Fast start-up, high efficiency, no toxic emissions into the atmosphere and good modularity are the key advantages of fuel cell applications. Despite the merits associated with fuel cells, the high cost of the technology remains a key factor impeding its widespread commercialization.
- Fuel Cells
1. Introduction
Energy is considered the driving force for all economy globally. Fossil fuels continue to dominate the energy industry due to the already established infrastructure available for harnessing energy via this medium [1,2,3,4,5,6,7,8,9,10]. Despite fossil fuels being the largest source of energy for industrial and domestic purposes, recent investigations have highlighted the need for alternative power generation media [10,11,12,13,14,15,16,17,18,19,20,21]. This clarion call by the scientific community is due to the harmful effect of fossil commodities on the environment coupled with their unstable prices [22]. Similarly, most of the fossil reserves are currently depleting hence the urgent need for a paradigm shift in how energy can be harnessed for industrial as well as domestic purposes [23,24,25,26,27]. Renewable energy is considered as the suitable replacement for fossil fuel because it is abundant and environmentally friendly, but the intermit nature of the renewable energy sources the key factor impeding their commercialization and possible competition with existing forms of power generation [28]. As an energy conversion device, fuel cells have also been reported as suitable to make these renewable systems efficient in terms of reducing losses during peak and off-peak times during the day [29,30,31,32].
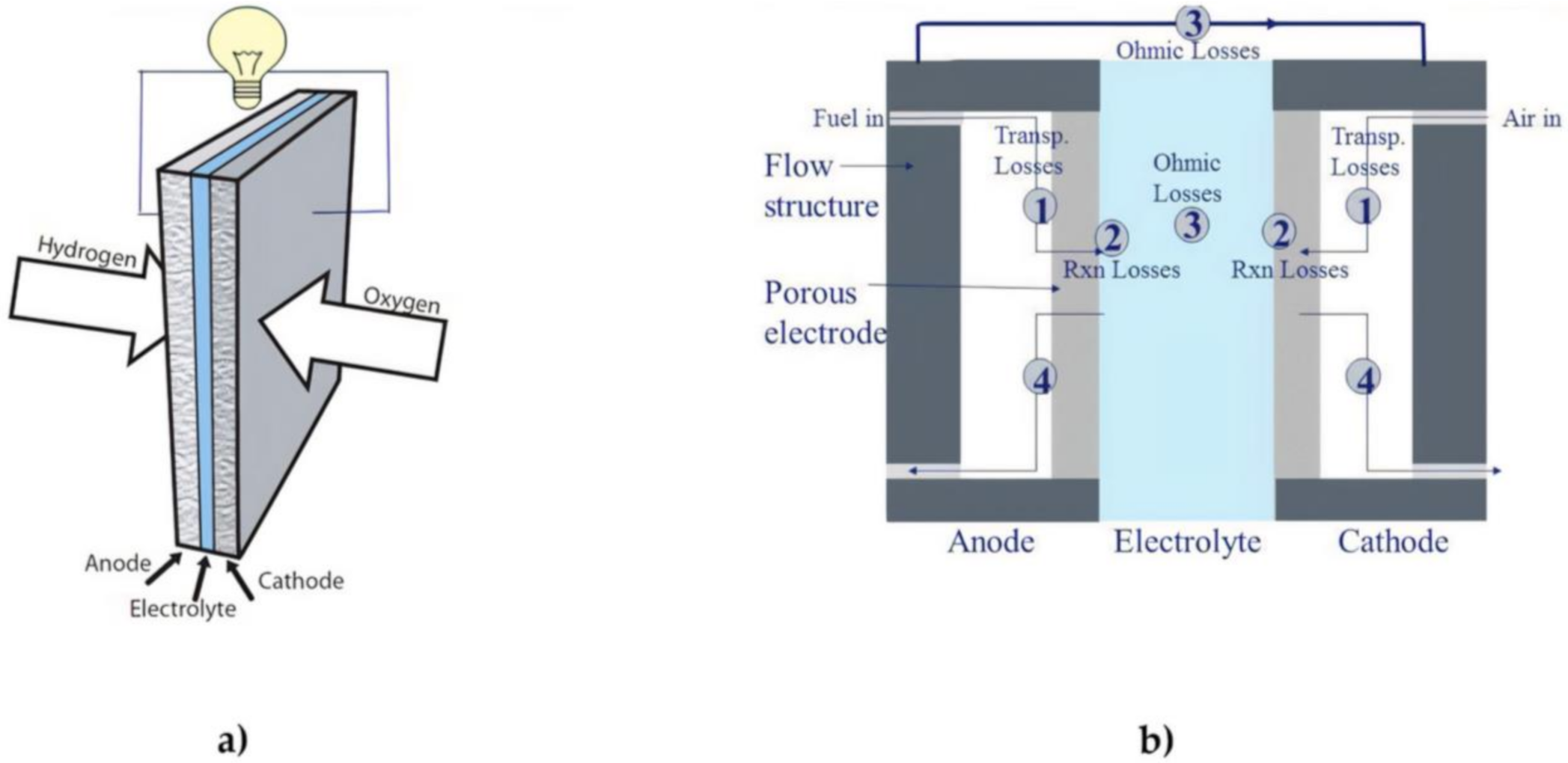
A fuel cell is a device which allows the direct conversion of chemical energy into an electrical form [33]. A fuel cell is usually powered by hydrogen as fuel and air as oxidant. This is a considerable advantage compared to any thermal machine since the losses regarding the combustion and the conversion in mechanical energy systems are not observable in fuel cells [34]. Besides the higher efficiency, a fuel cell can guarantee completely free emission energy production [35,36]. While a fuel cell is working, there are no local emissions since the only by-products of the reaction are water and heat [37]. Nevertheless, hydrogen is not available in free form and it needs to be extracted from hydrogen-containing compounds such as water and hydrocarbons. The extraction process generates carbon dioxide, so the pollution issue is just shifted. In order to avoid any emission in the whole fuel cell lifecycle, hydrogen can be extracted from electrolysis by electricity produced from green energy, as an example [38]. Figure 1 captures the various individual components of fuel cells in a simplified schematic. Fuel cells usually have two electrodes which is the anode and the cathode. Electricity is generated as a result of an electrochemical reaction between an oxidant and a fuel leading to the evolution of heat and water as by product of the electrochemical reaction. The various types of fuel cells differ based on the type of membrane/electrolyte used in the development of the cell. Most investigations being conducted in the area of fuel cells are mainly to ascertain the possibility of maximizing the electrochemical process to ensure higher cell efficiency is obtained at lower operating cost.
2. Advantages and Disadvantages of Fuel Cells
