β-amyloid (Aβ) is a peptide, 38 to 43 amino acids long, that derives from the proteolytic processing of amyloid precursor protein (APP) by the γ-secretase; Aβ40 and Aβ42 are the most studied Aβ peptide species.
- vaccine
- Alzheimer’s disease
- β-amyloid
1. Introduction
Vaccination is an extremely effective public health intervention for infectious diseases and represents the most remarkable contribution of immunology to medicine [1].
Mechanistically, vaccination relies on the phenomenon of immunity, a long-term change in the immunological response to subsequent encounters with the same pathogen that occurs after the recovery from some infectious diseases. During the immune response, antigens induce the activation and differentiation of antigen-specific clones of B and T lymphocytes, that recognize different portions of the antigen, or epitopes. B cells (and antibodies that represent the soluble version of the B cell receptor for antigen) recognize exposed portions of the antigen, the B cell epitopes. Instead, the epitopes recognized by T cells consist of linear peptide sequences that are 8–12 aminoacid long in the case of cytotoxic T cells and 12–17 aminoacid long in the case of helper and regulatory T cells. Immunity relies on the differentiation of B cells into long-lived plasma cells, that ensure a persistent production of antibodies; moreover, B and T cells differentiate into memory cells that afford enhanced responses to subsequent encounters with the same antigen.
Immunity is not an ‘all or nothing’ status. In sterilizing immunity, re-infection is completely prevented; in non-sterilizing immunity, the infection can occur but does not lead to disease, thanks to the mitigating effects of circulating antibodies against the pathogen and the enhanced speed, magnitude, and efficacy of the memory immune response. When a large fraction of a population is immune to an infectious pathogen, also members of the community that are not individually immune are protected from the disease, due to the reduced circulation of the pathogen. This phenomenon, termed herd immunity, only affects immunity to pathogens that are transmitted from one individual to another. Thus, vaccination against transmissible diseases consists of the induction of immunity, under conditions safer than the natural infection, and vaccination in general acts both at the level of the individual and the level of the community [2,3,4].
The last two decades have seen several attempts to harness the immune system’s power against Alzheimer’s disease (AD), by vaccinating against a peptide that has a central role in the pathogenesis, the β-amyloid peptide (Aβ). β-amyloid has been the target of several approaches to preventing and treating Alzheimer’s disease, including efforts to decrease the levels of Aβ monomers, oligomers, aggregates, and plaques using compounds that decrease production, antagonize aggregation, or increase brain clearance of Aβ [5]. Immunization against the β-amyloid peptide as a vaccination strategy for Alzheimer’s disease relies on the concept that antibodies against Aβ can interfere with its aggregation and accumulation, block its toxicity, or increase its catabolism, and on the hypothesis that these effects on brain Aβ may modify the course of the disease [6]. Obviously, the concept of herd immunity does not apply to vaccination against Alzheimer’s disease; in a vaccination for a non-infectious disease, only the vaccinated individuals that mount a response that meets the protective threshold are protected, and therefore the interindividual variability in the magnitude and quality of the immune response to vaccination is a particularly important issue.
An important difference between vaccination against pathogens and vaccination against β-amyloid is the fact that the β-amyloid is a self-peptide. The failure to respond to self-antigens, defined immunological tolerance, is an essential feature of the immune system; autoimmunity is physiologically avoided by several mechanisms—e.g., clonal deletion of high affinity autoreactive lymphocytes in the thymus, editing of autoreactive B cell receptors, induced unresponsiveness in mature lymphocytes, and suppression by regulatory T cells. Vaccination against the β-amyloid peptide aims at inducing a controlled type of autoimmunity. In this context, one risk is that a vaccine may be unable to break tolerance, and therefore may not be immunogenic, while the opposite risk is that the vaccine may induce a damaging autoimmune reaction, and therefore may prove unsafe. Therefore, the immunogenicity and safety of anti-Aβ vaccines need to be carefully evaluated.
There is no licensed anti-β-amyloid vaccine or monoclonal antibody yet for Alzheimer’s disease. In clinical trials, immunotherapy against Aβ has been repeatedly unsuccessful. Some candidate vaccines have been abandoned following safety issues, despite displaying some efficacy, whereas other vaccines displayed a good safety profile but no efficacy; several vaccine candidates are currently in clinical trials.
2. The β-amyloid Cascade Model of Alzheimer’s Disease Pathogenesis
β-amyloid (Aβ) is a peptide, 38 to 43 amino acids long, that derives from the proteolytic processing of amyloid precursor protein (APP) by the γ-secretase; Aβ40 and Aβ42 are the most studied Aβ peptide species (Figure 1). The β-amyloid cascade model of Alzheimer’s disease pathogenesis consists in the hypothesis that an imbalance between production and clearance of Aβ42 and related Aβ peptides is a very early, often initiating factor in Alzheimer’s disease [13].
