Antimicrobial peptides (AMPs) are the arsenals of the innate host defense system, exhibiting evolutionarily conserved characteristics that are present in practically all forms of life. Recent years have witnessed the emergence of antibiotic-resistant bacteria compounded with a slow discovery rate for new antibiotics that have necessitated scientific efforts to search for alternatives to antibiotics. Research on the identification of AMPs has generated very encouraging evidence that they curb infectious pathologies and are also useful as novel biologics to function as immunotherapeutic agents. Being innate, they exhibit the least cytotoxicity to the host and exerts a wide spectrum of biological activity including low resistance among microbes and increased wound healing actions. Notably, in veterinary science, the constant practice of massive doses of antibiotics with inappropriate withdrawal programs led to a high risk of livestock-associated antimicrobial resistance. Therefore, the world faces tremendous pressure for designing and devising strategies to mitigate the use of antibiotics in animals and keep it safe for posterity.
- anti-microbial peptide,AMP,farm animals
1. Introduction
Globally, intensive livestock farming has led to a rise in the consumption of antibiotics. Imprudent and excessive use of antibiotics in livestock has resulted in an increase in the incidences of antibiotic resistance in several pathogenic bacterial strains and contamination of dairy and meat products with higher levels of antibiotic residues, posing a very serious threat to human health. In 2010, China, the United States, Brazil, India, and Germany were the five top countries in terms of antimicrobial consumption in food animals with 23%, 13%, 9%, 3%, and 3% of total consumption, respectively. By 2030, the expected rise in this figure is projected to be China (30%), the United States (10%), Brazil (8%), India (4%), and Mexico (2%) [1]. The multi-cellular organism has the natural capacity to derive and devise defense strategies against pathogenic attacks. The majority of antimicrobial peptides (AMPs) originate from the cleavage of critical proteins during the process of protein turnover by several proteases. AMPs are evolutionarily conserved weapons against pathogens in almost every organism. These are also known as “host defense peptides” because of their involvement in conferring non-specific innate immunity to the host. Systematic screening of peptides in various species has led to the discovery of many AMPs, some of which are proven to have very effective broad-spectrum activities.
AMPs have been found in almost every stratum of life, ranging from arthropods, amphibians, and reptiles to mammals [2][3][4][5]. It is not surprising that, to date, more than 5000 AMPs have been documented so far, either discovered denovo or synthesized in the lab [6]. Farm animals have constantly been exposed to a heavy dose of antibiotics with improper withdrawal programs. The danger of livestock-associated antimicrobial resistance in humans is on the rise. Although there have been studies on understanding the innate immunity in farm animals, dedicated and systematic studies on AMPs derived from farm animals and their potential as a substitute to antibiotics are not available. In this review, we comprehensively updated the information about AMPs in farm animals, their diversity, chemical characteristics, modes of action, and challenges in their clinical applications.
2. Structural Diversity Among AMPs
Nuclear magnetic resonance (NMR) has played a significant role in determining the structural details of AMPs. An analysis of the peptide’s three-dimensional structure has offered a deeper insight into their functions. Two-dimensional NMR methods are mainly used for obtaining three-dimensional structures for small-sized AMPs that can be divided into five classes according to their secondary structure [7]. Although these peptides remain unstructured in the solution, they may adopt specific structural characteristics after coming in contact with the membrane. Four such structural characteristics have been observed, namely alpha-helical, beta stranded, beta-hairpin or loop, and extended conformation. The structures of AMPs, discussed in the later section, are summarised in Table 1. Most of the AMPs discussed here possess either α-helix or β-sheet structure. The structure is either confirmed by NMR, CD, or homology modeling studies, where the atomic structure of the target sequence is constructed from its amino acids and the three-dimensional structure of a template. Most of the AMPs belong to the α-helical conformations. A large number of sequences have been reported in this class, which includes both naturally isolated and chemically synthesized peptides. In an aqueous solution, α-helical AMPs have a linear structure, and upon contact with a bacterial membrane or organic solvent, it forms an amphipathic helical structure [8]. The amphipathic helical structure allows the α-helix AMPs to burrow deep into the phospholipid bilayer and disrupt the membrane integrity. The Myeloid antimicrobial peptide from different farm animals, namely BMAP-27, SMAP-29, and PMAP-23, all possess anα-helix as their secondary structure [8][9][10]. Cecropin P1, eCATH1, eCATH2, and eCATH3, members of the cathelicidin family, also possess α-helical conformation [11][12][13].
In general, β-sheet AMPs are cyclic molecules composed of at least two antiparallel β-sheets stabilized by intramolecular disulfide bonds. The β-sheet peptides are more ordered in an aqueous solution due to their rigid structure and do not undergo a drastic conformation shift like helical peptides upon membrane interaction [14]. Unlike α-helix, the mechanistic model behind the β-sheet antimicrobial activity is not well understood. The β-sheet peptides contain primarily plant defensins, mammal α defensins and β defensins (BNBDs, Bovine β defensin-1, porcine β-defensin), insect defensins, proline-rich antibacterial peptides, protegrin, and tachyplins. Defensins are the well-researched β-sheet peptides that are formed in neutrophils, macrophages, and epithelial cells as inactive precursors [7][15]. Protegrin groups of AMPs from porcine have a well-elucidated structure. Members of the protegrin group, PG-1 PG-2 PG-3, and PG-5are composed of two antiparallel β-sheets connected by a β turn [16][17][18].
Loop AMPs have a loop structure stabilized by amide, disulfide, and isopeptides bonds. Thanatin, a prominent member of this group is isolated from the spinning soldier bug. A single disulfide bond between residue 11 and 18 stabilizes and offers thanatin its characteristic structure [19].
Extended peptides lack the classical secondary structure. Oftentimes, they are rich in certain amino acids, including residues of glycine, arginine, tryptophan, proline, and histidine [20][21]. The structure is stabilized by hydrogen bond and Vander Waal interactions with the phospholipid bilayer. Indolicidin has an extended poly-L-proline II helix as a secondary structure; however, in a neutral DPC environment, the peptide backbone takes a boat shape [22][23]. Several AMPs do not belong to any of these classes, and some appear only in aggregated form or when communicating with the membrane [24]. The plant-derived circulin A is a clear example of this, consisting of a combined α-helix and β-sheet structure that forms the cyclic cysteine knot [20]. Proline-rich Bactenecins such as Bac-5 and Bac-7, identical to cathelicidins and arginine-rich PR-39, fall into this category [25].
3. Biological function and mode of action:
Farm animals harbor a variety of AMPs that help them to counter invading pathogens. Some of these AMPs have been utilized in studies where they were characterized, and considerable experimental evidence, discussed in later sections, suggest that these AMPs have activity against a wide range of pathogens. Some AMPs of animal origin, such as Indolicidin from bovine, have reached clinical II/III phase trials against several conditions. While most of the peptides have been actively tested against human-related conditions in clinical trials, their involvement in veterinary medicine is almost negligible. Most of the studies concerning AMPs against animal diseases and conditions are in preclinical studies.
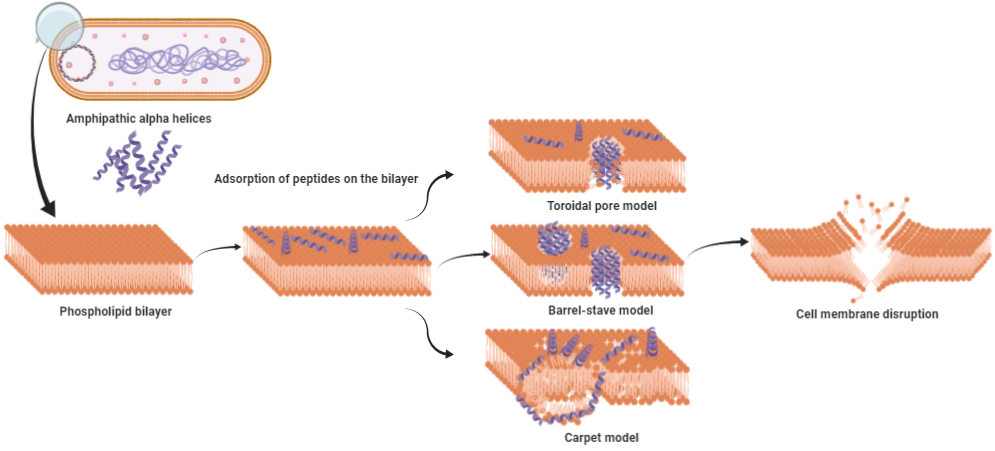
Depending on the nature of AMPs, the targets can be Gram-positive or G negative bacteria, fungi, or viruses [26][27][28][29][30]. The propensity to bind membranes is a conclusive feature of AMPs, which may or may not be associated with membrane permeabilization [31]. These AMPs employ several mechanisms to wipe out pathogens; most of them rely on the disruption of the cell membrane or alteration in cell membrane permeability, which ultimately relies on various physicochemical parameters possessed by the sequence. While most of the AMPs mainly adopt this approach for killing, others interfere with intracellular functions like DNA and protein synthesis, protein folding, or cell wall synthesis. Physicochemical parameters such as assize, charge, hydrophobicity, and amino acid composition directly affect the ability of the AMPs to insert in the membrane and induce pore formation. The slightest tweak in these parameters might augment the activity of AMPs. These properties facilitate amphipathic α-helix peptides to disrupt bacterial cell membranes by different models, namely, the barrel stave, carpet, and toroidal pore model (Figure 1). In the barrel stave model, peptides interact with one another to form a channel-like structure, while in the toroidal pore model, such peptide-peptide interaction is lacking; toroid pores are rather formed by peptides in a cooperative manner. In the carpet model, instead of forming pores peptides spread across the membrane in parallel orientation disordering the structure, at higher concentrations, these peptides act as a detergent, causing micellization of the phospholipid bilayer.
