Mitochondria are energy-producing structures and the main site for aerobic respiration in cells, and are therefore called the “powerhouse of the cell”.
- natural products,mitochondria
1. The Role of Mitochondria in Cancer Cells
Mitochondria are associated with many diseases, such as Parkinson’s disease [13], diabetic nephropathy [14], acute kidney injury [15], and Down syndrome [16]. Mitochondria also play an important role for cell signaling, apoptosis regulation, and energy metabolism in drug-induced cancer cells death; therefore, they are considered a significant target in cancer chemotherapy [17]. Some scholars have reviewed the mitochondrion as a target of anticancer therapy over the years [18,19,20,21]. Moreover, modulation of mitochondrial-dependent pathways by natural compounds is diverse (Figure 1). However, few researchers have reviewed natural products that regulate mitochondrial pathway in cancers.
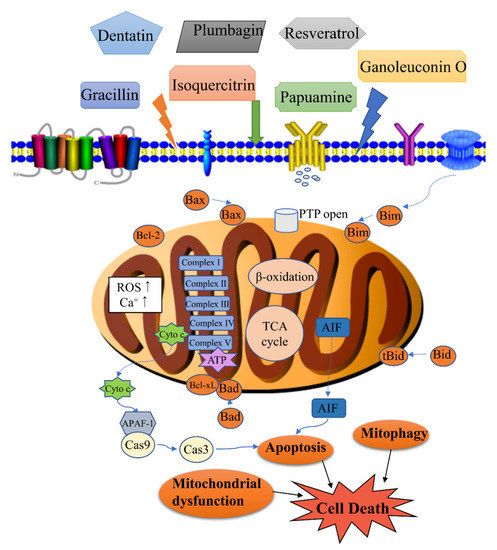
Figure 1. Modulation of mitochondrial-related cell death by natural products. Cell death associated with the activity of natural products includes apoptosis, mitophagy, mitochondrial dysfunction, etc. Apoptosis is regulated by the levels of Bcl-2 (B-cell lymphoma-2) family proteins, release of cytochrome c, and caspase activation. Mitophagy is the targeted phagocytosis and destruction of mitochondria by the autophagy machinery, and it is generally considered as the main mechanism of mitochondrial quality control. A decrease in energy production, an increase of reactive oxygen species (ROS) and permeability transition pore (PTP) opening can lead to mitochondrial dysfunction.
2. Mitochondrial Control of Apoptosis
Mitochondrial involvement is an important pathway in the process of apoptosis. The Bcl-2 protein family regulates apoptosis by controlling mitochondrial permeability. Anti-apoptotic proteins B-cell lymphoma-2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xL) reside in the outer membrane of mitochondria and inhibit the release of cytochrome c. Pro-apoptotic proteins Bax, Bad, Bid, and Bim can reside in the cytoplasm, translocating to mitochondria after receiving a death signal, and promote cytochrome c release into the cytoplasm. Released cytochrome c binds to apoptotic protease activating factor-1 (Apaf-1) to form apoptosome, amplifying the apoptotic cascade [22,23,24].
Necrotic stimulation leads to increased mitochondrial Ca2+ uptake and ROS production. High levels of Ca2+ and ROS induce the opening of the Cyclophilin-D (Cyp-D) sensitive permeability transition pore (PTP), leading to matrix swelling and Ca2+ release. Swelling damages the outer membrane and releases Ca2+ activating proteases, phosphatases, and nucleases, leading to necrotic degradation [12].
Fission or fusion rates may change under different growth conditions, and result in an increase or decrease in the number of mitochondria. When mitochondria become damaged, their connectivity is reduced, and mitochondria become shorter and rounder. The change from highly branched to fragmented morphologies may be induced by altered fission or fusion rates. At the early stage of apoptosis, the transition from a mitochondrial network to vesicular punctiform mitochondria was detected [25]. Mitochondrial fragmentation occurs in parallel to the formation of apoptotic bodies, increasing the number of the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive nuclei and cleavage of the caspase substrate polymerase (PARP) [26].