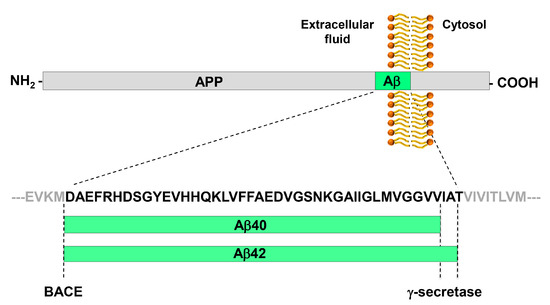
Figure 1. Generation of the Aβ peptide from the processing of APP by BACE and γ-secretase. APP is a type I trans-membrane glycoprotein. The β-secretase BACE has a single cleavage site on APP and generates the N-terminus of Aβ peptides. The γ-secretase has multiple cleavage sites on APP, which leads to the generation of Aβ peptides of variable length that differ for their C-terminus. The most abundant peptides are Aβ40 and Aβ42. Aβ42 is particularly prone to aggregation.
Strong evidence of the amyloid cascade model comes from the genetics of the ‘dominantly inherited forms of Alzheimer’s disease’ (DIAD). DIAD is caused by mutations that impact on the Aβ pathway, affecting either the amount of Aβ production or the ratio between the different forms of the peptide, in particular, by mutations within and immediately flanking the Aβ region of APP and by missense mutations in presenilin 1 and presenilin 2, the catalytic subunit of γ-secretase, that results in a relative increase in the production of Aβ42/43 peptides. In agreement with the concept that an excessive dose of Aβ causes Alzheimer’s, an enhanced gene dose of the precursor protein APP can cause the disease. In fact, APP is located on chromosome 21, and people with Down’s syndrome, who harbor three copies of APP, develop the typical neuropathology of Alzheimer’s disease at a young age [13].
The physiological functions of APP, the closely related APP-like proteins (APLPs), and their multiple processing products are still not well understood; there is evidence for a role in the development of the central nervous system, the formation and function of synapses, and neuroprotection following brain injury [15]. Aβ42, when excised from its precursor, is prone to undergo a conformational change that renders it able to self-aggregate into oligomers that can further assemble into fibrils and amyloid plaques (Figure 2). Amyloid fibrils are a structure that several proteins can adopt, characterized by a cross-β-sheet conformation in which β-strands run transversely to the main fiber axis and form an intermolecular network of hydrogen bonds [16].
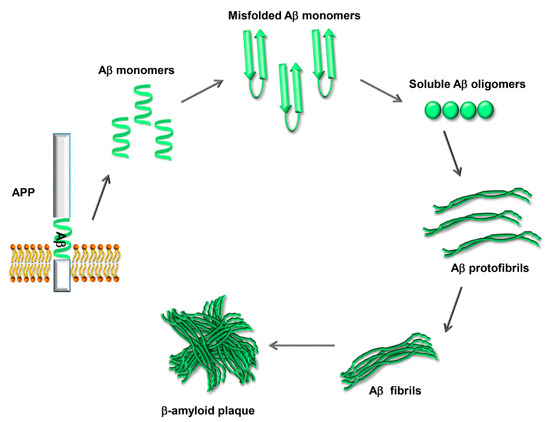
Figure 2. Process of Aβ aggregation and β-amyloid plaque formation. The Aβ peptide, once excised from APP, is prone to misfolding and self-aggregation. Misfolded Aβ monomers aggregate into small soluble oligomers. The oligomers interact to form protofibrils, which grow to form mature fibrils. Eventually the fibrils aggregate, forming the β-amyloid plaques.