Cecropin P1 is a prominent example of the carpet model. It preferentially adopts a parallel orientation when reconstituted into a phosphatidylethanolamine/phosphatidylglycerol lipid membrane, as shown by Polarized Attenuated Total Reflectance-Fourier transform infrared(ATR-FTIR) spectroscopy. [11][32]. SMAP-29 from the cathelicidin family also uses the same carpet model as a mode of action as confirmed by fluorescence and molecular dynamics simulations [33]. Collectively, membrane disruption by pore formation is the basic mechanism employed by AMPs to neutralize the bacterial cell. This mechanism is similar to the complement system, which uses membrane attacking complexes to create pores in the cell membrane. Both bacteria and host cells possess phospholipid bilayer cell membrane, so what stops AMPs from attacking the host cell?
The fundamental basis for antimicrobial action is the difference between bacterial and mammalian cell composition. The outer leaflets of bacterial membranes are populated mainly by negatively charged phospholipids, whereas mammalian cells are mostly composed of neutral zwitterionic phospholipids, such as phosphatidylcholine and sphingomyelin [34]. The Shai-Matsuzaki-Huang model describes the interaction of α-helical AMPs targeting bacterial membranes. It shows the stepwise accumulation of AMPs followed by either integration into the membrane resulting in membrane disruption or diffusion from membrane onto intracellular targets [35][36][37]. These intracellular targets are components of vital processes such as replication and translation and enzymes for sustenance (Figure 2). PR 39, a porcine AMP, exerts its lethal effect by halting the process of translation and DNA synthesis [38]. Similarly, Indolicidin exerts its antimicrobial activity by halting DNA synthesis [39][40]. Marchand and co-workers reported that it inhibits DNA synthesis as well as topoisomerase I, thus employing multiple mechanisms at the DNA level for its antimicrobial activity. It exerts antimicrobial activity in gram-negative bacteria by disrupting the cytoplasmic membrane by forming channels and membrane thinning [22][41]. A similar mechanism for the peptide was reported for a pathogenic fungus Trichosporon beigelii [42]. Nevertheless, a different study suggested that even though the peptide caused permeabilization of the bacterial membrane, it was inefficient in lysing bacterial cells [39]. DNA level for its antimicrobial activity. It exerts antimicrobial activity in gram-negative bacteria by disrupting the cytoplasmic membrane by forming channels and membrane thinning [22][41]. A similar mechanism for the peptide was reported for a pathogenic fungus Trichosporon beigelii [42]. Nevertheless, a different study suggested that even though the peptide caused permeabilization of the bacterial membrane, it was inefficient in lysing bacterial cells [39].
Figure 1. Models proposed for cell membrane disruption by amphipathic α-helix antimicrobial peptides (AMPs). (A) The toroidal pore model: hydrophilic region of AMPs are consistently in contact with the polar head groups of the phospholipid bilayer, causing the membrane to curve inward. (B) The barrel-stave model: hydrophobic region of AMPs interact with the lipid core of phospholipid bilayer, while the hydrophilic region points inward forming a channel-like structure. (C) The carpet model: above critical threshold concentration, AMPs distort the lipid bilayer structure resulting in the micellization of the structure.
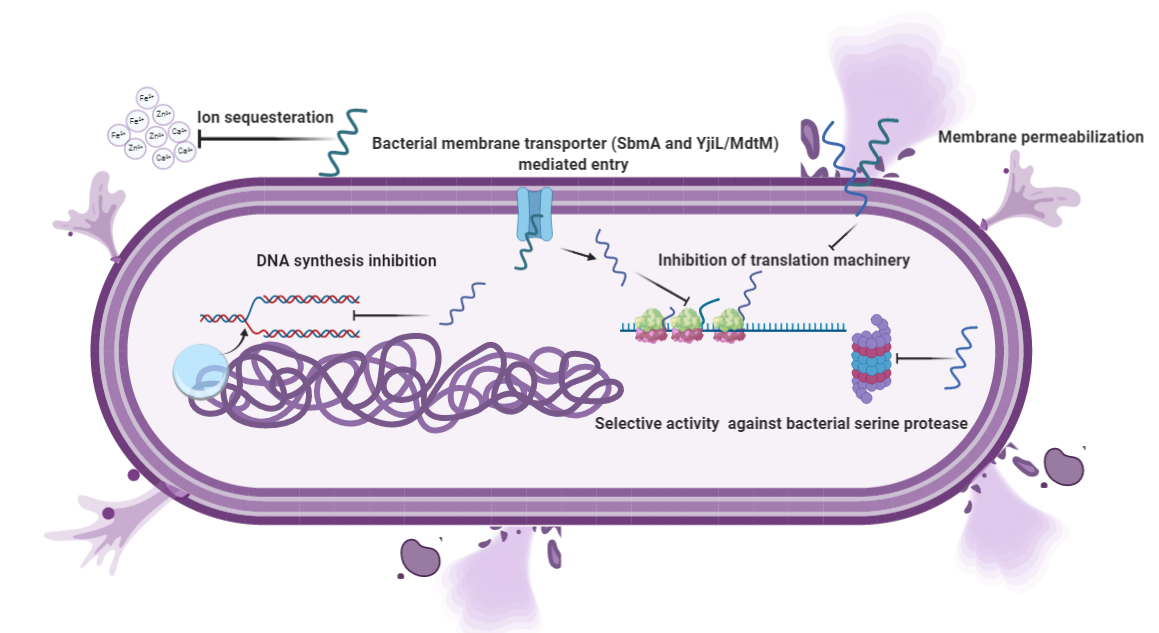
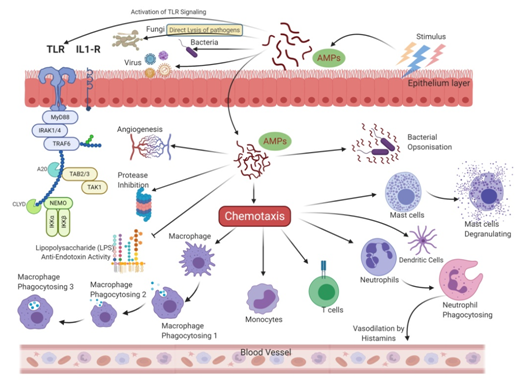
More recently, Indolicidin has been shown to exert its effect by the translocation mechanism, where it induces the release of negatively charged fluorescent dyes such as carboxyfluorescein (CF), calcein, and sulforhodamine B by forming a peptide-dye complex and not by the formation of pores [43]. Some AMPs tend to work by sequestration of ions vital for bacterial survival. Hepcidin and Psoriasin reduce the concentration of iron and zinc ions, respectively, and thus restrict the growth of pathogens [44][45]. It is remarkable that unlike antibiotics, many AMPs are more bactericidal than being bacteriostatic [46]. There are several unique features in the sequence of AMPs, such as net charge, hydrophobicity, secondary structure, etc., which stimulates additional anti-infective support in the host to fight against infection. In addition to having a direct effect on the target organism, AMPs can also act as a potent immunomodulator (Figure 3). They act as effector molecules of the innate immune system and influence diverse processes such as phagocytosis, cytokine release, and cell apoptosis [47][48]. They also act as a chemotactic factor, assisting in the recruitment and aggregation of immune cells at the site of inflammation [49][50], promoting angiogenesis [51][52], and inducing wound healing [53] by cytokine release and cell proliferation [54][55].
Figure 3. Allied and indirect bioactivities are associated with the AMPs sequence. In addition to direct antimicrobial activity, AMPs also trigger intracellular changes resulting in the activation of anti-inflammatory cytokines, immunomodulation, and promotion of non-specific immunity and wound healing.
