes virus with even better bioavailability. Oral antivirals are also recommended as a prophylaxis treatment in patients with frequent recurrence of HSK, or undergoing any excimer laser photokeratectomy procedure. However, resistance to acyclovir has become more prevalent, especially among immunocompromised patients. Alvarez et al. explained that approximately 3.5–10% of immunocompromised patients and 1% of immunocompetent patients reported resistance. A novel approach in real-time cell analysis (RTCA) with a rapid result test compared to the gold standard phenotypic plaque reduction assay (PRA) is now emerging in the clinical field to encourage the screening of resistance to antivirals and provide a better treatment to patients [23][24][25].
Meanwhile, a topical corticosteroid is used in combination with oral antiviral agents in managing stromal HSK and endotheliitis. As discussed in the pathophysiology section, stromal HSV and endothelitiitis are complicated by underlying immune reaction and viral antigens. Corticosteroids are able to inhibit cellular immune response, opacification, scarring, and neovascularisation. Available corticosteroids include prednisolone acetate 1% suspension, prednisolone sodium phosphate 1% suspension, fluorometholone 0.1% suspension, rimexolone 1% suspension, and difluprednate 0.05% emulsion. HEDS I reported that the corticosteroid treatment group showed a greater reduction (68%) in stromal keratouveitis progression compared to a placebo group. However, the judicious use and dose tapering of corticosteroids is warranted to prevent adverse effects such as exacerbation of infection, corneal thinning, and steroid-induced glaucoma and cataract. Some studies suggest topical cyclosporine or topical tacrolimus as supplementary immunosuppressive agents but require further clinical validation [26]. Debridement and cryopreserved amniotic membrane (CAM) may aid in re-establishing epithelial healing [27][28]. Lastly, corneal transplant may be an option for patients with severe corneal scarring, yet corneal graft rejection is another treatment challenge [29]. At present, research on the vaccine and new therapeutic molecules against the latent virus is still underway, which may take a substantial amount of time before they can be used in clinical settings.
In brief, topical antivirals are essential first-line agents to treat epithelial HSK at the earliest onset to suppress viral replication, maintain latency, and prevent complications. In oral formulations, a small amount of the drug will reach the posterior chamber of the eye, limited by the retinal blood barrier [30]. Besides, both oral acyclovir and valacyclovir may cause nephrotoxicity in renal impairment patients. However, there are several limitations with current topical antiviral agents. Due to a lack of optimal sustained drug release properties, all of them require multiple doses (five to nine times). Particularly, ophthalmic solution is the most rapidly eliminated through the nasolacrimal route [31][32]. Ointment can cause blurred vision and sticky, greasy, and gritty sensations after application, thus reducing patient compliance. Pre-formed gel can be difficult to use due to a thicker consistency compared to eye drops. Moreover, none of the current topical antiviral agents supports drug permeation into deeper ocular layers. The possibility of using topical agents in HSK prophylaxis without causing resistance is an area worthy of investigation. Hence, the following discussions aim to give insights into the novel strategies that can surpass current treatment challenges of HSK epithelial keratitis. The ultimate goal of ocular formulations is to efficiently cross both static and dynamic barriers of the eye and reach the targeted infection site without excessive exposure of patients to systemic toxicities [30][31].
Therefore, the physical barriers and the problems associated with conventional ocular formulations are the major challenges in ophthalmic drug development. The mucoadhesive polymeric approaches have gained much importance in recent years as they offer several advantages which minimise the challenges. Moreover, the mucoadhesive delivery system enables drug release at the site of action, which avoids enzymatic degradation in the gastrointestinal tract as well as the first-pass effect [33]. This system also promotes local absorption due to the rich blood supply of the mucosa, provides a localised effect, and subsequently improves ocular bioavailability along with therapeutic efficacy [33][34][35].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13010001
References
- White, M.L.; Chodosh, J. Herpes Simplex Virus Keratitis: A Treatment Guideline - 2014 - American Academy of Ophthalmology. Available online: https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline (accessed on 4 September 2020).