Aβ plaque deposition is followed by neuritic and glial cytopathology in surrounding areas. A sequential association has been reported between brain β-amyloid accumulation, subsequent tau change, and resulting cognitive decline in individuals with dominant inherited AD [17]. While the amyloid plaques have been the target of many therapeutic approaches, the Aβ oligomers, which are diffusible and neurotoxic and exist in equilibrium with plaques, play the major role in neurodegeneration [18]. Aβ42 oligomers induce tau hyperphosphorylation and cause neuritic dystrophy in cultured neurons. In animal models, Aβ oligomers decrease synapse density, inhibit long-term potentiation, enhance long-term synaptic depression, and impair memory [19]. The most likely sequence of events leading to the disease comprises a neocortical Aβ accumulation, followed by a microglial inflammatory reaction to Aβ, neuritic dystrophy and spread of tau from the limbic system to the neocortex, and progressive tau accumulation and spread resulting in neurodegeneration [8,17]. Different therapeutic targets could be required for different stages of the disease process: Aβ for primary prevention, microglia for secondary prevention, and tau for established disease [8]. The β-amyloid peptide displays a highly complex self-assembly behavior, and it is challenging to define the aggregation process in terms of molecular events. The process is modeled as comprising primary and secondary pathways, that is, pathways that generate aggregates at a rate dependent on the concentration of monomers alone and independent of the concentration of existing fibrils (primary) and pathways that generate new aggregates at a rate dependent on the concentration of fibrils (secondary) [20]. Secondary pathways include mechanisms that depend only upon the concentration of fibrils, such as the fragmentation of fibrils, and mechanisms that depend on the concentration of both monomers and fibrils, such as secondary nucleation, whereby the surface of existing fibrils catalyzes the formation of oligomers from monomers. Secondary nucleation is a positive feedback loop in the aggregation process [20].
In agreement with this model of the aggregation process, oligomeric Aβ42 aggregates are particularly abundant around plaques; the neurodegeneration that is observed, in a gradient, around plaques is believed to derive mainly from the gradient of concentration of the diffusible, toxic oligomers [19,21]. Strategies to suppress the production of toxic oligomers need to consider both primary and secondary nucleation pathways of oligomer production. In this respect, while the oligomers and not the plaques are the direct culprit of the neurodegeneration, removing existing plaques makes sense as a step toward the reduction in the concentration of oligomers.
The early phases of the aggregation process reflect the fact that Aβ is a prion-like peptide, namely a peptide that adopts alternative conformations, which are self-propagating [22]. The self-propagating conformers, also known as ‘Aβ seeds’, can be quantified in cellular assays and in mouse models [22,23]. Aβ seeding potency is greatest early in the pathogenic cascade and diminishes as Aβ accumulates in the brain [22,24]. Interestingly, Aβ, as prions, can assemble into distinct strains of aggregates. Such strains may drive some of the phenotypic heterogeneity observed in Alzheimer’s disease [25].
3. Mechanisms of Action of Anti-β-Amyloid Antibodies
Many studies in vitro, in cell cultures and animal models, have shown that antibodies against β-amyloid can exert potentially useful effects to counteract β-amyloid dependent neurodegeneration, such as interfering with β-amyloid aggregation, blocking β-amyloid toxicity, or reducing the amount of β-amyloid in the brain (Figure 3).
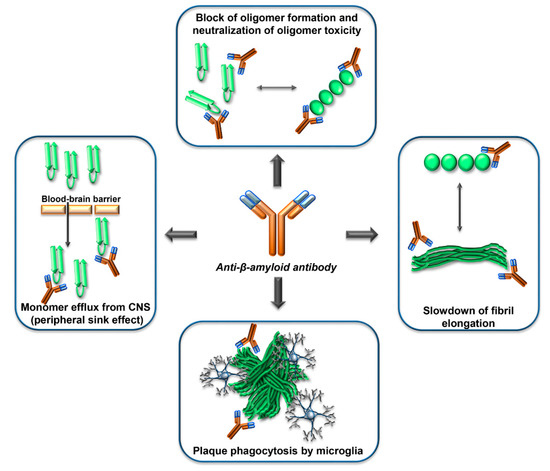
Figure 3. Mechanisms of action of anti-Aβ-antibodies. Anti-Aβ-antibodies can interfere with the β-amyloid cascade at several levels, by interacting with the Aβ monomers, the Aβ oligomers, the Aβ-fibrils, or the Aβ-plaques. The epitope specificity and concentration of antibody required for the different mechanisms are probably different. In human clinical trials, evidence of phagocytosis of plaques by microglia has been reported. The ‘peripheral sink effect’ is a hypothesis that has been disproven.
The entry of antibodies in the brain is restricted by the blood–brain barrier [26]; the concentration of an antibody in the CSF is 0.1 to 0.2% of the concentration in blood plasma [27,28]. Peripherally administered antibodies against the β-amyloid peptide can enter the central nervous system and reduce amyloid load; this was first documented in mouse models of Alzheimer’s Disease [29], and more recently also observed in clinical trials of passive immunization [30].
Different mechanisms of action of anti-β amyloid antibodies have been hypothesized or documented, including allosteric effects, the induction of plaque phagocytosis by microglia, the promotion of efflux of Aβ from the CNS to the circulation, the neutralization of β-amyloid toxicity (Figure 3). It is reasonable to expect that different mechanisms can become relevant depending on the stage of the amyloid deposition process, and on the concentration, isotype, and epitope specificity of the antibodies.