AMPs in farm animals can be broadly grouped into two leading families, namely cathelicidins and defensins. The cathelicidin family comprises small cationic AMPs that are stored in neutrophils and macrophages. They are part of the innate immune system and are generally proteolytically active proteins. Members of this family have a conserved cathelicidin domain. Defensin is a family of AMPs consisting of six cysteine residues that form three disulfide bonds. It includes three subfamilies, namely α-defensins, β-defensins, and θ-defensins, and these differ from each other in the arrangement of disulfide bonds. These are found in tissues that are involved in host immune response against microbial infections and are abundant in leukocyte granules. The following sections describe the AMPs in these classes in different farm animals comprehensively, which are summarised in Table 1.
| AMPs | Source | Activity | Structure | Mode of action |
CATHELICIDIN |
||||
Indolicidin |
Bovine neutrophils |
Gram-positive and Gram-negative bacteria, yeast, and fungi |
Extended helix |
Membrane thinning, disruption of the membrane by channel formation, inhibition of DNA synthesis, and topoisomerase 1 |
BMAP-18, BMAP-27, BMAP-28 & BMAP-34 |
Bovine myeloid cells |
Gram-positive and Gram-negative bacteria, viruses, fungi, trypanosomes, tumor cell |
α-helix |
Formation of small channels in the bacterial membrane resulting in the release of small ions, transition pores in mitochondria, and LPS neutralization |
Bac-5 & Bac-7 |
Bovine neutrophil |
Gram-positive & Gram-negative |
Combination of α-helix & β-sheet |
inhibit protein synthesis by entering via bacterial inner membrane transporter SbmA and YjiL/MdtM |
eCATH1, eCATH2 & eCATH3 |
Equine neutrophils |
Broad-spectrum |
α-helix |
N.A. |
ChBac3 & ChBac5 |
Caprine leucocytes |
Broad-spectrum |
N.A. |
Membrane disruption |
OaBac5, OaBac7, and variants |
Ovine leucocytes |
Broad-spectrum |
N.A. |
depolarization of the cytoplasmic membranes |
SMAP29 |
Ovine myeloid cells |
Gram-positive and Gram-negative and yeast |
α-helix |
Permeabilize bacterial membrane |
DEFENSINS |
||||
TAP |
Bovine mucosal epithelial cells and mammary epithelial cells |
Broad-spectrum |
β-sheet(homology modeling) |
N.A. |
LAP |
Bovine squamous epithelial cells tongue, esophagus, rumen reticulum, omasum, chief cells of gastric glands |
Broad-spectrum |
β-sheet(homology modeling) |
N.A. |
BNBDs |
Bovine neutrophils alveolar tissue & pulmonary macrophages |
Broad-spectrum |
β-sheet(homology modeling) |
N.A. |
Equine β defensin 1 |
Hepatic tissue and respiratory epithelial tissue |
Broad-spectrum |
N.A |
N.A |
Equine α-defensinDEFA1 |
small intestine |
Gram-positive, Gram-negative & Fungi |
β-sheet |
membrane permeabilization |
Ovine β-defensin(SBD1 & SBD2) |
trachea, tongue, and gastrointestinal tract |
Gram-positive, Gram-negative, parainfluenza virus, M. haemolytica |
β-sheet(homology modeling) |
N.A. |
|
|
|
NEUTROPHIL ANTIMICROBIAL PEPTIDE |
|
|
eNAP-1 & eNAP-2 |
equine neutrophils |
Broad-spectrum |
N.A. |
Selective activity against microbial serine proteases |
|
|
|
PSORIASIN |
|
|
Bovine Psoriasin |
Udder |
Gram-negative |
N.A. |
Reduces bacterial survival by zinc sequestration |
|
|
|
HEPICIDIN |
|
|
Equine hepcidin |
Liver |
N.A. |
N.A. |
Induces hypoferrimia by iron sequestration |
N.A.-not available
Homology modeling-information collected from ModBase: Database of comparative protein structure models
4. Bovine AMPs
A large number of peptides have been identified in bovines that showed immunity against the broad-spectrum activity of pathogens. The lethal action towards pathogens is mostly due to membrane permeabilization activity, whereas other possesses secondary activities. These secondary activities help the peptides to disrupt and halt the vital functions of the pathogen The bovine AMPs can be broadly classified into two groups, namely, cathelicidins and β-defensins. We have already discussed structural diversity and their mode of action in an earlier section and will explore the in vitro studies carried out against a variety of pathogens.
4.1. Bovine Cathelicidins
4.1.1. Indolicidin
Indolicidin belongs to the family of cathelicidin AMPs. It was first discovered in bovine neutrophils as a tridecapeptide and showed a bactericidal effect [56], including its action on Staphylococcus aureus [57]. Thus far, seven protein-coding cathelicidin genes have been identified, which are traced to a single cluster on chromosome 22 [58]. Indolicidins are rich in tryptophan residues and cause cytoplasmic
disruption in bacteria by forming channels. When compared to its phenylalanine analog, it was found that the presence of a tryptophan residue gives hemolytic activity to the peptide [59]. Treatment of pathogenic C. albicans with Indolicidin coated gold nanoparticles (Indolicidin–AuNP) inhibits its ability to produce biofilm by reducing the expression of biofilm-related genes [60]. Another in vitro study reported that Indolicidin–AuNP increases the level of Reactive Oxygen Species (ROS), which results in DNA damage in S. Cerevisiae [61].
4.1.2. Bovine MyeloidAntimicrobialPeptides (BMAPs)
Bovine Myeloid AMPs are a series of variable length peptides that belong to the cathelicidin family and show potent antimicrobial activity against a wide range of pathogens. BMAP-18, BMAP-27, and BMAP-28 are the main AMPs belonging to this group. BMAP-27 and BMAP-28 are encoded by CATHL6 and CATHL5 genes, respectively. These peptides kill bacteria by forming small channels in the membrane resulting in the release of small ions rather than by membrane disruption [38].
BMAP-27
BMAP-27 is a bovine cathelicidin; it exhibits broad-spectrum antimicrobial activity against Gram-negative and Gram-positive bacteria, viruses, and fungi, but may also induce some toxicity to erythrocytes [62][63]. In addition to its strong LPS (Lipopolysaccharides) neutralizing activity, the peptide also inhibited the formation of biofilm of multidrug-resistant bacterial strains collected from Cystic fibrosis patients [64]. These roles of BMAP-27 were considered useful for the treatment of Gram-negative sepsis [65][66]. Thus, BMAP-27 has a significant role as a potential therapeutic agent against antibiotic-resistant bacteria. The peptide exerts cytotoxicity against human tumor cells by disrupting the membrane, which leads to a Ca2+ influx into the cytosol, ultimately leading to apoptosis [67]. Leucine and phenylalanine zippers are responsible for the cytotoxicity displayed by the peptide, it facilitates the assembly of BMAP 27 on the mammalian cell [68].
BMAP-28
BMAP-28 effectively inhibited the growth of pan-drug-resistant Acinetobacter baumannii (PDRAB) by damaging the cell surface with the rapid killing ability [69]. In another study, Lynn and co-workers found that the two stereoisomers of BMAP-28, D-BMAP-28, and the retro-inverso form (RI-BMAP-28), were highly effective against promastigotes and the intracellular amastigotes stages of Leishmania life cycles. RI and D isomers of BMAP-28 were unaffected by the proteolytic activity of GP63, a metalloprotease covering the parasite surface. The L-isomer of BMAP-28 was susceptible to degradation by GP63. Takagi et al. (2011) observed the effect of BMAP-28 on methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) [70]. They concluded that for MSSA, the minimal inhibitory concentration (MIC) ranged from 1.25 to 20µg/mL, and for MRSA, the MIC range was 5–20 µg/mL. Similar to BMAP-27, it also exerted cytotoxicity against human tumor cells and healthy proliferating cells [67]. In a cell culture experiment involving RAW 264.7 macrophages, BMAP-28 obstructed the LPS-induced expression of the cytokine gene [71]. The cytotoxic effect of the peptide depends upon its ability to form various transition pores in mitochondria, causing depolarization of the inner mitochondrial membrane [72].
BMAP-18
BMAP-18 is derived from its parent peptide BMAP-27. Unlike BMAP-27, it lacks the hydrophobic C-terminal sequence. It showed strong inhibitory activity against several species and life cycle stages of African trypanosomes, fish trypanosomes, and Leishmania with reduced cytotoxicity towards mammalian cells as compared to BMAP-27 [73]. A comparative study of BMAP-27 with that of BMAP18 showed that BMAP-18 exerts only antibacterial activity, whereas BMAP-27 showed potent antibacterial as well as anticancer activity. The parent peptide was capable of neutralizing bacteria within 20 min of membrane disruption [8]. BMAP-34, a peptide with α helical structure, is stored as a proform in cytoplasmic granules of neutrophil and exerts broad-spectrum activity against Gram-positive and Gram-negative bacteria [74].
