Glycocyclodextrins (glycoCDs) comprise a family of cyclodextrin-based multivalent glycoclusters in which unique 3D displays of glycoepitopes can be achieved. As a consequence of the conformational rigidity and dense functional display of cyclodextrins, and taking advantage of selective chemical functionalization and "click chemistry" schemes, the upper/lower distribution of glycoepitopes and the inward/outward hydrophobic/hydrophilic balance can be precisely tailored and tunned to achieve complex functional properties such as programed self-(dis)assembling or biological recognition processes.
- cyclodextrins
- drug delivery
1. Introduction
The property confers CDs distinctive advantages to form inclusion complexes with lipophilic guest molecules as far as them entirely or partially entering the CD macro-ring cavity. The stability and water solubility of the guest can be improved in this manner, or the target guest can be captured, removed or masked, broadening application scopes. Indeed, the academic and (bio)technological interest for cyclodextrins has been historically dominated by their inclusion complex-formation ability, which has translated into numerous applications in fields like food [1][2], cosmetics [3], environment [4][5], and medicine [3][6][7][8][9][10].
Structurally, CDs are composed by α(1→4)-linked glucose units that feature a characteristic toroidal truncated-cone shape, in which glucose hydroxyls orient to the outer space flanking the upper and lower rims, while methinic protons (H-5 and H-3) point to the inner cavity. The hexamer (αCD), heptamer (βCD) and octamer (γCD) representatives are commercially available. All three are nanometric objects that share the same torus height (7.8 Å), as defined by the monosaccharide dimensions, and differ on the external (13.7, 15.3 and 16.7 Å) and internal diameters (5.7, 7.8 and 9.5 Å, respectively), the later determining their affinity towards size-variable guests (Figure 1) [11][12]. Conceptually, CDs can be considered as molecular nanoparticles in the sense advanced by Cheng and coworkers: nanometric molecular systems of natural or technological origin exhibiting persistent topology (shape and volume) [13][14]. They are susceptible to selective chemical manipulation to obtain sugary molecular nanosystems with tailored capabilities, or they can be used as functional components to engineer CD-based nanosystems benefiting from the CD inclusion properties [15][16][17]. Alternatively, they can be incorporated into polymeric, magnetic, lipid, metallic or mesoporous NPs to enhance their characteristics [18][19]. All these examples highlight the potential of CDs to galvanize positive synergies between research disciplines, such as synthetic chemistry, supramolecular chemistry, pharmaceutical formulation or materials science.
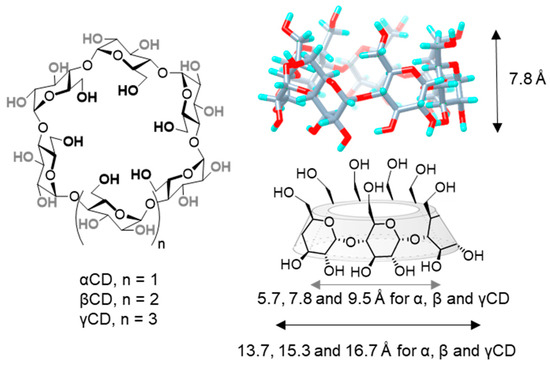
Figure 1. Structures of α, β and γCD (left), 3D view of βCD (upper-right) and schematic representation of CD basket-shape architecture (lower right), with indication of the height, and average internal and external diameters for the three commercially available representatives. CD, cyclodextrin.
Strictly speaking, CD-based nanomaterials can be considered as functional glyconanomaterials (CDs are definitely glyco) [20]. However, in the context of this thematic issue, this concept is reserved for nanomaterials exposing carbohydrate moieties mediating interactions with other biomolecular partners. By CD-based functional glyconanomaterials, here, we mean nanosized materials combining CD and glycan modules, either with an organic or inorganic core, obtained by self-assembly, template-assisted assembly or covalent conjugation.