Amyloid deposition appears to follow a sigmoidal trajectory over time [31]. The time window where the slope of the amyloid load versus time curve is greatest represents a potential therapeutic window for secondary preventive interventions [31]. On the other hand, therapeutic interventions designed to reduce the rate of new amyloid deposition, rather than removing previously deposited amyloid, may be less effective in patients who have already reached plateau levels of amyloid deposition [31].
In vitro, anti-Aβ monoclonal antibodies can prevent Aβ monomers from forming fibrillar aggregates, and can convert fibrillar aggregates into an amorphous state [32]. Interestingly, the efficacy of these mechanisms depends on the concentration of antibody. At low concentrations of AMY-33, a monoclonal antibody raised against β-amyloid fragment 1–28, only amorphous aggregates are formed; increasing the concentration of antibody to equimolar antigen/antibody ratios maintained the solubility of β-amyloid [32]. The solubilization of already formed aggregates also required an equimolar ratio of antigen/antibody ratio, and prevented the neurotoxicity of β-amyloid [33].
Some epitopes of Aβ are preferentially available within plaques, whereas other epitopes are only available on the soluble peptide. Therefore, the epitope specificity of an antibody affects its binding affinity against different β-amyloid species. For epitopes exposed both in the monomer and in the aggregate forms, the presence of two antigen-biding sites generates avidity effects for aggregates. Therefore, the preferential binding of an antibody to the aggregate forms does not necessarily imply the recognition of a conformational epitope only present in the aggregate species. For instance, the sera of mice immunized with a multimeric protein that displays the Aβ(1–11) peptide [34,35], recognize the synthetic Aβ(1–11) peptide in ELISA assay, but display a marked preference for oligomeric and fibrillar species of β-amyloid in dot blot assays [36].
It has been hypothesized that soluble β-amyloid levels in the brain and the peripheral blood are in equilibrium so that blocking or degrading β-amyloid in blood should increase its efflux from the brain, and reduce brain amyloid. This hypothesis, known as the peripheral sink hypothesis [37], has been refuted. In various experimental systems, treatments that substantially decreased peripheral Aβ levels failed to affect brain and cerebrospinal fluid levels of Aβ; these findings suggest a lack of a significant peripheral sink effect through which brain amyloid burdens can be therapeutically reduced [38,39,40]. In clinical trials, a monoclonal antibody that binds soluble Aβ in blood, solanezumab, did not affect brain levels of amyloid [41]. When Aβ in blood is bound to solanezumab, the half-life of the Aβ-antibody complex is much longer than the half-life of free Aβ; therefore, treatment with solanuzemab leads to an increase in of the concentration of Aβ in blood, but the increase is not due to an efflux of Aβ from the brain.
While the fragment of the antibody that binds the epitope determines the specificity of the antibody, the constant region of the antibody, or Fc, is responsible for the capacity of an antibody to activate the complement cascade or to bind the Fc receptors that are expressed on a wide variety of cell types of the immune system, including microglia in the central nervous system; different antibody isotypes differ in their affinity for Fc receptors and ability to activate complement. The antibody isotype, therefore, affects Fc- or complement-mediated phagocytosis of plaques by microglial cells. An analysis of the epitope and isotype specificity of antibodies against β-amyloid able to protect against Alzheimer’s disease concluded that epitopes within the N terminus of Aβ are important for plaque clearance and neuronal protection via an Fc-mediated mechanism, and that IgG2a antibodies against Aβ are more efficient than IgG1 or IgG2b antibodies in reducing neuropathology [42].
4. Effect of Anti-β-amyloid Vaccination in Mouse Models of β-Amyloid Deposition
Genetically engineered mouse models have been instrumental to Alzheimer’s disease research and preclinical drug development. Transgenic mouse strains that overexpress mutant human APP linked to familial AD progressively develop many of AD’s pathological hallmarks—including senile plaques, synaptic loss, astrocytosis, and microgliosis—and have been largely used as preclinical research models. Recently, new mouse models have been generated that contain humanized sequences and clinical mutations in the endogenous mouse APP gene [43].
The first report of a disease-modifying effect of immunization against β-amyloid was published over 20 years ago. A transgenic mouse model of Alzheimer’s disease, the PDAPP mouse, was immunized with Aβ42, either before the onset of neuropathology, or at an older age, when amyloid-β deposition and neuropathology were well established. Immunization of the young animals prevented the development of β-amyloid-plaque formation, neuritic dystrophy, and astrogliosis, and treatment of the older animals markedly reduced the neuropathology [44].