4.2. Bovine β-Defensins
4.2.1. Tracheal Antimicrobial Peptide (TAP)
Tracheal AMPs (TAP) is a 38-amino acid peptide that belongs to the β-defensin family and is produced by mucosal epithelial cells of cattle [75]. It was the first defensin to be identified in cattle. One study reported its expression in bovine Mammary Epithelial Cells (bMEC), which was downregulated following Staphylococcus aureus infection [76]. Quantitative RT-PCR analysis showed that Pam3CSK4 (a TLR2/1 agonist), IL-17A, and LPS significantly induce the expression of the TAP gene in tracheal epithelial cells [77]. Activation of NF-κB is necessary for the LPS-, Pam3CSK4-, or IL-17A-mediated induction of TAP gene expression [78]. In a study, basal mRNA expression was upregulated in lung tissues from neonatal calves with acute Mannheimia haemolytica pneumonia.TAP, NF-κB, and intercellular adhesion molecule 1 expression were also upregulated after infection, among which the expression of TAP and IL-8 were highly correlated [79]. The expression of TAP and β-defensin in a tightly regulated manner counter bacterial pneumonia.
Bovine origin TAP was expressed in transgenic mice using an expression vector under the control sequences from the murine whey acidic protein (WAP) gene. The bTAP was then later purified by acid precipitation, RP-HPLC, and ion-exchange chromatography with proposed activity against Escherichia coli [80]. Studies have shown the in vitro antibacterial activity of the peptide against Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia, Pseudomonas aeruginosa, Candida albicans, Mannheimia haemolytica, Histophilus somni, Pasteurella multocida, and Mycoplasma bovis [75][80][81].
4.2.2. Lingual Antimicrobial Peptide (LAP)
LAP, a β-defensin, was first isolated from the squamous epithelium of the bovine tongue. It exerts broad-spectrum antimicrobial activities against Gram-positive and Gram-negative micro-organisms, as well as antifungal activity [81]. Immunolocalization studies showed the presence of LAP in the stratum corneum of the stratified squamous epithelium of the tongue, esophagus, rumen reticulum, omasum, chief cells of gastric glands of the abomasum, and mammary alveolar tissue of cattle [82][83]. It is constitutively expressed with other β defensins in mammary lymph nodes [84]. Its expression increased in the mammary gland following coagulase-positive or negative staphylococci infection and vitamin D treatment [85][86].
Studies confirmed a positive correlation between Somatic Cell Count (SCC) in milk and LAP expression, suggesting it as an indicator of SCC [87][88]. Long term high concentrate diet feeding in lactating cows led to an increased translocation of rumen derived LPS into the bloodstream activating enhanced LAP synthesis via the NF-κB signaling pathway [89]. Moreover, challenging mammary glands with LPS increased the LAP level, which exerted a synergistic effect with the lactoperoxidase enzyme [90]. All these studies indicate the decisive role played by LAP in providing innate immunity against invading pathogens in the mammary gland and digestive tract.
4.2.3. BNBD (Bovine Neutrophil β-Defensins)
A variety of β-defensins have been identified in bovine neutrophils. Until now, 13 bovines neutrophil β-defensins have been identified and isolated from neutrophil granules. These AMPs were active against Staphylococcus aureus and Escherichia coli [91]. BNBD3 or DEFB3 is expressed in various tissues. Treatment of bovine monocyte culture with lipopolysaccharide increased its expression. It enhanced the cell-mediated immune response when co-administered as a fusion with glycoprotein D, a protective agent [92].
Another variant, BNBD4, also known as DEFB4, is constitutively expressed in bovine alveolar tissues in a large amount. It is derived from a prepropeptide that is 63 amino acids long and is converted to a 41 amino acid long mature peptide. Its expression was higher in both the early and late lactation stages of bovine. Its expression increased after infecting mammary glands with coagulase-positive Staphylococci as compared to coagulase-negative Staphylococci [93]. Another β-defensin known as BNBD5 or DEFB5 was reported, and it resembled BNBD4. It showed increased expression during intramammary infections. The expression of BNBD5 was observed to be high during late lactation stages. Besides their role in countering mammary infections, both BNBD4 and BNBD5 are expressed constitutively in the pulmonary macrophages [94]. BNBD12 and BNBD13 are derived from a common precursor of 60 amino acids, and the mature peptides are composed of 38 and 42 residues, respectively [95].
4.3. Bovine Psoriasin
Psoriasin is an important bovine antimicrobial protein. It is homologous to the human Psoriasin, another name for the calcium-binding S100A7 protein. It was first identified as a respiratory allergen in cattle but played an essential role in providing local defense against bacteria in the udder. It also shows antimicrobial activity, which is observed to be limited against Escherichia coli, a mastitis-causing agent. It can be used as an important agent in preventing coliform mastitis [45].
4.4. Proline-Rich AMPs: Bac-5 and Bac-7
Two bactenecins, Bac5 and Bac7, were isolated from bovine neutrophils. These sequences are rich in proline and have a molecular mass of 5 and 7 kDa. Both are efficient in suppressing the growth and killing of Gram-negative bacteria, Escherichia coli, Salmonella typhimurium, and Klebsiella pneumoniae. They are also responsible for arresting the growth of Enterobacter cloacae. An in vitro assay showed that Pseudomonas aeruginosa and Staphylococcus epidermis were susceptible to Bac7 but not to Bac5 [96]. Instead of disrupting the bacterial membranes, the proline-rich AMPs (prAMPs) use a non-lytic mode of action. They exert their effects by entering the bacterial cytoplasm via inner membrane transportersSbmA and YjiL/MdtM and inhibit protein synthesis. Bacteria lacking these membrane transporters were resistant to prAMPs [97]. Following their entry into the bacterial cell, peptides bind to the ribosome and prevent the process of translation from proceeding to the elongation phase by blocking the entry site of aminoacyl-tRNA; however, they do not affect DNA and RNA synthesis. Bac5 and Bac7 had little effect on eukaryotic translation as compared to bacterial translation,
suggesting that they cause minimal damage to eukaryotic cells [98].
Another study showed that Bac-7 utilized a stereospecificity-dependent mode of action at near MIC value, where the L-enantiomer was more actively internalized into the bacteria. However, it depends on a non-stereospecific lytic mechanism at concentrations higher than MIC value [99].
5. Equine AMPs
Unlike others, some AMPs in equine cannot be classified as either cathelicidins or β-defensins, such as hepcidin and Neutrophil Antimicrobial Peptides. These AMPs provide broad-spectrum immunity against a wide range of pathogens. Some of these peptides can be a therapeutic alternative in veterinary medicine such as the treatment of rhodococcosis by eCATH1(discussed in the following section). While many sequences have therapeutic potential, none of them represent the group in the clinical phase of development.
5.1. Equine Cathelicidin
Skerlavaj and co-workers reported three putative horse myeloid cathelicidin, namely, eCATH1, eCATH2, and eCATH3, and their cDNA sequences, each peptide having α-helical conformation confirmed by circular dichroism measurement. In vitro studies revealed potent broad-spectrum antimicrobial activity for eCATH1; however, the activity of eCATH2 was somewhat restricted. Both eCATH2 and eCATH3 were produced as propeptides in neutrophils and were cleaved to form mature proteins after neutrophil activation. The activity of eCATH3 showed a strong dependence on salt concentration, as revealed by the efficient killing of bacterial and fungal species in low ionic strength media, which was inhibited in the presence of physiological salt medium [13].
The eCATH1 showed antimicrobial activity against equine isolates of Rhodococcus equi, the causal agent of rhodococcosis in foals and showed, E. coli, S. enterica, K. pneumoniae, and Pseudomonas spp. with MICs ranging from 0.5–16 µg/mL [100]. eCATH1 showed an in vitro IC50 of 9.5μM against Trypanosoma brucei, Trypanosoma evansi, and Trypanosoma equiperdum [101].
Equine cathelicidin-derived AMPs, EA-CATH1 and EA-CATH2, were isolated from a constructed lung cDNA library of a donkey using nested PCR-based cloning. Chemically synthesized EA-CATH1 had MIC against Gram-positive bacteria in the range of 0.3–2.4 μg/mL. CD measurement studies showed that EA-CATH1 adopts an α-helical conformation in a 50% trifluoroethanol/water solution, but a random coil in an aqueous solution. The sequence showed serum stability and had no hemolytic activity against erythrocytes. Scanning electron microscope studies showed that the treatment of EA-CATH1 caused a rapid disruption of the Staphylococcus aureus (ATCC2592) membrane [102].