- Sugar, A.; Jacobs, D.; Hirsch, M.; Givens, J. Herpes simplex keratitis. Available online: https://www.uptodate.com/contents/herpes-simplex-keratitis (accessed on 4 Septebmber 2020).
- Farooq, A. V.; Shukla, D. Herpes Simplex Epithelial and Stromal Keratitis: An Epidemiologic Update. Surv. Ophthalmol. 2012, 57, 448–462, doi:10.1016/j.survophthal.2012.01.005.
- Ahmad, B.; Patel, B.C. Herpes Simplex Keratitis; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Valerio, G.S.; Lin, C.C. Ocular manifestations of herpes simplex virus. Curr. Opin. Ophthalmol. 2019, 30, 526–531, doi:10.1097/ICU.0000000000000618.
- Johnston, C.; Wald, A.; Hirsch, M.; Mitty, J. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection. Available online: https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection (accessed on 4 September 2020).
- Kalezic, T.; Mazen, M.; Kuklinski, E.; Asbell, P. Herpetic eye disease study: Lessons learned. Curr. Opin. Ophthalmol. 2018, 29, 340–346, doi:10.1097/ICU.0000000000000482.
- Khadr, L.; Harfouche, M.; Omori, R.; Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J. The epidemiology of herpes simplex virus type 1 in Asia: Systematic review, meta-analyses, and meta-regressions. Clin. Infect. Dis. 2019, 68, 757–772, doi:10.1093/cid/ciy562.
- Chou, T.Y.; Hong, B.Y. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: Background, effectiveness, tolerability, safety, and future applications. Ther. Clin. Risk Manag. 2014, 10, 665–681, doi:10.2147/TCRM.S58242.
- Lobo, A.M.; Agelidis, A.M.; Shukla, D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul. Surf. 2019, 17, 40–49.
- Cabrera-Aguas, M.; Robaei, D.; McCluskey, P.; Watson, S. Clinical translation of recommendations from randomized trials for management of herpes simplex virus keratitis. Clin. Exp. Ophthalmol. 2018, 46, 1008–1016, doi:10.1111/ceo.13319.
- Vadoothker, S.; Andrews, L.; Jeng, B.H.; Levin, M.R. Management of Herpes Simplex Virus Keratitis in the Pediatric Population. Pediatr. Infect. Dis. J. 2018, 37, 949–951, doi:10.1097/INF.0000000000002114.
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Rupenthal, I.D.; Al-Kassas, R. Ocular delivery systems for topical application of anti-infective agents. Drug Dev. Ind. Pharm. 2016, 42, 1–11, doi:10.3109/03639045.2015.1070171.
- Tsatsos, M.; MacGregor, C.; Athanasiadis, I.; Moschos, M.M.; Hossain, P.; Anderson, D. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin. Exp. Ophthalmol. 2016, 44, 824–837, doi:10.1111/ceo.12785.
- Koganti, R.; Yadavalli, T.; Shukla, D. Current and emerging therapies for ocular herpes simplex virus type-1 infections. Microorganisms 2019, 7, 429–429, doi:10.3390/microorganisms7100429.
- Reynaud, C.; Rousseau, A.; Kaswin, G.; M’garrech, M.; Barreau, E.; Labetoulle, M. Persistent Impairment of Quality of Life in Patients with Herpes Simplex Keratitis. Ophthalmology 2017, 124, 160–169, doi:10.1016/j.ophtha.2016.10.001.
- Álvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farías, M.A.; Henríquez, A.; Agurto-Muñoz, C.; González, P.A. Current Antivirals and Novel Botanical Molecules Interfering With Herpes Simplex Virus Infection. Front. Microbiol. 2020, 11, 139, doi:10.3389/fmicb.2020.00139.
- Roozbahani, M.; Hammersmith, K.M. Management of herpes simplex virus epithelial keratitis. Curr. Opin. Ophthalmol. 2018, 29, 360–364, doi:10.1097/ICU.0000000000000483.