2. Multitopic Guest-Driven Clusterization of GlycoCDs
Molecular scaffolds displaying two or more CD cavity-fitting motifs can behave, in principle, as multitopic guests, with the potential of forming high-order inclusion complexes. If the CD partner is a glycoCD, the total glycoligand valency of the resulting supramolecular species will grow with complex stoichiometry, which is expected to enhance the binding affinity towards complementary lectin receptors by virtue of the so-called multivalent or cluster effect [21][22][23]. The potential of this strategy in glycotargeting schemes was anticipated in 2004, in the context of a work aiming at developing an efficient carrier for the delivery of the taxane anticancer drug docetaxel (DTX, Taxotère®) to macrophages [24]. DTX possesses two phenyl fragments that are well-suited for βCD inclusion, whereas active uptake by macrophages can be promoted by targeting the macrophage mannose receptor (MMR). On such grounds, a trivalent βCD-mannosyl dendron conjugate was initially conceived. MMR binding studies evidenced a statistically significant increase in affinity in the presence of the drug, which is in agreement with the DTX-promoted clusterization of the conjugate. The association constant (Ka) of phenyl derivatives and βCD (Ka ≈ 103 M) [25] is, however, too modest to warrant a medically useful concentration of the 2:1 complex in biological media, which was overcome by engineering a dimeric βCD-hexamannosyl dendron conjugate that formed a very strong 1:1 chelate-type complex with DTX (Figure 2).
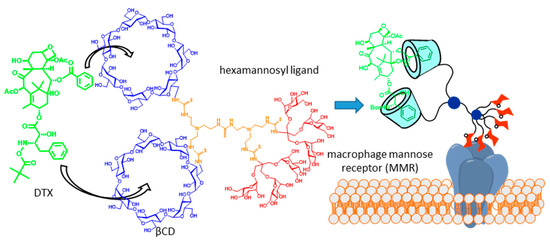
Figure 2. Docetaxel (DTX) carrier consisting of a hexamannosylated dimeric βCD derivative designed on the basis of the drug clusterization concept [24]. Adapted with permission from Reference [3]. Copyright 2013 Royal Society of Chemistry.
Adamantane (Ad) derivatives form much more robust inclusion complexes with βCD hosts than phenyl derivatives (Ka ≈ 105 M). This property has been amply used for macromolecular materials design [26]. Notwithstanding, reports on βCD/Ad host–guest supramolecular ligation chemistry using discrete molecular entities, for the purpose of assembling functional glyconanomaterials, are relatively scarce. In 2011, Seeberger and coworkers showed that the incorporation of up to six Ad moieties onto a fluorescent ruthenium(II)-bipyridine complex core provided a very convenient platform for the rapid construction of high-valent glyconanosensors for the visualization of carbohydrate–lectin interactions, just by mixing with sugar-appended βCD derivatives (Figure 3a) [27]. A follow-up of this proof of concept was reported by Kikkeri and coworkers [28], who synthesized the two enantiomerically pure versions of the hexa-Ad guest (denoted Δ and Λ for the positive and negative sign of the circular dichroism signal, respectively) and used them to build diastereomeric metallo-glycodendrimers by anchoring a heptamannosylated βCD conjugate. Evaluation of the binding affinities towards different mannose-binding C-type lectins, including the human Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (CD-SIGN), the mouse-Specific Intracellular adhesion molecule-3 Grabbing Non-integrin homolog-Related 3 (SIGNR3) and Dectin-1, evidenced amazing differences that revealed a major impact of the chiral microenvironment in the recognition process, in spite of the similar topology of the supramolecular diastereomers. Such dissimilar behaviors were also confirmed in cellulo as well as in vivo. The same authors prepared metallo-glycodendrimers from a Ru(II) bipyridine complex that has attached only two Ad motifs and either a heptamannosylated or a heptagalactosylated βCD component [29]. The glycodendrimers demonstrated selective carbohydrate–protein interaction properties and mediated the delivery of the Ru(II) complexes into cancer cells expressing specific mannose- or galactose-selective lectins, respectively. Once internalized, they showed cytotoxic activity by interacting with the endoplasmic reticulum (ER) and triggering caspase-mediated apoptosis, whereas they were not cytotoxic to normal cells (Figure 3b).
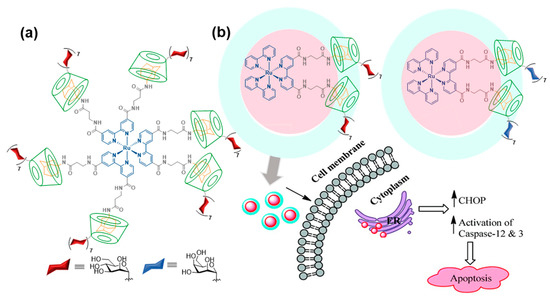
Figure 3. Heptamannosyl-βCD/Ru(II)-scaffolded hexa-adamantyl host–guest complex reported by Seeberger and coworkers [27] (a) and bis-adamantyl analogs with anti-apoptotic activity reported by Kikkeri and coworkers [29] (b). Adapted with permission from Reference [29]. Copyright 2016 Royal Society of Chemistry.