5.2. Equine Neutrophil Antimicrobial Peptides
The eNAP-1 is a 7.2 kDa cysteine-rich endogenous AMP that was purified from the extracts of cytoplasmic granules of equine neutrophils. The peptide was found to be effective against Streptococcus zooepidemicus, an equine uterine pathogen. However, it showed less activity against Escherichia coli and Pseudomonas aeruginosa. The AMP plays an essential role in phagocyte-mediated host defense during equine infections [103].
Another peptide in this series is eNAP-2, a 6.5 KDa endogenous AMP, which was also isolated from the extracts of cytoplasmic granules of equine neutrophils. It showed activity similar to eNAP-1, as it efficiently killed Streptococcus zooepidemicus. Studies showed that eNAP-2 exerted bacteriostatic activity against Klebsiella pneumoniae by exerting selective activity against microbial serine protease, namely, Subtilisin A and Proteinase K, without any inhibitory effect on mammalian serine protease, ensuring the killing of pathogens without any cytotoxicity to host cells [104][105].
5.3. Equine Hepcidin
Hepcidin is a 25 amino acid peptide produced in the liver. In addition to its role in iron homeostasis, it also showed antifungal and antibacterial effects. Hepcidin is synthesized as 84 amino acid prepropeptide with a 24 amino acid N-terminal signal sequence, targeting the peptide in the endoplasmic reticulum. Posttranslational processing of hepcidin is carried out by proprotein convertases, such as furin, PC7/LPC, PACE4, and PC5/6 [106]. During acute inflammation, the increase in levels of iron supports the growth of pathogens, and hepcidin reduces bacterial survival by restricting the availability of iron. In one study, healthy horses were administered with Freund’s complete adjuvant by intramuscular injection at two-time point, namely, 0 h and 12 h. It was observed that 6 h post-infection, hepcidin mRNA increased and remained high until 18 h. The plasma iron concentration decreased significantly between 16 h and 72 h post-infection as compared to control, suggesting the role of hepcidin in the rapid onset of hypoferremia to restrict the availability of iron for the growth of pathogens [44]. Overexpression of hepcidin in transgenic mice resulted in decreased body iron levels and severe microcytic hypochromic anemia, confirming its role in iron sequestration is central to its activity [107].
A research on donkey identified that the high expression of hepcidin in the liver was found to be similar to the reference gene expression. The study also stated that the mature hepcidin sequence from the donkey exhibited 100% sequence homology to the mature hepcidin sequence from the horse [108].
5.4. Equine α and β Defensin
Equine β defensin 1 (eBD-1) expression was first reported in hepatic tissue, after a database search for Expressed Sequence Tags ESTs presumed its expression to be in hepatic tissue. Another study showed its expression in respiratory epithelial tissue [109]. The peptide sequence is conserved among human and porcine orthologues, sharing the highest sequence similarity with porcine β defensin [110].
The horse is the only α-defensin expressing species in the Laurasiatheria group [111]. DEFA1 was the first α-defensin to be characterized in the equine and its expression was found in the small intestine. It displayed homology with Panethcell-specific α-defensins from primates. It showed antimicrobial activity against Gram-positive and Gram-negative bacteria and Candida albicans by employing membrane permeabilization as the mechanistic approach [111][112].
6. CaprineAMPs
Exploration studies of AMPs in caprine are still in its early phase, although attempts have been made to characterize and define them. Mostly, these studies were focused on Bactenecin homologous to the bovine Bac5. Proline-rich AMPChBac5 was isolated from elastase treated extracts of goat leucocytes. It is homologous to Bac5 from bovine and showed broad-spectrum activity against diverse pathogens [113].
ChBac3, another proline-rich AMP, was isolated from the leukocytes of Capra hircus. It had a 50% sequence similarity to Bac5, with a broad-spectrum antimicrobial activity even at low salt concentrations. The sequence showed high microbial membrane damaging ability as compared to other proline-rich AMPs. However, it does not exhibit any hemolytic activity against human erythrocytes [114]. Two AMPs designated as mini bactenecin, namely, mini-ChBac7.5Nα and mini-ChBac7.5Nβ, were isolated from the neutrophils of the domestic goat. These AMPs possessed antimicrobial activity against Gram-negative bacteria as wells as drug-resistant strains of bacteria (Klebsiella spp., Pseudomonas aeruginosa, and Acinetobacter baumanii). They also showed activity against some Gram-positive bacteria (Listeria monocytogeness and Micrococcus luteus) and claimed to have no hemolytic effect on human red blood cells [115].
7. Ovine AMPs
The genes coding for ovine defensins and cathelicidins were mapped to chromosome 26 and chromosome 19, respectively. Two exons on chromosome 26 code for two defensins SBD-1 and SBD-2, and four exons on ch19 code for four cathelicidins, namely, OaBac5, OaBac7.5, OaBac6, and OaBac11 [116].
7.1. Ovine Cathelicidins
In addition to ChBac3, Shamova and co-workers also isolated OaBac5α from sheep leukocytes via elastase treatment. It was homologous to caprine ChBac3 and bovine Bac5 and showed broad-spectrum antimicrobial activity as well. OaBac5α was considered as a variant of OaBac5. OaBac5β was another variant isolated from sheep leukocytes and was 20–30% more abundant as compared to OaBac5α. OaBac5 and OaBac7.5 are present in truncated forms OaBac5mini and OaBac7.5mini, respectively [113]. These are proline and arginine-rich peptides that were isolated from sheep neutrophils. OaBac5mini exerted a strong response against Gram-negative bacteria but was not effective against Gram-positive bacteria and yeast. On the other hand, OaBac7.5mini was not effective against most of them. Once inside the bacterial cell, they exerted a bactericidal effect by depolarizing the cytoplasmic membrane [117].
SMAP-29, an AMP from the cathelicidin family, was derived from sheep myeloid RNA. It was observed that the N-terminal amphipathic α-helical region and the C-terminal hydrophobic regions were essential for antibacterial and hemolytic activities of SMAP-29 [9]. It also possessed antibacterial activity against antibiotic-resistant clinical isolates such as MRSA and VERF isolates and was also active against Cryptococcus neoformans and Pseudomonas aeruginosa [118].
7.2. Ovine β-Defensins
Ovine β-defensins have two prominent members SBD-1 and SBD-2 located on chromosome-26 [119]. SBD-1 and SBD-2 are small cationic AMPs that are active against Gram-negative and Gram-positive bacteria. SBD-1 expression was found in the trachea, tongue, and gastrointestinal tract, whereas SBD-2 was present in the only ileum and colon [116]. Expression of SBD-1 and SBD-2 along with IL-8 occurred during M. haemolytica infections occurred during. However, the expression of SBD-1 was low in M. haemolytica infection as compared to parainfluenza virus type 3 (PI-3)infections. SBD-1 was primarily expressed in respiratory epithelium instead of leukocytes [120].
Zhao et al. (2011) used the Pichia pastoris vector to express 6-His tagged mature SBD-1. The peptide produced had similar activity as mature SBD-1 and had inhibitory effects on Escherichia coli, Staphylococcus aureus, Proteus vulgaris, Pseudomonas aeruginosa, and Shigella flexneri [121].
This entry is adapted from the peer-reviewed paper 10.3390/vetsci7040206
References
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654.
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Insects, arachnids and centipedes venom: A powerful weapon against bacteria. A literature review. Toxicon 2017, 130, 91–103.
- Patocka, J.; Nepovimova, E.; Klimova, B.; Wu, Q.; Kuca, K. Antimicrobial Peptides: Amphibian Host Defense Peptides. Curr. Med. Chem. 2018, 26, 5924–5946.
- Van Hoek, M.L. Antimicrobial peptides in reptiles. Pharmaceuticals 2014, 7, 723–753.
- Dutta, P.; Das, S. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets Against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2015, 16, 99–129.
- Zhao, X.; Wu, H.; Lu, H.; Li, G.; Huang, Q. LAMP: A Database Linking Antimicrobial Peptides. PLoS ONE 2013, 8, e66557.
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171.
- Yang, S.; Lee, C.W.; Kim, H.J.; Jung, H.H.; Kim, J.I.; Shin, S.Y.; Shin, S.H. Structural analysis and mode of action of BMAP-27, a cathelicidin-derived antimicrobial peptide. Peptides 2019, 118, 170106.
- Shin, S.Y.; Park, E.J.; Yang, S.T.; Jung, H.J.; Eom, S.H.; Song, W.K.; Kim, Y.; Hahm, K.S.; Kim, J. Il Structure-activity analysis of SMAP-29, a sheep leukocytes-derived antimicrobial peptide. Biochem. Biophys. Res. Commun. 2001, 285, 1046–1051.