3. Mitochondrial Control of Energy Metabolism
Mitochondria provide considerable flexibility for the growth and survival of tumor cells, and play a key role in harsh conditions, such as nutrient depletion and hypoxia. The rapid proliferation of cancer cells requires more mitochondria than normal cells. Therefore, the development of chemotherapeutic drugs for mitochondria is a breakthrough in the fight against cancer. Many scholars have clarified that the mechanical drive of mitochondrial respiration involves the tricarboxylic acid (TCA) cycle, and fatty acid β-oxidation enzymes in the mitochondrial matrix that generate electron donors to fuel respiration and electron transport chain (ETC) complexes, and ATP synthase in the inner mitochondrial membrane (IMM) that carry out oxidative phosphorylation [27]. Some natural products inhibit electron transport chain complexes. Four such complexes are NADH-ubiquinone reductase(complex I), succinate-ubiquinone reductase (complex II), ubiquinol-cytochrome c reductase (complex III), and cytochrome c oxidase (complex IV) [28]. Complex V, which is called ATP synthase, together with the above four complexes, completes oxidative phosphorylation to produce ATP. Inhibition of mitochondrial ETC complex activity can lead to significant mitochondrial dysfunction.
Cardiolipin, which consists of two phosphatidyl residues linked by a glycerol bridge, is a unique phospholipid dimer in the inner mitochondrial membrane in all eukaryotes. Cardiolipins play an important role in preserving mitochondrial structure and function. They support membrane dynamics and stabilize the lateral organization of protein-rich membranes in mitochondria [29]. Cardiolipins are involved in mitochondrial cristae morphology and stability [30], mitochondrial quality control, and dynamics by fission and fusion [31,32] and mitophagy [33]. They can also serve as a binding platform to recruit apoptotic factors in the apoptotic process [34,35]. However, it is still not clear how these events are interconnected and cooperate. In addition, cardiolipins are very susceptible to damage from ROS because of their high content of unsaturated acyl chains. Thus, the stability and function of mitochondria can be impaired by the biophysical properties of the membranes that are altered [36].
In this paper, we attempt to summarize the mechanisms through which natural products exert anticancer effects, as published in the past five years, by using a structural classification, with emphasis on the molecular mechanisms of mitochondrial involvement. Through all the reports, we found that most natural products regulate a series of proteins, such as Bax, Bcl-2, and caspases-3 and -9. Moreover, inhibitors of electron transport chain complexes can also exert anticancer activity. Details can be found in Table 1.
Table 1. Natural products (1–81) regulated mitochondria by different mechanisms in cancer cells.
| No. | Isolated Compound | Origin | Cell Line | Mechanism | Reference |
|---|---|---|---|---|---|
| Terpenoids | |||||
| 1 | Ganoleuconin O | Ganoderma leucocontextum | Huh7.5 | Fatty acid immobilization, loss of the mitochondrial lipid cardiolipin | [30] |
| 2 | Lupeol | Bombax ceiba | SK-RC-45 | Mitochondrial hyper fission | [31] |
| 3 | Betulinic acid | Betula alba | HeLa | Cardiolipin modification, ROS generation, Bad, caspase 9 | [32,33] |
| 4 | Alisol B-23-acetate | Alisma orientale | A549, NCI-H292 | ROS generation, Bcl-2↓, Bax↑, activation of caspase-3, -9, release of cytochrome c/AIF | [34] |
| 5 | Genipin | Gardenia jasminoides | N18TG2 | Activation of dicarboxylate carrier, decreased activity of UCP1, UCP3, and complex III of the respiratory chain, UCP2 inhibition | [35] |