A study performed in a different transgenic mouse model of Alzheimer’s disease, the TgCRND8 mouse, reported that Aβ immunization reduced both the deposition of cerebral fibrillar Aβ and cognitive dysfunction without, however, altering total levels of Aβ in the brain. The authors suggested that either a 50% reduction in dense-cored Aβ plaques was sufficient to affect cognition, or vaccination may modulate the activity/abundance of a small subpopulation of especially toxic Aβ species [45].
In yet another transgenic mouse strain, the Tg 2576 APP transgenic mice, and in a double mutant including both the APP and a PSEN mutation, vaccination against Aβ afforded protection from memory impairment, in the presence of reduced—but still substantial—Aβ deposits. The authors hypothesized that the antibodies could neutralize Aβ in some restricted compartment or deplete a non-deposited form of Aβ (for example, a soluble form) responsible for the memory loss [46].
The perspective of injecting the Aβ1–42 peptide in healthy, young humans to vaccinate them against Alzheimer’s raises in principle a safety concern over the possibility that the injected peptide itself, being able to promote oligomerization and fibrillogenesis, may start the pathogenic cascade in some healthy vaccinees, causing an enhanced probability of incurring the disease decades later. This scenario still cannot be formally excluded. Therefore, it is important to bear in mind that, ideally, the molecules used to immunize against β-amyloid should not be able to initiate the amyloid cascade. So far, all human immunizations have been performed in individuals that already had aggregated β-amyloid in their body. Importantly, in mice, it is unnecessary to immunize with the entire, pre-aggregated Aβ1–42 to observe an effect on brain amyloid. Immunization of transgenic APP mice with a soluble nonamyloidogenic, nontoxic Aβ homologous peptide, consisting of the first 30 amino acid residues of Aβ with six additional lysine residues at the N-terminus, reduced cortical and hippocampal brain amyloid burden and brain levels of soluble Aβ1–42, and reduced neuroinflammation [47].
6. Anti-β-Amyloid Vaccination in Humans with Vaccine AN1792
The Aβ1–42 peptide, in a pre-aggregated form, has been tested as a vaccine in humans under the name AN1792. AN1792 is the anti-β amyloid active immunization attempt on which more data has been published, with the longest follow-up. Therefore, we will review the results of the human immunizations here in detail.
AN1792 was formulated with QS21, an adjuvant able to enhance antibody responses and to favor a Th1 polarization of the T cell response [48]. Activated T cells can differentiate into subsets characterized by the productions of different sets of cytokines and different functions. The Th1 subset is characterized by its ability to secrete IFN-γ, a pro-inflammatory cytokine.
In the phase I trial, patients with mild to moderate AD received injections of AN1792 + QS-21 on day 0 and at weeks 4, 12, and 24. Patients could receive up to four additional injections of a polysorbate 80 modified formulation at weeks 36, 48, 60, and 72 [49]. During the period of the first four injections, 23.4% of AN1792-treated patients had an anti-AN1792 antibody titer of ≥1:1000. This increased to 58.8% after additional injections with the polysorbate 80 modified formulation. Disability Assessment for Dementia scores showed less decline among active compared with control patients at week 84 [49].
Thus, the phase I trial of AN1792 demonstrated that AN1792 + QS-21 was able to elicit an antibody response to Aβ42 [49], and also indicated some efficacy on the progression of the disease. The safety of the immunization was considered acceptable. One patient developed meningoencephalitis, that was diagnosed after death, and at the time was not considered to be related to the study treatment. AN1792 therefore proceeded to phase II trials. However, all clinical trials of AN1792 were interrupted when, in the phase II trial, meningoencephalitis occurred in 6% (18/300) of immunized patients [50].
In the phase II trial, the dosing protocol included intra muscular injections at baseline and at months 1, 3, 6, 9, and 12. As patients started to develop the meningoencephalitis reactions, however, dosing was discontinued, after only one to three injections. The predefined serum antibody response (anti-AN1792 IgG titer ≥ 1:2200) was achieved in 59 out of 300 patients (19.7%) [51]. Of the 18 patients that developed signs of meningoencephalitis, one had received one dose, 16 had received two doses, and one had received three doses before the symptoms of meningoencephalitis occurred [51].
6.1. Short Term Effects of AN1792 Immunization—One Year Follow-Up
In a Zurich cohort of 30 patients who had participated to the multicenter phase IIa trial the generation of antibodies against β-amyloid plaques correlated with clinical stabilization. Over the observation period of one year, patients with strong increases in anti-plaque antibodies remained clinically and cognitively stable, whereas the cognition of patients that had not generated the antibodies worsened [52]. The anti-plaques antibodies measured by tissue amyloid plaque immunoreactivity (TAPIR) [53] predicted outcome, whereas the anti β-amyloid titer measured by ELISA did not predict outcome [52]. This observation suggests that the antibody response to AN1792 may be qualitatively different in different individuals, and that the quality of the antibody response can affect the outcome.