- Park, K.; Oh, D.; Kim, Y.; Yub Shin, S.; Hahm, K.S. Structural studies of porcine myeloid antibacterial peptide PMAP-23 and its analogues in DPC micelles by NMR spectroscopy. Biochem. Biophys. Res. Commun. 2002, 290, 204–212.
- Gazit, E.; Boman, A.; Boman, H.G.; Shai, Y. Interaction of the Mammalian Antibacterial Peptide Cecropin PI with Phospholipid Vesicles. Biochemistry 1995, 34, 11479–11488.
- Sipos, D.; Andersson, M.; Ehrenberg, A. The structure of the mammalian antibacterial peptide cecropin P1 in solution, determined by proton-NMR. Eur. J. Biochem. 1992, 209, 163–169.
- Skerlavaj, B.; Scocchi, M.; Gennaro, R.; Risso, A.; Zanetti, M. Structural and functional analysis of horse cathelicidin peptides. Antimicrob. Agents Chemother. 2001, 45, 715–722.
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55.
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141.
- Fahrner, R.L.; Dieckmann, T.; Harwig, S.S.L.; Lehrer, R.I.; Eisenberg, D.; Feigon, J. Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes. Chem. Biol. 1996, 3, 543–550.
- Cho, Y.; Turner, J.S.; Dinh, N.N.; Lehrer, R.I. Activity of protegrins against yeast-phase Candida albicans. Infect. Immun. 1998, 66, 2486–2493.
- Usachev, K.S.; Efimov, S.V.; Kolosova, O.A.; Filippov, A.V.; Klochkov, V.V. High-resolution NMR structure of the antimicrobial peptide protegrin-2 in the presence of DPC micelles. J. Biomol. NMR 2015, 61, 227–234.
- Powers, J.P.S.; Hancock, R.E.W. The relationship between peptide structure and antibacterial activity. Peptides 2003.
- Angélique, L.; Frederik, W.J.; Garmi, J.; Hester, D.P.L. The potential use of natural and structural analogues of antimicrobial peptides in the fight against neglected tropical diseases. Molecules 2015, 20, 15392–15433.
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011.
- Falla, T.J.; Nedra Karunaratne, D.; Hancock, R.E.W. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 1996, 271, 19298–19303.
- Rozek, A.; Friedrich, C.L.; Hancock, R.E.W. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774.
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575.
- Anbanandam, A.; Albarado, D.C.; Tirziu, D.C.; Simons, M.; Veeraraghavan, S. Molecular Basis for Proline- and Arginine-Rich Peptide Inhibition of Proteasome. J. Mol. Biol. 2008, 384, 219–227.
- Hein, K.Z.; Takahashi, H.; Tsumori, T.; Yasui, Y.; Nanjoh, Y.; Toga, T.; Wu, Z.; Grötzinger, J.; Jung, S.; Wehkamp, J.; et al. Disulphide-reduced psoriasin is a human apoptosisinducing broad-spectrum fungicide. Proc. Natl. Acad. Sci. USA 2015, 112, 13039–13044.
- Bai, F.; Town, T.; Pradhan, D.; Cox, J.; Ashish; Ledizet, M.; Anderson, J.F.; Flavell, R.A.; Krueger, J.K.; Koski, R.A.; et al. Antiviral Peptides Targeting the West Nile Virus Envelope Protein. J. Virol. 2007, 81, 2047–2055.
- Chang, K.Y.; Yang, J.R. Analysis and Prediction of Highly Effective Antiviral Peptides Based on Random Forests. PLoS ONE 2013, 8, e70166.
- Zhao, H.; Zhou, J.; Zhang, K.; Chu, H.; Liu, D.; Poon, V.K.M.; Chan, C.C.S.; Leung, H.C.; Fai, N.; Lin, Y.P.; et al. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci. Rep. 2016, 6, 22008.
- Sala, A.; Ardizzoni, A.; Ciociola, T.; Magliani, W.; Conti, S.; Blasi, E.; Cermelli, C. Antiviral Activity of Synthetic Peptides Derived from Physiological Proteins. Intervirology 2019, 61, 166–173.
- Varkey, J.; Singh, S.; Nagaraj, R. Antibacterial activity of linear peptides spanning the carboxy-terminal β-sheet domain of arthropod defensins. Peptides 2006, 27, 2614–2623.
- Gazit, E.; Miller, I.R.; Biggin, P.C.; Sansom, M.S.P.; Shai, Y. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J. Mol. Biol. 1996, 258, 860–870.
- Orioni, B.; Bocchinfuso, G.; Kim, J.Y.; Palleschi, A.; Grande, G.; Bobone, S.; Park, Y.; Kim, J.I.; Hahm, K.S.; Stella, L. Membrane perturbation by the antimicrobial peptide PMAP-23: A fluorescence and molecular dynamics study. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1523–1533.
- Graham, J.M.; Higgins, J.A. Membrane Analysis; BIOS Scientific Publishers: New York, NY, USA, 1997.
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta Biomembr. 1999.
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta Biomembr. 1999, 1462, 1–10.
- Yang, L.; Weiss, T.M.; Lehrer, R.I.; Huang, H.W. Crystallization of antimicrobial pores in membranes: Magainin and protegrin. Biophys. J. 2000, 79, 2002–2009.
- Boman, H.G.; Agerberth, B.; Boman, A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 1993, 61, 2978–2984.
- Subbalakshmi, C.; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96.
- Hsu, C.H.; Chen, C.; Jou, M.L.; Lee, A.Y.L.; Lin, Y.C.; Yu, Y.P.; Huang, W.T.; Wu, S.H. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: Evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005, 33, 4053–4064.
- Neale, C.; Hsu, J.C.Y.; Yip, C.M.; Pomès, R. Indolicidin binding induces thinning of a lipid bilayer. Biophys. J. 2014, 106, L29–L31.
- Lee, D.G.; Kim, H.K.; Kim, S.A.; Park, Y.; Park, S.C.; Jang, S.H.; Hahm, K.S. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun. 2003, 305, 305–310.
- Rokitskaya, T.I.; Kolodkin, N.I.; Kotova, E.A.; Antonenko, Y.N. Indolicidin action on membrane permeability: Carrier mechanism versus pore formation. Biochim. Biophys. Acta Biomembr. 2011, 1808, 91–97.
- Oliveira-Filho, J.P.; Badial, P.R.; Cunha, P.H.J.; Bordon, A.P.; Araujo, J.P.; Divers, T.J.; Winand, N.J.; Borges, A.S. Freund’s adjuvant-induced inflammation: Clinical findings and its effect on hepcidin mRNA expression in horses. Pesqui. Vet. Bras. 2014, 34, 51–56.
- Lee, K.C.; Eckert, R.L. S100A7 (Psoriasin) Mechanism of antibacterial action in wounds. J. Investig. Dermatol. 2007, 127, 945–957.
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547.
- Zuyderduyn, S.; Ninaber, D.K.; Hiemstra, P.S.; Rabe, K.F. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J. Allergy Clin. Immunol. 2006, 117, 1328–1335.
- Paredes-Gamero, E.J.; Martins, M.N.C.; Cappabianco, F.A.M.; Ide, J.S.; Miranda, A. Characterization of dual effects induced by antimicrobial peptides: Regulated cell death or membrane disruption. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1062–1072.
- Elsbach, P. What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J. Clin. Investig. 2003, 111, 1643–1645.
- Yang, D.; Biragyn, A.; Kwak, L.W.; Oppenheim, J.J. Mammalian defensins in immunity: More than just microbicidal. Trends Immunol. 2002, 23, 291–296.
- Torres, P.; DÍaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. FASEB J. 2017, 31, 4946–4958.
- Kanazawa, K.; Okumura, K.; Ogawa, H.; Niyonsaba, F. An antimicrobial peptide with angiogenic properties, AG-30/5C, activates human mast cells through the MAPK and NF-κB pathways. Immunol. Res. 2016, 64, 594–603.
- Mu, L.; Tang, J.; Liu, H.; Shen, C.; Rong, M.; Zhang, Z.; Lai, R. A potential wound-healing-promoting peptide from salamander skin. FASEB J. 2014, 28, 3919–3929.
- Wang, W.; Jia, J.; Li, C.; Duan, Q.; Yang, J.; Wang, X.; Li, R.; Chen, C.; Yan, H.; Zheng, Y. Antimicrobial peptide LL-37 promotes the proliferation and invasion of skin squamous cell carcinoma by upregulating DNA-binding protein A. Oncol. Lett. 2016, 12, 1745–1752.
- Wang, W.; Zheng, Y.; Jia, J.; Li, C.; Duan, Q.; Li, R.; Wang, X.; Shao, Y.; Chen, C.; Yan, H. Antimicrobial peptide LL-37 promotes the viability and invasion of skin squamous cell carcinoma by upregulating YB-1. Exp. Ther. Med. 2017, 14, 499–506.