- Cabrera-Aguas, M.; Kerdraon, Y.; Symes, R.J.; McCluskey, P.; Samarawickrama, C.; Rawlinson, W.; Watson, S.L. Development, Implementation, and Evaluation of Treatment Guidelines for Herpes Simplex Keratitis in Sydney, Australia. Cornea 2020, 39, 834–840, doi:10.1097/ICO.0000000000002273.
- Wilhelmus, K.R. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst. Rev. 2015, 2015.
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689, doi:10.1016/j.ophtha.2017.05.012.
- Sozen, E.; Avunduk, A.M.; Akyol, N. Comparison of Efficacy of Oral Valacyclovir and Topical Acyclovir in the Treatment of Herpes Simplex Keratitis: A Randomized Clinical Trial. Chemotherapy 2006, 52, 29–31, doi:10.1159/000090239.
- Vikas, R.; Prabhu, S.G.; Mudgal, P.P.; Shetty, U.; Karunakaran, K.; Jagadesh, A.; Auti, A.; Stansilaus, R.P.; Nair, S.; Arunkumar, G. HSV susceptibility to acyclovir – genotypic and phenotypic characterization. Antivir. Ther. 2019, 24, 141–145, doi:10.3851/IMP3279.
- Piret, J.; Goyette, N.; Boivin, G. Novel method based on real-time cell analysis for drug susceptibility testing of herpes simplex virus and human cytomegalovirus. J. Clin. Microbiol. 2016, 54, 2120–2127, doi:10.1128/JCM.03274-15.
- Caliaro, O.; Barbani, M.T.; Klenja, S.; Morfin, F.; Frobert, E.; Gorgievski, M.; Steinlin-Schopfer, J.; Suter-Riniker, F. Phenotypic testing of patient herpes simplex virus type 1 and 2 isolates for acyclovir resistance by a novel method based on real-time cell analysis. J. Clin. Virol. 2020, 125, 104303, doi:10.1016/j.jcv.2020.104303.
- Akbari, M.; Moghadam, R.S.; Elmi, R.; Nosrati, A.; Taghiabadi, E.; Aghdami, N. Topical tacrolimus as an adjunct to conventional therapy for stromal herpetic keratitis: A randomized clinical trial. J. Ophthalmic Vis. Res. 2019, 14, 400–411, doi:10.18502/jovr.v14i4.5437.
- Brocks, D.; Mead, O.G.; Tighe, S.; Tseng, S.C.G. Self-retained cryopreserved amniotic membrane for the management of corneal ulcers. Clin. Ophthalmol. 2020, 14, 1437–1443, doi:10.2147/OPTH.S253750.
- Field, A.J.; Gottsch, J.D. Persisting epithelial herpes simplex keratitis while on cyclosporin-A ointment. Aust. N. Z. J. Ophthalmol. 1995, 23, 333–334, doi:10.1111/j.1442-9071.1995.tb00186.x.
- Sharif, Z.; Sharif, W. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019, 63, 15–22, doi:10.22336/rjo.2019.4.
- Castro-Balado, A.; Mondelo-García, C.; Zarra-Ferro, I.; Fernández-Ferreiro, A. New ophthalmic drug delivery systems. Farm. Hosp. 2020, 44, 149–157, doi:10.7399/fh.11388.
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2020, 374, 602–624, doi:10.1124/jpet.119.256933.
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22, doi:10.1016/j.jconrel.2020.01.057.
- Tandel, H.T.; Parmar, H.K.; Pandya, K.K.; Pardasani, L.J.; Panchal, V.S. A Systematic Review on Mucoadhesive Drug Delivery System. World J. Pharm. Res. 2017, 6, 337–366, doi:10.20959/wjpr20179-9281.
- Anil, A.; Sudheer, P. Mucoadhesive Polymers: A Review. J. Pharm. Res. 2018, 17, 47–55, doi:10.18579/jpcrkc/2018/17/1/119566.
- Ibrahim, Y.H.E.Y.; Regdon, G.; Hamedelniel, E.I.; Sovány, T. Review of recently used techniques and materials to improve the efficiency of orally administered proteins/peptides. DARU, J. Pharm. Sci. 2020, 28, 403–416, doi:10.1007/s40199-019-00316-w.