Protein-protein interactions (PPIs) are involved in all aspects of cellular functions. The identification and characterisation of PPIs are essential for understanding the molecular mechanisms that regulate biological systems and for guiding drug design programs. PPIs can modify the kinetic properties of enzymes, form new binding sites, control the localisation, and change the specificity, among others. The spatial arrangement of protein complexes is determined by the composition of the amino acid of the proteins involved, their concentration, and the free energy of the complex.
- : protein-protein interactions
- protein-RNA
- cancer
- p53 pathway
- MDM2
1. Introduction
Studies on PPI networks and their topologies revealed the existence of nodes of proteins able to interact with a large number of partners [1]. These proteins are known as hubs and they have particular biological properties. They tend to be evolutionarily conserved to a larger extent compared to non-hubs [2]. Their ability to interact with multiple partners is often facilitated by intrinsically disordered regions (IDR), and it is regulated by post-translational modifications (PTMs) that govern different conformations and bound states [3][4]. Such conformational changes lead to allosteric structural modifications, which dynamically regulate the interactions with binding partners. Allosterically-induced interactions are vital for the regulation of several proteins, determining their function and orchestrating the physiological effect of the cognate signalling pathway [5]. Since hubs play central roles in signalling networks, they constitute exciting targets for drug development applications.
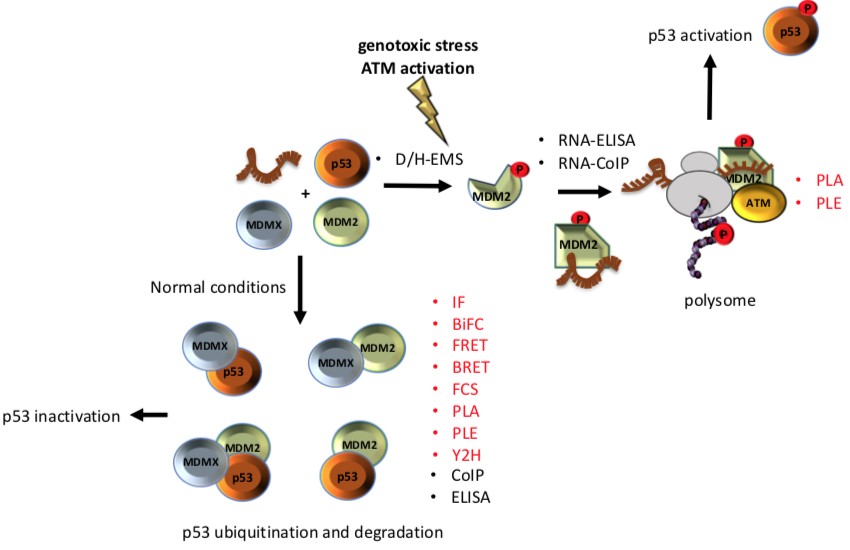
The p53-MDM2 pathway is a dynamic and well-characterised model used to study both protein-nucleic acid and PPIs since both Mouse double minute 2 (MDM2) and p53 are hubs in a vast network of interactions involved in several cellular pathways [6][7][8]. The p53 tumour suppressor is a crucial regulator of cellular homeostasis and it is tightly linked to cancer development. p53 function is lost in more than 50% of all types of human cancers and represents a main target of genetic diagnostics and therapeutic interventions. As a result, the p53 pathway regulation constitutes an ideal study-model for improving the current state of methodologies aiming to decipher the underlying mechanisms. Under normal conditions, p53 activity is low due to its interaction with MDM2, which exhibits an E3 ubiquitin ligase activity that targets p53 for degradation via the 26S proteasome, and with its homolog Mouse double minute 4 (MDMX) that blocks the transcriptional activity of p53 [9]. Following genotoxic stress, p53 levels increase to allow the cells to repair the damage before entering replication, or to trigger irreversible senescence or apoptosis, when the damage is too severe. Activation of p53 requires MDM2 to switch from binding the p53 protein to p53 mRNA and become a positive regulator of the p53 tumour suppressor protein. This involves the ATM kinase-dependent phosphorylation of MDM2 on Ser 395. This PTM induces a conformational change on MDM2, which results in allosteric changes allowing the formation of a p53 mRNA-binding site that stimulates p53 synthesis [10][11]. Together with ribosomal proteins, such as RPL5 and RPL11, the complex (MDM2-p53 mRNA-RPs) is transported to the cytoplasm where the p53-polysome is formed. ATM also phosphorylates MDMX at Ser 403, which promotes its RNA chaperone activity toward the p53 mRNA to create an mRNA structure suitable for the MDM2-p53 mRNA interaction [12]. Given this complexity, it becomes clear that the mechanistic description of the p53 regulation requires the application of multi-faceted techniques and methodologies. In addition, the study of p53 regulation needs be approached from several levels, including (a) in vivo, in vitro, and in situ techniques, adequately addressing the interactions and the expression levels, and (b) the effect of different conditions that alter those interactions and the expression of the partners involved. Diverse studies have employed proteomic techniques in an attempt to broadly identify binding partners and to unravel the mechanisms control, which are regulated by the p53-MDM2 pathway [13][14][15][16]. Some of the techniques that have contributed to understanding the dynamics of this pathway are: enzyme immunoassay/enzyme-linked immunosorbent assay, yeast 2-hybrid or 2 hybrid (in mammals), Bimolecular Fluorescence Complementation, Förster Resonance Energy Transfer, Bioluminescence Resonance Energy Transfer, Co-immunoprecipitation. as well as fluorescence cross-correlation spectroscopy, Proximity Ligation assay and Proximity Ligation ELISA.