| 6 | Alternol | Yew tree | PC-3 | Decrease of mitochondrial respiration, isocitric acid, fumaric acid and malic acid, ATP production | [36,37] |
| 7 | Cyathin Q | Cyathus africanus | HCT116 | Bcl-2↓, Bax↑, Bcl-xL↓, ROS generation, release of cytochrome c | [38] |
| 8 | 3α-hydroxy-19α-hydrogen-29-aldehyde-27-lupanoic acid | Potentilla discolor | HepG2 | Bcl-2↓, Bax↑, release of cytochrome c | [39] |
| 9 | Uvedafolin | Smallanthus sonchifolius | HeLa | MMP loss, release of cytochrome c | [40] |
| 10 | Heteronemin | Hippospongia sp. | Molt4 | ROS generation | [41] |
| 11 | Jatrogossone A | Jatropha gossypiifolia | KOPN-8 | MMP loss, ROS generation | [42] |
| 12 | Walsuronoid B | Walsura robusta | Bel-7402, HepG2 | ROS generation, mitochondrial and lysosomal dysfunction | [43] |
| 13 | Ferruginol | Podocarpus ferruginea | MDA-T32 | ROS generation, MMP loss, Bcl-2↓ | [44,45] |
| 14 | Lobocrassin B | Lobophytum crassum | CL1-5, H520, BEAS-2B |
Bcl-2↓, Bax↑, ROS generation, MMP loss, release of cytochrome c, activation of caspase-3 | [46] |
| 15 | Aellinane | Euphorbia aellenii | Caov-4 | Bcl-2↓, Bax↑, ROS generation, MMP loss | [47] |
| 16 | Tingenin B | Maytenus sp. | MCF-7s | Bcl-2↓, Bax↑, MMP loss | [48] |
| 17 | 3-O-trans-p-coumaroyl alphitolic acid | Ziziphus jujuba | PC-3 | ROS generation | [49] |
| 18 | Zerumbone | Zingiber zerumbet | PC-3, DU-145 | Tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult | [50,51] |
| Flavonoids | |||||
| 19 | Isoquercitrin | Hibiscus cannabinus | MDA-MB-231 | LSD1-induced mitochondrial-mediated apoptosis pathway | [52,53] |
| 20 | Luteolin | Cauliflower, peanut, and carrot | SW1990 | Inhibitor of Bcl-2, mitochondrial permeabilization | [54] |
| 21 | Dihydromyricetin | Ampelopsis grossedentata | HepG2 | Akt/Bad signal pathway, mitochondrial apoptotic pathway, Bax↑, Bad↑, inhibition of the phosphorylation of Bad at Ser136 and Ser112 | [55,56] |
| 22 | Artonin E | Artocarpus elasticus | SKOV-3 | Release of cytochrome c, Activation of caspases-3, -8, and -9, Bax↑, Bcl-2↓, HSP70↓, survivin↓ | [57] |
| 23 | Myricetin | Fruits and vegetables | SNU-80 | Bax/Bcl-2↑, release of AIF | [58] |
| 24 | Xanthones | Garcinia xanthochymus | HepG2 | Bax↑, Bcl-2↓, Bcl-xL↓, Mcl-1↓, and survivin↓ | [59] |
| 25 | Cycloartobiloxanthone | Artocarpus gomezianus | H460 | Bax↑, Bcl-2↓, Mcl-1↓ | [60] |
| 26 | Paratocarpin E | Euphorbia humifusa | MCF-7 | Bax↑, Bcl-2↓, release of cytochrome c | [61] |
| 27 | Puerarin 6′’-O-xyloside | Pueraria lobata | SW480 | Bax↑, Bad↑, Bcl-2↓, caspase-3 and -9 activation | [62] |
| 28 | α-mangostin | Cratoxylum arborescens | HeLa | ROS generation, MMP loss, release of cytochrome c | [63] |
| 29 | Chrysin | Honey and propolis | Mitochondria isolated from hepatocytes of HCC rats |
ROS generation, MMP loss, release of cytochrome c, swelling in mitochondria | [64,65] |
| 30 | Fisetin | Strawberries, apples, grapes, onions, and cucumbers | SCC-4 | ROS generation, Ca2+ production, MMP loss, Bcl-2↓, Bax↑, Bid↑, release of cytochrome c, AIF, and Endo G | [66,67] |
| 31 | Baicalein | Scutellaria baicalensis, Scutellaria radix | A2780 | Combination therapy with baicalein and taxol had much higher antitumor effects compared with the monotherapy. Release of cytochrome c, and caspase-3 and -9 activation |
[68,69] |
| 32 | Alpinetin | Zingiberaceous plants |
A549 | Bcl-2↓, Bax↑, Bcl-xL↓, XIAP↓, PI3K/Akt signaling pathway, sensitized drug-resistant lung cancer cells | [70,71] |
| 33 | Chamaejasmin B | Stellerachamaejasme | KB, KBV200 | Bcl-2↓, Bax↑, MMP loss, release of cytochrome c and AIF | [72] |