Ferrocene and boron-dipyrromethene (BODIPY) have also been used as core elements for the construction of Ad guests, in order to clusterize glycoCDs into functional glyconanomaterials through inclusion complex formation. For instance, a ferrocene-bis-Ad guest was found to form a supramolecular dimer with a heptalactosyl-βCD derivative that was employed to assess binding to the legume lectin peanut agglutinin (PNA), broadly used in fundamental studies on carbohydrate–protein recognition [30]. Zhang, Yin and coworkers prepared a BODIPY-tris-Ad derivative (BTA) and used it to prepare mannose-coated nanoparticles by the spontaneous supramolecular immobilization of a heptamannosylated-βCD conjugate (CD-Man7) onto the surface of BTA aggregates upon co-precipitation (Figure 4) [31]. Transmission electron microscopy (TEM) revealed the formation of spherical BTA@CD-Man7 nanoparticles with an average diameter of 117 ± 16 nm, whereas confocal laser scanning microscopy (CLMS) showed that the nanoparticles were efficiently internalized in MDA-MB-231 breast cancer cells overexpressing the mannose receptor (MR), but not in healthy MCF-10A cells. The nanoparticles accumulated in the lysosomes of the cancer cells, where they dissociated and revealed the photosensitizer (PS) character of BODIPY. Irradiation with 665 nm light emitting diode (LED) light then resulted in phototoxicity. The potential of the mannose-mediated PS system for targeted photodynamic therapy (PDT) was confirmed in a mouse model that was established by injecting MDA-MB-231 cancer cells into subcutaneous tissues, confirming the remarkable tumor inhibition effect of BTA@CD-Man7 under irradiation.
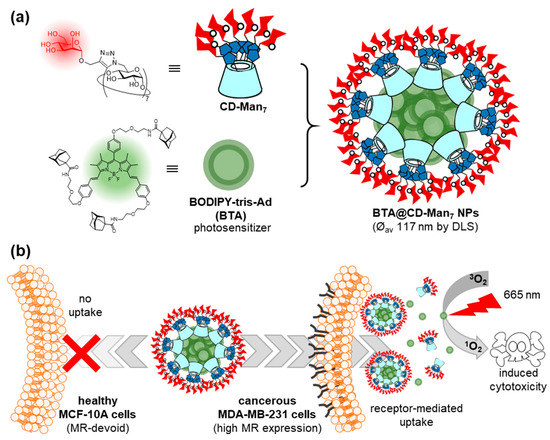
Figure 4. Structures of the CD-Man7 glycoCD host and BTA tritopic guest used by Zhang, Yin and coworkers (a) and schematic representation of their self-assembly into nanoaggregates for targeted photodynamic therapy against breast cancer (b) [31]. CD, cyclodextrin; Man, mannose; BODIPY, boron-dipyrromethene; BTA, BODIPY-tris-Ad derivative; LSD, dynamic light scattering; MR, mannose receptor.
3. Amphiphilicity as an Action Principle for GlycoCD Self-Assembly
Inclusion complex formation can be exploited to impart self-assembling properties to CD derivatives beyond multitopic guest-driven clusterization. For instance, if the guested molecule contains a hydrophobic tail, the resulting species will behave as a supramolecular amphiphile or “superamphiphile”, a concept that emerged during the last decade and refers to surfactants where the hydrophilic and hydrophobic moieties are linked by noncovalent connections [32]. The implementation of this notion in the elaboration of nanocomplexes for the targeted delivery of drugs is particularly appealing due to the unique stimuli responsiveness of host–guest interactions [33][34]. Recently, Zhang and coworkers reported a superamphiphile construct encompassing a polyethylene glycol-tethered lactobionic acid-βCD conjugate (LA-PEG-βCD) and a benzimidazole-modified doxorubicine (BM-DOX) as the hydrophilic and hydrophobic constituents respectively, intended for the treatment of hepatocellular carcinoma (HCC) [35]. LA is a ligand of the galactose-specific asialoglycoprotein receptor (ASGPR) at the surface of liver cancer cells. The BM module fits well in the cavity of βCD: it was connected to the anticancer drug DOX through an acid-labile hydrazone functionality that was expected to be cleaved in the intracellular acidic environment with rapid release of DOX from the prodrug. In aqueous media, host–guest association between BM and βCD brought together the targeted supramolecular prodrug (TSPD), which spontaneously self-assembled into spherical, biocompatible nanoparticles that exhibited a pH-dependent drug release profile and excellent effects on HCC tumor cell growth (Figure 5).