- Selsted, M.E.; Novotny, M.J.; Morris, W.L.; Tang, Y.Q.; Smith, W.; Cullor, J.S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 1992.
- Van Abel, R.J.; Tang, Y.-Q.; Rao, V.; Dobbs, C.H.; Tran, D.; Barany, G.; Selsted, M.E. Synthesis and characterization of indolicidin, a tryptophan-rich antimicrobial peptide from bovine neutrophils. Int. J. Pept. Protein Res. 1995, 45, 401–409.
- Tomasinsig, L.; Zanetti, M. The Cathelicidins Structure, Function and Evolution. Curr. Protein Pept. Sci. 2005, 6, 23–34.
- Subbalakshmi, C.; Krishnakumari, V.; Nagaraj, R.; Sitaram, N. Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett. 1996, 395, 48–52.
- De Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018, 11, 915–925.
- Ghosh, A.; Kar, R.K.; Jana, J.; Saha, A.; Jana, B.; Krishnamoorthy, J.; Kumar, D.; Ghosh, S.; Chatterjee, S.; Bhunia, A. Indolicidin targets duplex DNA: Structural and mechanistic insight through a combination of spectroscopy and microscopy. ChemMedChem 2014, 9, 2052–2058.
- Benincasa, M.; Skerlavaj, B.; Gennaro, R.; Pellegrini, A.; Zanetti, M. In vitro and in vivo antimicrobial activity of two α-helical cathelicidin peptides and of their synthetic analogs. Peptides 2003, 24, 1723–1731.
- Benincasa, M.; Scocchi, M.; Pacor, S.; Tossi, A.; Nobili, D.; Basaglia, G.; Busetti, M.; Gennaro, R. Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J. Antimicrob. Chemother. 2006, 58, 950–959.
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; Di Primio, A.; Fiscarelli, E.; Gennaro, R.; Di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814.
- Ciornei, C.D.; Sigurdardóttir, T.; Schmidtchen, A.; Bodelsson, M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob. Agents Chemother. 2005, 49, 2845–2850.
- Mookherjee, N.; Wilson, H.L.; Doria, S.; Popowych, Y.; Falsafi, R.; Yu, J.J.; Li, Y.; Veatch, S.; Roche, F.M.; Brown, K.L.; et al. Bovine and human cathelicidin cationic host defense peptides similarly suppress transcriptional responses to bacterial lipopolysaccharide. J. Leukoc. Biol. 2006, 80, 1563–1574.
- Risso, A.; Zanetti, M.; Gennaro, R. Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell. Immunol. 1998, 189, 107–115.
- Ahmad, A.; Azmi, S.; Srivastava, R.M.; Srivastava, S.; Pandey, B.K.; Saxena, R.; Bajpai, V.K.; Ghosh, J.K. Design of nontoxic analogues of cathelicidin-derived bovine antimicrobial peptide BMAP-27: The role of leucine as well as phenylalanine zipper sequences in determining its toxicity. Biochemistry 2009, 48, 10905–10917.
- Guo, Y.; Xun, M.; Han, J. A bovine myeloid antimicrobial peptide (BMAP-28) and its analogs kill pan-drug-resistant Acinetobacter baumannii by interacting with outer membrane protein A (OmpA). Medicine 2018, 97, e12832.
- Takagi, S.; Hayashi, S.; Takahashi, K.; Isogai, H.; Bai, L.; Yoneyama, H.; Ando, T.; Ito, K.; Isogai, E. Antimicrobial activity of a bovine myeloid antimicrobial peptide (BMAP-28) against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Anim. Sci. J. 2012, 83, 482–486.
- D’Este, F.; Tomasinsig, L.; Skerlavaj, B.; Zanetti, M. Modulation of cytokine gene expression by cathelicidin BMAP-28 in LPS-stimulated and -unstimulated macrophages. Immunobiology 2012, 217, 962–971.
- Risso, A.; Braidot, E.; Sordano, M.C.; Vianello, A.; Macrì, F.; Skerlavaj, B.; Zanetti, M.; Gennaro, R.; Bernardi, P. BMAP-28, an Antibiotic Peptide of Innate Immunity, Induces Cell Death through Opening of the Mitochondrial Permeability Transition Pore. Mol. Cell. Biol. 2002, 22, 1926–1935.
- Haines, L.R.; Thomas, J.M.; Jackson, A.M.; Eyford, B.A.; Razavi, M.; Watson, C.N.; Gowen, B.; Hancock, R.E.W.; Pearson, T.W. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl. Trop. Dis. 2009, 3, e373.
- Gennaro, R.; Scocchi, M.; Merluzzi, L.; Zanetti, M. Biological characterization of a novel mammalian antimicrobial peptide. Biochim. Biophys. Acta Gen. Subj. 1998, 1425, 361–368.
- Diamond, G.; Zasloff, M.; Eck, H.; Brasseur, M.; Lee Maloy, W.; Bevins, C.L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: Peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. USA 1991, 88, 3952–3956.
- López-Meza, J.E.; Gutiérrez-Barroso, A.; Ochoa-Zarzosa, A. Expression of tracheal antimicrobial peptide in bovine mammary epithelial cells. Res. Vet. Sci. 2009, 87, 59–63.
- Berghuis, L.; Abdelaziz, K.T.; Bierworth, J.; Wyer, L.; Jacob, G.; Karrow, N.A.; Sharif, S.; Clark, M.E.; Caswell, J.L. Comparison of innate immune agonists for induction of tracheal antimicrobial peptide gene expression in tracheal epithelial cells of cattle. Vet. Res. 2014, 45, 1–10.
- Taha-Abdelaziz, K.; Wyer, L.; Berghuis, L.; Bassel, L.L.; Clark, M.E.; Caswell, J.L. Regulation of tracheal antimicrobial peptide gene expression in airway epithelial cells of cattle. Vet. Res. 2016, 47, 44.
- Caverly, J.M.; Diamond, G.; Gallup, J.M.; Brogden, K.A.; Dixon, R.A.; Ackermann, M.R. Coordinated expression of tracheal antimicrobial peptide and inflammatory-response elements in the lungs of neonatal calves with acute bacterial pneumonia. Infect. Immun. 2003, 71, 2950–2955.
- Yarus, S.; Rosen, J.M.; Cole, A.M.; Diamond, G. Production of active bovine tracheal antimicrobial peptide in milk of transgenic mice. Proc. Natl. Acad. Sci. USA 1996, 93, 14118–14121.
- Schonwetter, B.S.; Stolzenberg, E.D.; Zasloff, M.A. Epithelial antibiotics induced at sites of inflammation. Science (80-.) 1995, 267, 1645–1648.
- Isobe, N.; Morimoto, K.; Nakamura, J.; Yamasaki, A.; Yoshimura, Y. Intramammary challenge of lipopolysaccharide stimulates secretion of lingual antimicrobial peptide into milk of dairy cows. J. Dairy Sci. 2009, 92, 6046–6051.
- Isobe, N.; Sugino, T.; Taniguchi, K.; Moriya, N.; Hosoda, K.; Yoshimura, Y. Differential localization of lingual antimicrobial peptide in the digestive tract mucosal epithelium of calves. Vet. Immunol. Immunopathol. 2011, 142, 87–94.
- Tetens, J.; Friedrich, J.J.; Hartmann, A.; Schwerin, M.; Kalm, E.; Thaller, G. The spatial expression pattern of antimicrobial peptides across the healthy bovine udder. J. Dairy Sci. 2010, 93, 775–783.
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Krzyzewski, J.; Zwierzchowski, L.; Bagnicka, E. Expression patterns of β-defensin and cathelicidin genes in parenchyma of bovine mammary gland infected with coagulase-positive or coagulase-negative Staphylococci. BMC Vet. Res. 2014, 10, 1–14.
- Téllez-Pérez, A.D.; Alva-Murillo, N.; Ochoa-Zarzosa, A.; López-Meza, J.E. Cholecalciferol (vitamin D) differentially regulates antimicrobial peptide expression in bovine mammary epithelial cells: Implications during Staphylococcus aureus internalization. Vet. Microbiol. 2012, 160, 91–98.
- Swanson, K.; Gorodetsky, S.; Good, L.; Davis, S.; Musgrave, D.; Stelwagen, K.; Farr, V.; Molenaar, A. Expression of a β-defensin mRNA, lingual antimicrobial peptide, in bovine mammary epithelial tissue is induced by mastitis. Infect. Immun. 2004, 72, 7311–7314.
- Kawai, K.; Akamatsu, H.; Obayashi, T.; Nagahata, H.; Higuchi, H.; Iwano, H.; Oshida, T.; Yoshimura, Y.; Isobe, N. Relationship between concentration of lingual antimicrobial peptide and somatic cell count in milk of dairy cows. Vet. Immunol. Immunopathol. 2013, 153, 298–301.