| 34 | Mensacarcin | Streptomyces bacteria | SK-Mel-28, SK-Mel-5, HCT-116 | Release of cytochrome c, energy production and mitochondrial function rapidly disturbed | [73] |
| Saponins | |||||
| 35 | Gracillin | Dioscorea gracillima | H226B, H460 | Targeting mitochondrial complex II, suppressing ATP synthesis, ROS generation | [74] |
| 36 | Polyphyllin I | Paris polyphylla | MDA-MB-231 | Mitochondrial translocation of DRP1, mitochondrial fission, release of cytochrome c, mitochondrial PTEN-induced kinase 1↑ | [75,76] |
| 37 | Frondoside A | Cucumaria frondosa | CA46 | Bcl-2↓, survivin↓, release of HtrA2/Omi and cytochrome c, ROS generation | [77] |
| 38 | 3β-O-α-l- arabinopyranoside |
Clematis ganpiniana | MCF-7, MDA-MB-231 | Release of cytochrome c and Apaf-1, upregulation of caspase-9 and caspase-3 | [78] |
| 39 | Sakuraso-saponin | Aegiceras corniculatum | LNcaP, 22RV-1, C4-2 | Bcl-xL↓ | [79,80] |
| 40 | Ginsenoside compound K | Panax ginseng | SK-N-BE(2), SH-SY5Y | Bcl-2↓, Bcl-xL↓ | [81] |
| 41 | Escin | Aesculus hippocastanum | 786-O, Caki-1 | G2/M arrest and ROS-modulated mitochondrial pathways | [82] |
| 42 | α-Hederin | Hedera helix | SW620 | NF-κB signaling pathway, Bcl-2↓, Bax↑, release of cytochrome c | [83,84] |
| Alkaloids | |||||
| 43 | Cathachunine | Catharanthus roseus | HL60 | ROS-dependent mitochondria-mediated intrinsic pathway, Bcl-2/Bax↓, ROS generation, MMP loss, release of cytochrome c | [85] |
| 44 | Berberine | Rhizoma coptidis | T98G, LN18 | ERK1/2-mediated impairment of mitochondrial aerobic respiration | [86,87] |
| 45 | Papuamine | Haliclona sp. | H1299 | Intracellular ATP depleted by causing mitochondrial dysfunction, mitochondrial superoxide production | [88] |
| 46 | Bis (2-ethyl hexyl) 1H-pyrrole-3, 4-dicarboxylate | Tinospora cordifolia | MDA-MB-231 | ROS generation, increase in intracellular calcium, phosphorylation of p53, mitochondrial membrane depolarization, MPTP, and cardiolipin peroxidation, Bcl-2↓, Bax↑, release of cytochrome c, caspase activation, DNA fragmentation | [89] |
| 47 | Unantimycin A | Found in the fraction library of microbial metabolites | Semi-intact cells with specific substrates for each complex of the mitochondrial electron transport chain |
Targeted inhibition of mitochondrial complex I | [90] |
| 48 | NPL40330 | Found in chemical library | Semi-intact cells with specific substrates for each complex of the mitochondrial electron transport chain |
Targeted inhibition of mitochondrial complex III | [90] |
| 49 | Boholamide A | Marine mollusks | U87MG | Influx of Ca2+ | [91] |
| 50 | Cernumidine | Solanum cernuum | T24 | Cytotoxicity and chemosensitizing effect of cernumidine to cisplatin. Bcl-2↓, Bax↑, MMP loss | [92] |
| 51 | Lycorine | Amaryllidaceae plant family | HepG2 | mPTP opening, MMP loss, ATP depletion, release of Ca2+ and cytochrome c, caspase activation | [93] |
| 52 | Lagunamides A | Lyngbya majuscule | A549 | MMP loss, ROS generation | [94] |
| 53 | Cordycepin | Cordyceps | OVCAR-3 | Downregulation of mitochondrial function and limitation of energy production; metastasis and migration suppressed | [95,96] |
| Coumarins | |||||
| 54 | 2,3-Dihydro-7- hydroxy-2R*,3R*- dimethyl-2-[4,8-dimethyl-3(E),7- nonadienyl]-furo[3,2-c]coumarin |
Ferula ferulaeoides | C6 | MMP loss, Bcl-xL↓, Bcl-2↓, Bax↑, cleavage of Bid, FAS↑, FADD↑ | [97] |
| 55 | Dentatin | Clausena excavate | HepG2 | Bcl-xL↓, Bcl-2↓, Bax↑, release of cytochrome c | [98,99] |
| 56 | Aesculetin | Cortex Fraxini | THP-1 | Bcl-2↓, Bax↑ | [100] |
| Quinones | |||||