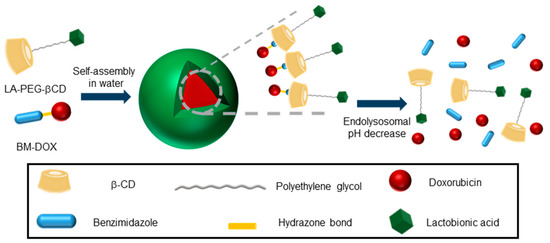
Figure 5. Schematic illustration of the superamphiphile strategy developed by Zhang and coworkers for the self-assembly of acid-sensitive ASGPR-targeted prodrug glyconanoparticles [35]. ASGPR, asialoglycoprotein receptor; LA, lactobionic acid; PEG, poly(ethylenglycol); βCD, β-cyclodextrin; BM, benzimidazole; DOX, doxorubicin.
The intrinsic two-face topology of CDs and the distinct reactivity of primary and secondary hydroxyls offers a convenient entry to covalent “classical” amphiphiles that can be tailored to self-assemble into different classes of aggregates (micelles, vesicles, nanospheres, nanocapsules) [17][36][37][38]. If the hydrophilic face in the amphiphilic CD is glyco-coated (glycoamphiphilic CDs; GaCDs), the corresponding self-assemblies will expose the glycan appendages to the bulk, available for their participation in biomolecular recognition processes. The interest of such systems for targeted drug delivery is obvious. Early work in the field was conducted by the groups of Nishimura, Darcy and Mazzaglia [39][40][41][42][43] and has already been reviewed [44]. Recent contributions by Seeberger, Yin and coworkers focus on the optimization of amphiphilic mannosylated βCD derivatives in terms of self-assembling properties and stability of the resulting mannose-functionalized nanoparticles [45]. The authors encountered that installation of seven mannosyl residues at the primary rim through copper(II)-catalyzed azide-alkyne cycloaddition (CuAAC) “click” coupling [46] and propanoyl ester groups at the fourteen secondary hydroxyls afforded conjugates (C3-CD-Man7) that self-assembled in water. In this manner, they obtained spherical nanoparticles with an average diameter of 45 nm (TEM), an average hydrodynamic diameter of 112 nm (DLS), low polydispersity index (PDI, 0.109) and high stability under physiological conditions. C3-CD-Man7 nanoparticles were able to encapsulate up to 12% of DOX, and the DOX-loaded system (DOX@C3-CD-Man7) efficiently delivered the drug to MR overexpressing MDA-MB-231 breast cancer cells in vitro and in vivo. The targeted drug delivery capabilities of C3-CD-Man7 were further confirmed for the treatment of visceral leishmaniasis (VL) [47]. The resulting AmB&DOX@C3-CD-Man7 nanomicelles were selectively taken up by RAW264.7 macrophages, with high expression of MR, and their therapeutic effect was established in vitro in RAW264.7 cells infected with L. donovani parasites. After treatment with AmB&DOX@C3-CD-Man7, almost no parasite was detected, supporting that the formulation is a promising solution for VL therapy (Figure 6).
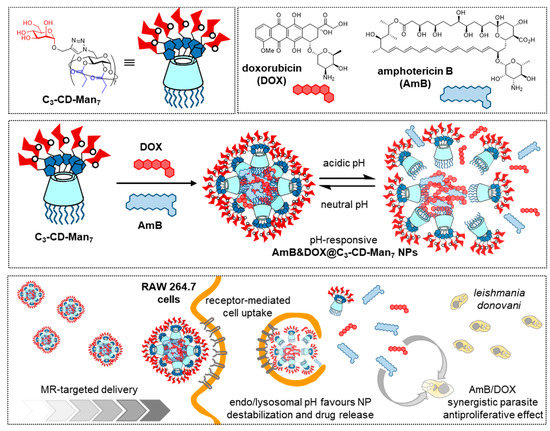
Figure 6. Structures of the GaCD derivative C3-CD-Man7, doxorubicin (DOX) and amphotericin B (AmB) (upper panel) and schematic representations of their co-assembly to afford pH-sensitive nanoparticles (Amb@DOX@C3-CD-Man7) (middle panel) and internalization in macrophages to treat visceral leishmaniasis (lower panel), as reported by Seeberger, Yin and coworkers [45][47]. GaCD, glycoamphiphilic cyclodextrin; Man, mannose; NP, nanoparticle.