2. Discussion
p53 is a very interesting molecule; it involves IDRs, dozens of PTMs sites and has tumour suppressor and transcription factor activities. These qualities make p53 a particularly challenging molecule, whose study requires the combination of several techniques. In the p53-MDM2 model, a single phosphorylation event in response to DNA damage mediates a conformational change on the MDM2 protein with dramatic functional physiological effects on the regulation of p53. Such induced changes and their functional effects may only be addressed by combining in cell, in vitro and in situ techniques, offering multifaceted insights (Figure 1). The techniques listed here constitute technical cornerstones which drove the current knowledge around p53 regulation. However, these techniques are individually suitable for the study of any interaction, expression or mechanism independently on whether it involves IDRs or PTMs inducing allosteric changes, in response to cellular stresses or exogenous factors. Clearly, each study may require a particular selection of the techniques to adequately address the regulatory mechanism.
Figure 1. Different techniques have been used to unravel the interaction among of p53-MDM2-MDMX A phosphorylation on Ser 395 of MDM2 change its conformation and allow the binding of the p53 mRNA that results in increased p53 protein synthesis after DNA damage. On the other hand, under normal conditions interaction of MDMX and/or MDM2 with p53 negatively controls the p53 levels. Techniques that allow to study the interactions in situ are shown in red.
Targeting structural epitopes of protein-protein interactions with small chemical compounds such as nutlin, is a promising strategy for the development of new therapeutics. In this context a challenging task is to see how one interaction affects the next and instead target specific conformations induced by PTMs. In the example of MDM2, targeting the phosphorylation of Ser 395 that prevents the interaction with p53 through its binding with the p53 mRNA, could result in less invasive therapies. Such strategies may allow the development on therapies targeting transitory interfaces. In the MDM2 model, this would consider the association of third factors determining the positive or negative regulation of p53. To address this regulatory network, in their complexity, we must be able to find other factors involved, where in the cell these interactions take place, and what innovative techniques would allow to predict the diverse dynamic and kinetic properties, which further depend on cell conditions or exogenous factors.
This entry is adapted from the peer-reviewed paper 10.3390/biom11010036
References
- Vallabhajosyula, R.R.; Chakravarti, D.; Lutfeali, S.; Ray, A.; Raval, A. Identifying hubs in protein interaction networks. PLoS ONE 2009, 4, e5344. [Google Scholar] [CrossRef]
- Wuchty, S.; Almaas, E. Peeling the yeast protein network. Proteomics 2005, 5, 444–449. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Bustos, D.M. Intrinsic disorder associated with 14-3-3 proteins and their partners. Prog. Mol. Biol. Transl. Sci. 2019, 166, 19–61. [Google Scholar] [CrossRef]
- Uversky, V.N. Analyzing IDPs in Interactomes. Methods Mol. Biol. 2020, 2141, 895–945. [Google Scholar] [CrossRef]
- Wang, J.; Jain, A.; McDonald, L.R.; Gambogi, C.; Lee, A.L.; Dokholyan, N.V. Mapping allosteric communications within individual proteins. Nat. Commun. 2020, 11, 3862. [Google Scholar] [CrossRef]
- Fahraeus, R.; Olivares-Illana, V. MDM2′s social network. Oncogene 2014, 33, 4365–4376. [Google Scholar] [CrossRef]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer. 2020, 20, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. P53 and The Immune Response: 40 Years of Exploration-A Plan for the Future. Int. J. Mol. Sci. 2020, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Karni-Schmidt, O.; Lokshin, M.; Prives, C. The Roles of MDM2 and MDMX in Cancer. Annu. Rev. Pathol. 2016, 11, 617–644. [Google Scholar] [CrossRef]
- Medina-Medina, I.; Garcia-Beltran, P.; de la Mora-de la Mora, I.; Oria-Hernandez, J.; Millot, G.; Fahraeus, R.; Reyes-Vivas, H.; Sampedro, J.G.; Olivares-Illana, V. Allosteric Interactions by p53 mRNA Govern HDM2 E3 Ubiquitin Ligase Specificity under Different Conditions. Mol. Cell Biol. 2016, 36, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, M.; Candeias, M.M.; Malbert-Colas, L.; Mazars, A.; Fujita, J.; Olivares-Illana, V.; Fahraeus, R. The p53 mRNA-Mdm2 interaction controls Mdm2 nuclear trafficking and is required for p53 activation following DNA damage. Cancer Cell 2012, 21, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Malbert-Colas, L.; Ponnuswamy, A.; Olivares-Illana, V.; Tournillon, A.S.; Naski, N.; Fahraeus, R. HDMX folds the nascent p53 mRNA following activation by the ATM kinase. Mol. Cell 2014, 54, 500–511. [Google Scholar] [CrossRef]
- Hupp, T.R.; Hayward, R.L.; Vojtesek, B. Strategies for p53 reactivation in human sarcoma. Cancer Cell 2012, 22, 283–285. [Google Scholar] [CrossRef]
- Cao, Z.; Xue, J.; Cheng, Y.; Wang, J.; Liu, Y.; Li, H.; Jiang, W.; Li, G.; Gui, Y.; Zhang, X. MDM2 promotes genome instability by ubiquitinating the transcription factor HBP1. Oncogene 2019, 38, 4835–4855. [Google Scholar] [CrossRef]
- Dickinson, E.R.; Jurneczko, E.; Nicholson, J.; Hupp, T.R.; Zawacka-Pankau, J.; Selivanova, G.; Barran, P.E. The use of ion mobility mass spectrometry to probe modulation of the structure of p53 and of MDM2 by small molecule inhibitors. Front. Mol. Biosci. 2015, 2, 39. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, T.; Chen, Y. LC-MS/MS-based targeted proteomics quantitatively detects the interaction between p53 and MDM2 in breast cancer. J. Proteom. 2017, 152, 172–180. [Google Scholar] [CrossRef]