In the general analysis of all the data from the phase IIa trial [51], although no significant differences were found between antibody responders and placebo groups for the various scales of cognitive assessment used, the Neuropsychological Test Battery (NTB) revealed differences favoring antibody responders. Greater improvements from baseline were associated with higher IgG antibody titers [51]. Moreover, tau in the CSF was decreased in antibody responders vs. placebo subjects [51].
6.2. Long Term Effects of AN1792 Immunization
The long term effects of AN1792 immunization was first reported on a small subset patients from the phase I trial, who consented to the clinical follow-up and post-mortem neuropathological examination [54]. In the immunized participants the mean Aβ load was lower than in unimmunized controls matched for age at death [54]. The mean antibody response attained during the treatment study period appeared to affect the degree of plaque removal [54]. Although immunization with Aβ42 resulted in clearance of amyloid plaques in patients with Alzheimer’s disease, this clearance did not prevent progressive neurodegeneration; also patients with the highest mean antibodies to Aβ and virtually complete plaque removal reached severe end stage dementia [54], implying that progressive neurodegeneration can occur in Alzheimer’s disease despite removal of plaques.
A larger follow-up study was conducted to assess the long-term outcomes 4.6 years after immunization with AN1792, to determine if benefits might accrue over time in patients from the phase IIa who had developed the pre-defined antibody titers (above 1:2200); patients originally identified as antibody responders were compared with placebo-treated patients [55]. Antibody responders retained low but persistent anti-AN1792 antibody titers after approximately 4.6 years. Compared with placebo-treated patients, antibody responders demonstrated significantly less impairment in activities of daily living and significantly less dependence on caregivers, and tended to perform better on the memory component of the Neuropsychological Test Battery [55].
A 15-year post-mortem neuropathological follow-up of patients from the phase I trial of AN1792 has investigated the relationships between the topographical distribution of amyloid-β removal from the cerebral cortex and tau pathology, cerebrovascular territories, anti-AN1792 antibody titers, and late cognitive status [56]. Fourteen of 16 (88%) Alzheimer’s patients who had received the active agent had evidence of plaque removal. Two Alzheimer’s patients who died 14 years after immunization had only very sparse or no detectable plaques in all regions examined. Despite modification of Alzheimer’s pathology, most patients had progressed to severe dementia, notably including those with very extensive plaque removal, possibly due to continued tau propagation [56]. Nevertheless, the study demonstrated that patients with Alzheimer’s disease actively immunized against amyloid-β can remain virtually plaque-free for 14 years. The extent of plaque removal was related to the anti-AN1792 antibody response [56].
6.3. Specificity of Antibodies Induced by AN1792
The immune sera from patients immunized with AN1792 specifically recognized β-amyloid plaques and diffuse Aβ deposits, as well as vascular amyloid in subarachnoidal and perforating brain vessels [53]. The immune sera did not cross-react with either denatured or native full-length APP [53]. Hock et al. reported no immunoreactivity of the sera against soluble Aβ42, dimers and trimers [53]. Epitope mapping with 10mer peptides mapped the antibody response, in 42 subjects, to the first 10 amino acids of Aβ42 (DAEFRHDSGY); the exposed N terminal amino acid appeared part of the epitope, as peptides extending N terminally to include APP sequence were poorly recognized by sera, confirming that the antibody response induced by Aβ42 does not cross-react with APP [57]. Pre-absorption with the amino terminal peptide Aβ(1–8) removed plaque-binding activity of sera, suggesting that the antibodies induced by AN1792 recognize a linear epitope, and not a specific conformation or multiple of Aβ unique to amyloid plaques [57]. At a difference with the data from Hock et al. [53], the data from Lee at al. [57] indicate that immune sera with high titer from patients immunized with AN1792 recognize also monomeric Aβ42, and that the antibodies generated by immunization with the pre-aggregated synthetic peptide recognize a linear epitope, not a conformational epitope [57].
6.4. Meningoencephalitis Reaction Induced by AN1792
The meningoencephalitis reactions that were observed during the AN1792 phase II experimentation were clearly associated to the treatment; none of the participants that had received placebo developed meningoencephalitis. Meningoencephalitis occurred without clear relation to serum anti-Aβ42 antibody titers [58]; five of the 18 patients that had experienced meningoencephalitis did not show the predetermined anti-β-amyloid antibody response, that is a titer higher than 1:2200, and one never developed a measurable antibody response [51].