4. Tailoring Glycopolymer Topology by CD Inclusion-Promoted Macromolecular Folding and Self-Assembly
The inclusion complexation between CDs, comprising CD units incorporated in polymers, and various guests has been extensively investigated in supramolecular chemistry [18]. However, although CD-containing polymers have been used in pharmaceutical applications since the 1980’s [1][5][48][49], the profitable features of inclusion complexation did not sufficiently draw attention and therefore were not employed in macromolecular self-assembly until about the beginning of this century [50]. CDs are particularly valuable and extensively employed in tuning the amphiphilicity of macromolecules. For instance, if a fragment of a macromolecule containing a guest moiety is connected to CDs via inclusion complexation, this area will become more hydrophilic and thus the amphiphilicity of the complete macromolecule as a whole is altered. This strategy allows to control the self-assembly and the morphology of the assemblies [15]. In 2015, Bercer and coworkers extended the inclusion complexation-driven macromolecule-associated micellization strategy to the use of glyco-coated CDs [51]. The authors prepared an ABA triblock copolymer architecture having precisely positioned random poly(N,N-dimethylacrylamide) (PDMA)/poly(adamantane-acrylate) (PAdac) blocks within the first and the third blocks, while the middle block consisted of thermo-responsive poly(N-isopropylacrylamide) (PNIPAM) that enables the formation of a self-assembled micellar structure above the cloud point. The Ad-functionalized thermo-responsive triblock copolymer provided supramolecular host–guest interactions with βCD as well as mono- and heptamannosylated βCD derivatives (CD-Man1 and CD-Man7). The resulting supramolecular amphiphilic macromolecule formed stable micelles in water as was clearly revealed by DLS. Quantitative concanavalin A (ConA; a mannose-specific plant lectin) precipitation assays demonstrated the accessibility of the mannosyl residues and their multivalent presentation at the surface of the assemblies, even in the case of the monomannosylated glycoCD, since lectin precipitation requires high carbohydrate density. Altogether, the results validated the Ad/βCD host–guest tactic for careful fine-tuning of the interaction between lectins and supramolecular glycopolymers (Figure 7).
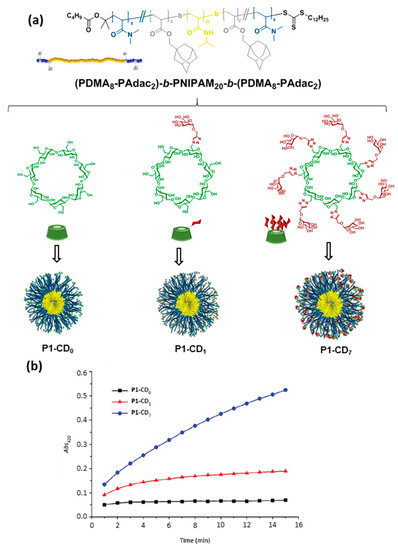
Figure 7. Average structure of the triblock copolymer (P1) and structures of the βCD derivatives used by Bercer and coworkers [51] to self-assemble glycomicelles trough equimolecular mixing in water (a) and turbidity plots obtained upon mixing with concanavalin A (ConA) at 40 °C (b). Adapted with permission from Reference [51]. Copyright 2016 Royal Society of Chemistry. PDMA, poly(N,N-dimethylacrylamide); PNIPAM, poly(N-isopropyl acrylamide); PAdac, poly(adamantane-acrylate).
The alliance between CD supramolecular chemistry and living polymerization has been particularly fruitful at providing fascinating polymer materials [52]. Bercer and coworkers applied one of the most popular of such reactions, reversible addition−fragmentation chain transfer (RAFT), to create triblock glycocopolymers bearing βCD, Ad and mannose residues, combined with PDMA, in different blocks [53]. At sufficiently high dilution in aqueous solution, intermolecular host–guest interactions were prevented and only single-chain folding, promoted by intramolecular CD/Ad interactions, were observable by bidimensional nuclear Overhauser effect spectroscopy (2D NOESY) nuclear magnetic resonance (NMR) and dynamic light scattering (DLS). Folded glycopolymers were unfolded at high temperatures and also by addition of the competitive guest molecule 1-adamantylamine hydrochloride. Interestingly, the folding state strongly affected the binding affinity towards the mannose-specific ConA, DC-SIGN and DC-SIGN-related (DC-SIGNR) lectins, as determined by turbidimetry and surface plasmon resonance (SPR) assays, providing direct evidence of the critical importance of controlling the secondary structures of glycopolymers for biological applications (Figure 8).