Modifications in fuel cell operation mode and material composition are necessary to make them fully commercialisable. Fuel cells can be fed without any dependency on fossil fuels but again that is subject to the source of hydrogen generation. This makes electricity produced from fuel cells environmentally friendly especially if the hydrogen gas was obtained from renewable sources. Another justification for the environmentally friendly nature of fuel cells is the fact that water is the byproduct of the electrochemical reaction in fuel cell, making it an ideal candidate in the quest for fighting climate change. Fuel cells are further designed to have quick start up times compared to other sources of energy generation [41,47]. The absence of moving parts in fuel cells is also another merit of these energy-converting devices. This implies that maintenance time and cost can be curbed compared to other conventional medium of energy generation. The operation of fuel cells is also very reliable, with virtually no form of vibration due to the absence of moving parts. The power density and efficiency of fuel cells are also higher when compared to batteries and heat engines. Fuel cells generally have longer life spans because they only produce electricity based on the introduction of the reactants into the cell. Other electrochemical devices like battery tend to have shorter life span because electrochemical reactions occur in the battery even when they are not generating any electricity. Fuel cells are not usually susceptible to corrosion like other energy device [42,48].
The main downside of fuel cells has to do with cost. The membrane which is the heart of the cell is often coated with a catalyst, mainly to speed up the electrochemical reaction. Some of the catalysts used are platinum and ruthenium but loading these catalysts on the membrane contributes significantly to the overall cost of the cell. The other major challenge in fuel cells are thermal and water management. Due to the fact that fuel cells’ performance is directly proportional to their cell operational temperature, the cell performance is likely to decrease significantly if the cell is operated below or above its required range of operating temperatures. This can sometimes be very challenging as maintaining a constant cell operating temperature often becomes very tedious in the management of the cell [43,49,50]. Mitigation strategies like increasing the relative humidity of the reactants in cell have been proposed in some references, but this equally comes with a cost that often increases the overall cost of the system. The availability of the fuel coupled with its storage is also another challenge that need to be factored into future research activities. A summary of the advantages and disadvantages of proton-exchange membrane fuel cells is summarised in Table 2.
Table 2. Advantages and disadvantages of proton-exchange membrane fuel cells.
| Advantages | Disadvantages | Reference |
|---|---|---|
| Higher energy density | Limited hydrogen infrastructure | [51] |
| No toxic emissions | Requirement of continuous stream of fuel and air | [52] |
| No noise produced during operation | Need of auxiliaries to run, thus requiring power | [53] |
| Simple design and operation | Still lavish due to lack of bulk productions | [54] |
| Quick start-up | Need of a proper control system, which adds to the production cost | [55] |
| Multiple applications | Desiccation of membrane and submerging of the cathode layer is also a challenge | [56] |
| Operate under stop-start driving circumstances | [57] |
This entry is adapted from the peer-reviewed paper 10.3390/en14010144