| 57 | Quambalarine B | Quambalaria cyanescens | Jurkat E6.1 | Inhibition of mitochondrial complex I and II, inhibition of mitochondrial respiration, metabolism reprogramming | [101,102] |
| 58 | Plumbagin | Plumbago zeylanica | MG63 | ROS generation, Bcl-2↓, Bax↑, Bcl-xL↓, and Bak↓, endoplasmic reticulum stress | [103] |
| 59 | Shikonin | Lithospermum erythrorhizon | HGC-27 | Bcl-2↓, Bax↑, survivin↓ | [104] |
| 60 | 2,7-dihydroxy-3-methylanthraquinone | Hedyotis diffusa | SGC-7901 | Bcl-xl↓, Bcl-2↓, Bax↑, Bad↑, release of cytochrome c | [105] |
| 61 | 3-hydroxy-1,5,6-trimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris connate | A549, H1299 | Bcl-2↓, Mcl-1↓, Bax↑ | [106] |
| 62 | Thymoquinone | Nigella sativa | T24, 253J | Bcl-2↓, Bax↑, release of cytochrome c and AIF | [107] |
| Miscellanea | |||||
| 63 | Methylsulfonylmethane | Fruits and vegetables | YD-38 | Bcl-xL↓, Bcl-2↓, Bax↑, release of cytochrome c, MMP loss | [108,109] |
| 64 | Parameritannin A-2 | Urceola huaitingii | HGC27 | Enhanced doxorubicin-induced mitochondria-dependent apoptosis, inhibition of the PI3K/Akt, ERK1/2 and p38 pathways, Bcl-2↓, Bcl-xl↓, Bax↑, Bid↑, release of cytochrome c, caspase activation | [110] |
| 65 | Resveratrol | Polygonum cuspidatum, Veratrum nigrum, Cassia obtusifolia |
H838, H520; K562 |
Enhanced antitumor activities of cisplatin; Induced apoptosis |
[111,112] |
| 66 | Oleuropein | Olea europaea | H1299 | Bcl-2/Bax↓, release of cytochrome c, activation of caspase-3 | [113,114] |
| 67 | Homoisoflavanone-1 | Polygonatum odoratum | A549 | Mitochondria-caspase-dependent and ER stress pathways, Bcl-2/ Bak↓ | [115] |
| 68 | Gallic acid | Green tea, grapes, red wine |
H446 | ROS-dependent mitochondrial apoptotic pathway | [116] |
| 69 | Hierridin b | Cyanobium sp. | HT-29 | Proteomics identified 21 differentially expressed proteins belonging to the categories protein folding/synthesis and cell structure and reduced mitochondrial activity and as confirmed by morphological analysis of mitochondrial parameters |
[117,118] |
| 70 | Deoxyarbutin | Ecklonia cava | B16F10 | MMP loss, ATP depletion and ROS overload generation | [119] |
| 71 | Magnolol | Magnolia officinalis | OS-RC-2, 786-O | P53, Bcl-2/Bax↓, release of cytochrome c, caspase activation, ROS generation | [120] |
| 72 | Oblongifolin C | Garcinia yunnanensis | QBC939 | Mitochondrial dysfunction | [121] |
| 73 | Amorfrutin C | Glycyrrhiza foetida | HT-29 | mPTP opening, mitochondrial oxygen consumption and extracellular acidification increased | [122] |
| 74 | Allyl isothiocyanate | Cruciferous vegetables | MCF-7, MDA-MB-231 | ROS and Ca2+ production, MMP loss, release of cytochrome c, AIF, and Endo G, Bcl-2↓, Bax↑ | [123,124] |
| 75 | α-conidendrin | Taxus yunnanensis | MCF-7 and MDA-MB-231 | ROS generation, p53↑, Bax↑, Bcl-2↓, MMP loss, release of cytochrome c, activation of caspases-3 and -9 | [125] |
| 76 | Dehydrobruceine B | Brucea javanica | A549, NCI-H292 | MMP loss, release of cytochrome c, cleavage of caspase-9, caspase-3, and poly (ADP-ribose) polymerase (PARP) | [126] |
| 77 | Frugoside | Calotropis procera | M14, A375 | ROS generation | [127,128] |
| 78 | Methyl caffeate | Solanum torvum | MCF-7 | Bcl-2↓, Bax↑, Bid↑, p53↑, cleavage of caspase-3 and PARP, release of cytochrome c | [129] |
| 79 | Tetrahydrocurcumin | Curcuma longa | MCF-7 | ROS generation, Bcl-2↓, PARP↓, Bax↑, release of cytochrome c, MMP loss | [130] |
| 80 | Phloretin | Apple tree leaves and Manchurian apricot | EC-109 | Bcl-2↓, Bax↑ | [131] |
| 81 | Sesamol | Sesame seeds | HepG2 | Bcl-2↓, Bax↑, MMP loss, H2O2 production, PI3K Class III/Belin-1 pathway | [132] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules26010092