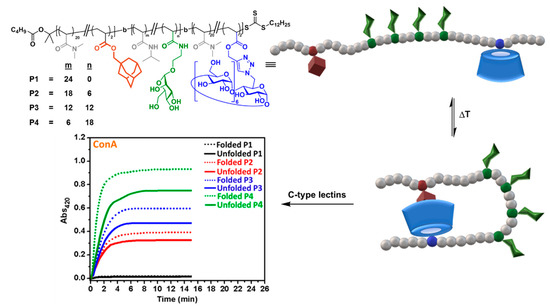
Figure 8. Triblock glycocopolymers bearing βCD units designed by Bercer and coworkers [53] to modulate the C-type lectin binding properties through intramolecular host–guest control of the folding state. Adapted with permission from Reference [53], https://pubs.acs.org/doi/abs/10.1021/acs.biomac.8b00600. Copyright 2018 American Chemical Society. ConA, concanavalin A.
This entry is adapted from the peer-reviewed paper 10.3390/nano10122517
References
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; García Fernández, J.M.; Benito, J.M. Cyclodextrins for pharmacological and biomedical applications. In Supramolecular Systems in Biomedical Fields; Schneider, H.-J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; Chapter 5; pp. 94–139. [Google Scholar]
- Fakayode, S.O.; Lowry, M.; Fletcher, K.A.; Huang, X.; Powe, A.M.; Warner, I.M. Cyclodextrins host-guest chemistry in analytical and environmental chemistry. Curr. Anal. Chem. 2007, 3, 171–181. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Cyclodextrin as a magic switch in covalent and noncovalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, A.; Garcia-Fandino, R. The Lord of the nanorings: Cyclodextrins and the battle against SARS-CoV-2. J. Pharm. Sci. 2020, 588, 119689. [Google Scholar]
- García Fernández, J.M.; Ortiz Mellet, C.; Defaye, J. Glyconanocavities: Cyclodextrins and beyond. J. Incl. Phenom. Macrocyl. Chem. 2006, 56, 149–159. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Yu, X.; Wang, C.-L.; Sun, H.-J.; Hsieh, I.-F.; Li, Y.; Dong, X.-H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular nanoparticles are unique elements for macromolecular science: From “nanoatoms” to giant molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Zhang, W.-B.; Cheng, S.Z.D. Giant molecules: Where chemistry, physics, and bio-science meet. Sci. China Chem. 2017, 60, 338–352. [Google Scholar] [CrossRef]
- Fernández, M.A.; Silva, O.F.; Vico, R.V.; de Rossi, R.H. Complex systems that incorporate cyclodextrins to get materials for some specific applications. Carbohydr. Res. 2019, 480, 12–34. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Liu, Y.-H.; Liu, Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 2020, 32, 1806158. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. The classification and application of cyclodextrin polymers: A review. New J. Chem. 2020, 44, 9137–9148. [Google Scholar] [CrossRef]
- Shelley, H.; Babu, R.J. Role of cyclodextrins in nanoparticle-based drug delivery systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Gim, S.; Zhu, Y.; Seeberger, P.; Delbianco, M. Carbohydrate-based nanomaterials for biomedical applications. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1558. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate-protein interactions: Basis of glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Jayaraman, N. Multivalent ligand presentation as a central concept to study intricate carbohydrate–protein interactions. Chem. Soc. Rev. 2009, 38, 3463–3483. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef]
- Benito, J.M.; Gómez-García, M.; Ortiz Mellet, C.; Baussanne, I.; Defaye, J.; García Fernández, J.M. Optimizing saccharide-directed molecular delivery to biological receptors: Design, synthesis, and biological evaluation of glycodendrimer-cyclodextrin conjugates. J. Am. Chem. Soc. 2004, 126, 10355–10363. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic macromolecular material design—The versatility of cyclodextrin-based host–guest chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef]
- Grünstein, D.; Maglinao, M.; Kikkeri, R.; Collot, M.; Barylyuk, K.; Lepenies, B.; Kamena, F.; Zenobi, R.; Seeberger, P.H. Hexameric supramolecular scaffold orients carbohydrates to sense bacteria. J. Am. Chem. Soc. 2011, 133, 13957–13966. [Google Scholar] [CrossRef]