- Jin, D.; Chang, G.; Zhang, K.; Guo, J.; Xu, T.; Shen, X. Rumen-derived lipopolysaccharide enhances the expression of lingual antimicrobial peptide in mammary glands of dairy cows fed a high-concentrate diet. BMC Vet. Res. 2016, 12, 1–10.
- Isobe, N.; Hosoda, K.; Yoshimura, Y. Immunolocalization of lingual antimicrobial peptide (LAP) in the bovine mammary gland. Anim. Sci. J. 2009, 80, 446–450.
- Selsted, M.E.; Tang, Y.Q.; Morris, W.L.; McGuire, P.A.; Novotny, M.J.; Smith, W.; Henschen, A.H.; Cullor, J.S. Purification, primary structures, and antibacterial activities of β- defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 1993, 271, 16430.
- Mackenzie-Dyck, S.; Kovacs-Nolan, J.; Snider, M.; Babiuk, L.A.; Van Drunen Littel-Van Den Hurk, S. Inclusion of the bovine neutrophil beta-defensin 3 with glycoprotein D of bovine herpesvirus 1 in a DNA vaccine modulates immune responses of mice and cattle. Clin. Vaccine Immunol. 2014, 21, 463–477.
- Gurao, A.; Kashyap, S.K.; Singh, R. β-defensins: An innate defense for bovine mastitis. Vet. World 2017, 10.
- Ryan, L.K.; Rhodes, J.; Bhat, M.; Diamond, G. Expression of β-defensin genes in bovine alveolar macrophages. Infect. Immun. 1998, 66, 878–881.
- Yount, N.Y.; Yuan, J.; Tarver, A.; Castro, T.; Diamond, G.; Tran, P.A.; Levy, J.N.; McCullough, C.; Cullor, J.S.; Bevins, C.L.; et al. Cloning and expression of bovine neutrophil β-defensins: Biosynthetic profile during neutrophilic maturation and localization of mature peptide to novel cytoplasmic dense granules. J. Biol. Chem. 1999, 274, 26249–26258.
- Gennaro, R.; Skerlavaj, B.; Romeo, D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect. Immun. 1989, 57, 3142–3146.
- Mattiuzzo, M.; Bandiera, A.; Gennaro, R.; Benincasa, M.; Pacor, S.; Antcheva, N.; Scocchi, M. Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 2007, 66, 151–163.
- Mardirossian, M.; Barrière, Q.; Timchenko, T.; Müller, C.; Pacor, S.; Mergaert, P.; Scocchi, M.; Wilsona, D.N. Fragments of the nonlytic proline-rich antimicrobial peptide Bac5 kill Escherichia coli cells by inhibiting protein synthesis. Antimicrob. Agents Chemother. 2018, 62.
- Podda, E.; Benincasa, M.; Pacor, S.; Micali, F.; Mattiuzzo, M.; Gennaro, R.; Scocchi, M. Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1732–1740.
- Schlusselhuber, M.; Guldbech, K.; Sevin, C.; Leippe, M.; Petry, S.; Grötzinger, J.; Giguère, S.; Cauchard, J. In vitro effectiveness of the antimicrobial peptide eCATH1 against antibiotic-resistant bacterial pathogens of horses. FEMS Microbiol. Lett. 2014, 350, 216–222.
- Cauchard, S.; Van Reet, N.; Büscher, P.; Goux, D.; Grötzinger, J.; Leippe, M.; Cattoir, V.; Laugier, C.; Cauchard, J. Killing of trypanozoon parasites by the equine cathelicidin eCATH1. Antimicrob. Agents Chemother. 2016, 60, 2610–2619.
- Lu, Z.; Wang, Y.; Zhai, L.; Che, Q.; Wang, H.; Du, S.; Wang, D.; Feng, F.; Liu, J.; Lai, R.; et al. Novel cathelicidin-derived antimicrobial peptides from Equus asinus. FEBS J. 2010, 277, 2329–2339.
- Couto, M.A.; Harwig, S.S.L.; Cullor, J.S.; Hughes, J.P.; Lehrer, R.I. Identification of eNAP-1, an antimicrobial peptide from equine neutrophils. Infect. Immun. 1992, 60, 3065–3071.
- Couto, M.A.; Harwig, S.S.; Cullor, J.S.; Hughes, J.P.; Lehrer, R.I. eNAP-2, a novel cysteine-rich bactericidal peptide from equine leukocytes. Infect. Immun. 1992, 60, 5042–5047.
- Couto, M.A.; Harwig, S.S.L.; Lehrer, R.I. Selective inhibition of microbial serine proteases by eNAP-2, an antimicrobial peptide from equine neutrophils. Infect. Immun. 1993, 61, 2991–2994.
- Valore, E.V.; Ganz, T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells, Mol. Dis. 2008, 40, 132–138.
- Nicolas, G.; Bennoun, M.; Porteu, A.; Mativet, S.; Beaumont, C.; Grandchamp, B.; Sirito, M.; Sawadogo, M.; Kahn, A.; Vaulont, S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA 2002, 99, 4596–4601.
- Oliveira-Filho, J.P.; Marques, J.A.; Cunha, P.H.J.; Medeiros, G.X.; Riet-Correa, F.; Machado, V.M. V.; Borges, A.S. Sequencing and expression analysis of hepcidin mrna in donkey (Equus asinus) liver. Pesqui. Vet. Bras. 2012, 32, 1050–1054.
- Quintana, A.M.; Landolt, G.A.; Annis, K.M.; Hussey, G.S. Immunological characterization of the equine airway epithelium and of a primary equine airway epithelial cell culture model. Vet. Immunol. Immunopathol. 2011, 140, 226–236.
- Davis, E.G.; Sang, Y.; Blecha, F. Equine β-defensin-1: Full-length cDNA sequence and tissue expression. Vet. Immunol. Immunopathol. 2004, 99, 127–132.
- Bruhn, O.; Paul, S.; Tetens, J.; Thaller, G. The repertoire of equine intestinal α-defensins. BMC Genomics 2009, 10, 631.
- Shomali, M.R. Recombinant Expression of the NOD2 CARD2 Domain and Determination of the Solution Structure of Equine Alpha-Defensin 1 (DEFA1). Ph.D. Thesis, Kiel University, Kiel, Germany, 2012.
- Shamova, O.; Brogden, K.A.; Zhao, C.; Nguyen, T.; Kokryakov, V.N.; Lehrer, R.I. Purification and properties of proline-rich antimicrobial peptides from sheep and goat leukocytes. Infect. Immun. 1999, 67, 4106–4111.
- Shamova, O.; Orlov, D.; Stegemann, C.; Czihal, P.; Hoffmann, R.; Brogden, K.; Kolodkin, N.; Sakuta, G.; Tossi, A.; Sahl, H.G.; et al. ChBac3.4: A novel proline-rich antimicrobial peptide from goat leukocytes. Int. J. Pept. Res. Ther. 2009, 15, 31–42.
- Shamova, O.V.; Orlov, D.S.; Zharkova, M.S.; Balandin, S.V.; Yamschikova, E.V.; Knappe, D.; Hoffmann, R.; Kokryakov, V.N.; Ovchinnikova, T.V. Minibactenecins ChBac7.Nα and ChBac7. Nβ Antimicrobial Peptides from leukocytes of the goat Capra hircus. Acta Naturae 2016, 8.
- Huttner, K.M.; Brezinski-Caliguri, D.J.; Mahoney, M.M.; Diamond, G. Antimicrobial Peptide Expression Is Developmentally Regulated in the Ovine Gastrointestinal Tract. J. Nutr. 1998, 128, 297S–299S.
- Anderson, R.C.; Hancock, R.E.W.; Yu, P.L. Antimicrobial Activity and Bacterial-Membrane Interaction of Ovine-Derived Cathelicidins. Antimicrob. Agents Chemother. 2004, 48, 673–676.
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999, 463, 58–62.
- Huttner, K.M.; Lambeth, M.R.; Burkin, H.R.; Burkin, D.J.; Broad, T.E. Localization and genomic organization of sheep antimicrobial peptide. Gene 1998, 206, 85–91.
- Ackermann, M.R.; Gallup, J.M.; Zabner, J.; Evans, R.B.; Brockus, C.W.; Meyerholz, D.K.; Grubor, B.; Brogden, K.A. Differential expression of sheep beta-defensin-1 and -2 and interleukin 8 during acute Mannheimia haemolytica pneumonia. Microb. Pathog. 2004, 37, 21–27.
- Zhao, P.; Cao, G. Production of bioactive sheep β-defensin-1 in Pichia pastoris. J. Ind. Microbiol. Biotechnol. 2012, 39, 11–17.