The first neuropathological case report of an AD patient immunized against β-amyloid who had developed meningoencephalitis, proposed that the vaccination had caused Aβ plaques’ clearance [59].The patient had participated to a phase I trial of immunogenicity. After the first dose and subsequent doses at 4, 12, and 24 weeks, the woman had suffered no apparent adverse effects. Thirty-six weeks after the first injection, the woman had received a fifth injection with a reformulated preparation containing polysorbate-80. Six weeks later, she had become unwell, deteriorating such that cognitive tests could not be performed; her conditions remained relatively unchanged until she died, one year after the last injection. Comparison with unimmunized cases of AD revealed that, in the immunized patient, there were extensive areas of neocortex with very few Aβ plaques. These areas contained tangles, neuropil threads and cerebral amyloid angiopathy similar to unimmunized AD, but lacked plaque-associated dystrophic neurites and astrocyte clusters. In some of these plaque-free areas, Aβ-immunoreactivity was associated with microglia, the resident macrophages of the central nervous system; during development and homeostasis, microglial phagocytosis is essential for the refinement of synapses, and for the removal of apoptotic cells and debris [60].
All these findings suggested that the immune response generated against the peptide had elicited clearance of Aβ plaques, and that microglia cells had phagocytosed plaques. In the analysis, T-lymphocyte meningoencephalitis and infiltration of cerebral white matter by macrophages were also observed, and identified as possible correlates of the adverse reaction [59]. In this case report, extensive and persistent plaque removal clearly had afforded no clinical benefit, whereas the effect of the adverse reaction had been long-lasting. A neuropathological analysis of the brain of another trial participant who had received two intramuscular injections of AN1792 with adjuvant QS-21, separated by one month, and had experienced meningoencephalitis 6 months later, also reported a reduction of β-amyloid and T cell infiltration, and also multiple small hemorrages [61]. The absence of plaques was also reported in a patient immunized with AN-1792 who did not experience meningoencephalitis [62]. In this case, there were no amyloid plaques in the frontal cortex and abundant Aβ-immunoreactive macrophages, but tangles and amyloid angiopathy were present. The white matter appeared normal and minimal lymphocytic infiltration in the leptomeninges was observed [62]. This case demonstrated that Aβ immunization can affect brain amyloid in the absence of overt meningoencephalitis and leukoencephalopathy.
Overall, the meningoencephalitis reactions were attributed to the T cell response. In the phase IIa study, in which meningoencephalitis reactions were more frequent than in the phase I study, T-cell responses to Aβ were Th1-biased, and it was hypothesized that the meningoencephalitis might be associated with Th1 CD4 T cells, which are known to be pro-inflammatory, or with CD8, cytotoxic T cells. The epitope of antibodies were similar in the phase I study and the phase II [63]. Polysorbate 80, used in the phase II study, was considered a possible explanation for the different polarization of the T cell response in the different studies [63].
In the Zurich cohort, two patients with aseptic meningoencephalitis and who generated antibodies against β-amyloid experienced a transient worsening of cognition, but then recovered and remained cognitively stable one year after the immunizations, suggesting that the beneficial effects of antibodies against β-amyloid on cognitive functions are maintained even after transient episodes of meningoencephalitis [52].
6.5. Effect of AN1792 Immunization on Brain Volume and Brain Vasculature
In Alzheimer’s disease, progressive neurodegeneration involves brain atrophy over time, which is detectable in vivo by magnetic resonance imaging (MRI), and can be used as a marker of disease progression. Quite surprisingly, the MRI findings of the Phase IIa trial revealed that, one year after the start of immunization, antibody responders had greater brain volume decrease, and greater ventricular enlargement than placebo patients [64]. These increased losses in brain volume were not reflected in worsening cognitive performance [64]. The decrease in brain volume was transient. Placebo-treated patients and antibody responders did not demonstrate significant differences in loss of brain volume approximately 3.6 years from the end of the phase IIa study [55]. These observations revealed that amyloid removal and associated cerebral fluid shifts can result in macroscopic effects on brain volume, not due to neuronal degeneration [64].