- Bavireddi, H.; Vasudeva Murthy, R.; Gade, M.; Sangabathuni, S.; Madhukar Chaudhary, P.; Alex, C.; Lepenies, B.; Kikkeri, R. Understanding carbohydrate–protein interactions using homologous supramolecular chiral Ru(II)-glyconanoclusters. Nanoscale 2016, 8, 19696–19702. [Google Scholar] [CrossRef]
- Bavireddi, H.; Vasudeva Murthy, R.; Gade, M.; Sangabathuni, S.; Kikkeri, R. Supramolecular metalloglycodendrimers selectively modulate lectin binding and delivery of Ru(II) complexes into mammalian cells. Org. Biomol. Chem. 2016, 14, 10816–10821. [Google Scholar] [CrossRef]
- Bavireddi, H.; Bharatez, P.; Kikkeri, R. Use of Boolean and fuzzy logics in lactose glycocluster research. Chem. Commun. 2013, 49, 9185–9187. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Wang, X.-J.; Xu, J.-L.; Ye, Z.; Wang, S.; Seeberger, P.H.; Yin, J. Targeted photodynamic killing of breast cancer cells employing heptamannosylated β-cyclodextrin-mediated nanoparticle formation of an adamantane-functionalized BODIPY photosensitizer. ACS Appl. Mater. Interfaces 2016, 8, 33405–33411. [Google Scholar] [CrossRef]
- Kang, Y.; Tang, X.; Cai, Z.; Zhang, X. Supra-amphiphiles for functional assemblies. Adv. Funct. Mater. 2016, 26, 8920–8931. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Q.; Bi, Y.; Chen, X.; Liu, L.; Ruan, C.; Zhao, Z.; Jiang, C. Supramolecular amphiphiles based on cyclodextrin and hydrophobic drugs. J. Mater. Chem. B 2017, 5, 2644–2654. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.P.; Song, X.; Zhuo, L.; Bu, H.; Tian, W. Photo- and pH- dual-responsive β-cyclodextrin-based supramolecular prodrug complex self-assemblies for programmed drug delivery. Chem. Asian J. 2018, 13, 3903–3911. [Google Scholar] [CrossRef]
- Yang, T.; Du, G.; Cui, Y.; Yu, R.; Hua, C.; Tian, W.; Zhang, Y. pH-sensitive doxorubicin-loaded polymeric nanocomplex based on β-cyclodextrin for liver cancer-targeted therapy. Int. J. Nanomed. 2019, 14, 1997–2010. [Google Scholar] [CrossRef]
- Varan, G.; Benito, J.M.; Ortiz Mellet, C.; Bilensoy, E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein J. Nanotechnol. 2017, 8, 1457–1467. [Google Scholar] [CrossRef]
- Méndez-Ardoy, A.; Gomez-Garcia, M.; Geze, A.; Putaux, J.L.; Wouessidjewe, D.; Ortiz Mellet, C.; Defaye, J.; Garcia Fernandez, J.M.; Benito, J.M. Monodisperse nanoparticles from self-assembling amphiphilic cyclodextrins: Modulable tools for the encapsulation and controlled release of pharmaceuticals. Med. Chem. 2012, 8, 524–532. [Google Scholar] [CrossRef]
- Sallas, F.; Darcy, R. Amphiphilic cyclodextrins—Advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008, 957–969. [Google Scholar] [CrossRef]
- Sallas, F.; Niikura, K.; Nishimura, S.-I. A practical synthesis of amphiphilic cyclodextrins fully substituted with sugar residues on the primary face. Chem. Commun. 2004, 596–597. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Forde, D.; Garozzo, D.; Malvagna, P.; Ravoo, B.J.; Darcy, R. Multivalent binding of galactosylated cyclodextrin vesicles to lectin. Org. Biomol. Chem. 2004, 2, 957–960. [Google Scholar] [CrossRef]
- Micali, N.; Villari, V.; Mazzaglia, A.; Monsú Scolaro, L.; Valerio, A.; Rencurosi, A.; Lay, L. Cyclodextrin nanoaggregates and their assembly with protein: A spectroscopic investigation. Nanotechnology 2006, 17, 3239–3244. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Valerio, A.; Villari, V.; Rencurosi, A.; Lay, L.; Spadaro, S.; Monsú Scolaro, L.; Micali, N. Probing specific protein recognition by size-controlled glycosylated cyclodextrin nanoassemblies. New J. Chem. 2006, 30, 1662–1668. [Google Scholar] [CrossRef]
- McNicholas, S.; Rencurosi, A.; Lay, L.; Mazzaglia, A.; Sturiale, L.; Perez, M.; Darcy, R. Amphiphilic N-glycosyl-thiocarbamoyl cyclodextrins: synthesis, self-assembly, and fluorimetry of recognition by Lens culinaris lectin. Biomacromolecules 2007, 8, 1851–1857. [Google Scholar] [CrossRef]
- Martínez, A.; Ortiz Mellet, C.; García Fernández, J.M. Cyclodextrin-based multivalent glycodisplays: Covalent and supramolecular conjugates to assess carbohydrate–protein interactions. Chem. Soc. Rev. 2013, 42, 4746–4773. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-targeted drug delivery with mannose-functionalized nanoparticles self-assembled from amphiphilic β-cyclodextrins. Chem. Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; Méndez-Ardoy, A.; García Fernández, J.M. Click multivalent glycomaterials: Glycoclusters, glycodendrimers, glycopolymers, hybrid glycomaterials, and glycosurfaces. In Click Chemistry in Glycoscience: New Developments and Strategies; Witczak, Z.J., Bielski, R., Eds.; Wiley: Hoboken, NJ, USA, 2013; Chapter 6; pp. 143–182. [Google Scholar]
- Wei, P.; Ye, Z.; Cao, S.; Bai, S.; Seeberger, P.H.; Yin, J.; Hu, J. Combination therapy with amphotericin B and doxorubicin encapsulated in mannosylated nanomicelles for visceral leishmaniasis. Coll. Surf. A 2020, 598, 124804. [Google Scholar] [CrossRef]
- Fenyvesi, É. Cyclodextrin polymers in the pharmaceutical industry. J. Incl. Phenom. 1988, 6, 537–545. [Google Scholar] [CrossRef]
- Simoes, S.M.N.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- Cakir, N.; Hizal, G.; Becer, C.R. Supramolecular glycopolymers with thermoresponsive self-assembly and lectin binding. Polym. Chem. 2015, 6, 6623–6631. [Google Scholar] [CrossRef]
- Seidi, F.; Shamsabadi, A.A.; Amini, M.; Shabanian, M.; Crespy, D. Functional materials generated by allying cyclodextrin-based supramolecular chemistry with living polymerization. Polym. Chem. 2019, 10, 3674–3711. [Google Scholar] [CrossRef]
- Yilmaz, G.; Uzunova, V.; Napier, R.; Becer, C.R. Single-chain glycopolymer folding via host−guest interactions and its unprecedented effect on DC-SIGN binding. Biomacromolecules 2018, 19, 3040–3047. [Google Scholar] [CrossRef]
- McNicholas, S.; Rencurosi, A.; Lay, L.; Mazzaglia, A.; Sturiale, L.; Perez, M.; Darcy, R. Amphiphilic N-glycosyl-thiocarbamoyl cyclodextrins: synthesis, self-assembly, and fluorimetry of recognition by Lens culinaris lectin. Biomacromolecules 2007, 8, 1851–1857. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-targeted drug delivery with mannose-functionalized nanoparticles self-assembled from amphiphilic β-cyclodextrins. Chem. Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; Méndez-Ardoy, A.; García Fernández, J.M. Click multivalent glycomaterials: Glycoclusters, glycodendrimers, glycopolymers, hybrid glycomaterials, and glycosurfaces. In Click Chemistry in Glycoscience: New Developments and Strategies; Witczak, Z.J., Bielski, R., Eds.; Wiley: Hoboken, NJ, USA, 2013; Chapter 6; pp. 143–182. [Google Scholar]
- Wei, P.; Ye, Z.; Cao, S.; Bai, S.; Seeberger, P.H.; Yin, J.; Hu, J. Combination therapy with amphotericin B and doxorubicin encapsulated in mannosylated nanomicelles for visceral leishmaniasis. Coll. Surf. A 2020, 598, 124804. [Google Scholar] [CrossRef]
- Fenyvesi, É. Cyclodextrin polymers in the pharmaceutical industry. J. Incl. Phenom. 1988, 6, 537–545. [Google Scholar] [CrossRef]
- Simoes, S.M.N.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- Cakir, N.; Hizal, G.; Becer, C.R. Supramolecular glycopolymers with thermoresponsive self-assembly and lectin binding. Polym. Chem. 2015, 6, 6623–6631. [Google Scholar] [CrossRef]
- Seidi, F.; Shamsabadi, A.A.; Amini, M.; Shabanian, M.; Crespy, D. Functional materials generated by allying cyclodextrin-based supramolecular chemistry with living polymerization. Polym. Chem. 2019, 10, 3674–3711. [Google Scholar] [CrossRef]
- Yilmaz, G.; Uzunova, V.; Napier, R.; Becer, C.R. Single-chain glycopolymer folding via host−guest interactions and its unprecedented effect on DC-SIGN binding. Biomacromolecules 2018, 19, 3040–3047. [Google Scholar] [CrossRef]