Since antibodies can dissolve aggregated Aβ, an important issue about anti-Aβ immunotherapy is the fate of the solubilized Aβ. In aged APP-transgenic mice treated with passive immunotherapy against Aβ, as solubilized Aβ drains via the vascular pathway, vascular amyloid and microhemorrhages increase [65]. In mice treated with passive immunotherapy against Aβ, clearance of Aβ plaques and clearance of Aβ from vessels follows distinct kinetics [66]. It has been hypothesized that solubilized Aβ drains via the perivascular pathway, causing a transient increase in the severity of cerebral amyloid angiopathy [67]. This hypothesis is supported by the neuropathological examination on nine patients who died between four months and five years after their first immunization with AN1792. Compared with non-immunized Alzheimer’s patients, immunized patients had more blood vessels containing Aβ42 in the cerebral cortex and the leptomeninges, a significantly higher level of cerebrovascular Aβ40, and a higher density of cortical microhemorrhages and microvascular lesions. Two of the longest survivors, who had lived four to five years after first immunization, had virtually complete absence of both plaques and cerebral amyloid angiopathy, raising the possibility that the increase in the severity of CAA is transient, and that immunotherapy at later timepoints clears Aβ also from the cerebral vasculature. A similar observation has been reported in the context of passive immunization; a massive vascular amyloid burden has also been reported in a patient treated with the monoclonal anti-β-amyloid antibody solanezumab [68]. It remains to be established if it represented solubilized β-amyloid mobilized to the brain vasculature.
In subsequent clinical trials of anti-β-amyloid immunotherapy, the effects on brain vasculature have been monitored in vivo, by magnetic resonance imaging (MRI). The side effects of anti-β-amyloid treatment that can be detected by MRI have been named amyloid-related imaging abnormalities (ARIA). In particular, two types of ARIA have been defined: ARIA-E, or vasogenic edema; and ARIA-H, indicating microhemorrhages and hemosiderosis [69].
ARIA-E and ARIA-H are induced by anti-Aβ antibodies [70,71], and therefore can occur both in passive and in active anti-Aβ immunotherapy. In the prospect of developing a safe immunotherapy, it is important to identify the patients more at risk of incurring serious events, and to understand how to best manage these events. The APOE 4 allele, in a study with anti-Aβ monoclonal Bapineuzumab, appeared to be a risk factor for ARIA [71].
7. Anti-β-Amyloid Vaccination in Humans with Second Generation Vaccines
Despite the serious adverse events, the results of the AN1792 clinical trials encouraged further research into active anti-Aβ immunotherapy. Since the meningoencephalitis was attributed to the T cell response, mapped to the central part of the β-amyloid peptide, and the effects on disease progression were attributed to the antibody response, mapped to the N-terminus of Aβ, second generation vaccines mainly used the strategy of directing the immune response to the N-terminal B cell epitope, without inducing a concomitant T cell response to β-amyloid.
The active immunogens that have entered clinical trials are shown in Table 1. Four anti-Aβ vaccines are currently being tested in phase II trials: CAD106, ACI-24, UB-311, and ABVac40.
Table 1. Vaccines against β-amyloid that entered phase II clinical trials.

The T cell response is important for the generation of high affinity IgG; several second generation anti-Aβ vaccines include exogenous T cell epitopes. Some candidate vaccines rely on T cell epitopes present in a carrier, for instance vaccine CAD106 consists of the B cell epitope Aβ1–6 linked to the capsid of the Qβ bacteriophage, and vaccine ABvac40 consists of the B cell epitope Aβ33-40 conjugated to a carrier protein, keyhole limpet hemocyanine (KLH) (Table 1).
Other candidate vaccines consist of synthetic peptides that include T cell epitopes from pathogens: vaccine UB-311 is a mixture of two synthetic peptides, each including the B cell epitope Aβ1–14 and a T cell epitope either from the hepatitis virus or the measles virus; vaccine Lu AF20513 includes B cell epitope Aβ1–12 and T cell epitopes from tetanus toxin (Table 1).
None of the second-generation vaccines that entered clinical trials have induced meningoencephalitis, whereas antibody-mediated adverse effects, such as ARIA, were reported. No clinical benefit from the second-generation vaccines has been reported so far.
Although no direct comparison has been published, the duration of the anti-Aβ response elicited by second generation vaccines appears shorter than the duration of the response elicited by the AN1792; vaccine-induced antibodies become undetectable a few months after the last injection.
In the case of ACC-001 [72], substantial interindividual variability in anti-Aβ IgG titer was observed, and no correlation was found between IgG titer levels and cognitive or functional efficacy results, or biomarker results [72].
CAD106 induced an antibody response against Aβ in most patients. No meningoencephalitis was observed. CAD106-induced antibodies reacted with amyloid plaque cores and Aβ oligomers ex vivo. The best reactivity was seen with the entire Aβ1–6 epitope. No statistically significant differences were observed in CSF biomarkers [73].
This entry is adapted from the peer-reviewed paper 10.3390/biology9120425
