Coronavirus disease 2019 (COVID-19), a respiratory illness caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has claimed over one million lives worldwide since December 2019. The complement system, while a first-line immune defense against invading pathogens, has off-target effects that lead to increases in inflammation, tissue damage, and thrombosis; these are common, life-threatening complications seen in patients with COVID-19. The potential impact of complement activation in COVID-19 and possible treatments targeting the complement system are discussed.
- COVID-19
- complement system
- C3
- C5
1. Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1] and was first recognized in December 2019 in Wuhan, China [2]. The virus quickly spread throughout the world and has been reported in over 180 countries [1]. As of December 14, 2020, there have been 73,188,395 cases and 1,627,783 deaths worldwide [3]. There is currently only one medication approved by the Food and Drug Administration (FDA) for the treatment of COVID-19 as well as two additional antiviral therapies available through emergency use authorization [1][2]. New data on treatment possibilities are emerging daily, and many investigations and clinical trials are underway. There is an urgent need for information on treatment of COVID-19. Many of the symptoms associated with COVID-19 can be related to immune response and complement activation.
2. Complement as a Therapeutic Target
2.1. C3 Inhibition
Compstatin is a highly selective inhibitor of C3 [4]. A potent compstatin-based C3 inhibitor, AMY-101, has been developed [5]. The molecule is a small peptide that is selective for human and primate C3. AMY-101 is currently in phase II clinical trials after showing favorable safety and tolerability in humans in phase I trials. A case report has demonstrated that AMY-101 may facilitate clinical improvement in severe COVID-19 patients [5]. The case presents a 71-year-old male patient diagnosed with ARDS caused by SARS-CoV-2. The patient was eligible as a candidate for experimental treatments of COVID-19, including compassionate use of AMY-101. The therapy was administered as a one-time loading dose, followed by 13 daily maintenance doses for a total of 14 days of treatment. No side effects were recorded after the loading dose and no infusion reactions were reported during the entire duration of therapy. The patient showed dramatic clinical improvement within 48 h of AMY-101 treatment, demonstrated by significantly improved respiratory performance and a gradual decrease in oxygen requirements. By day 19 after initiation of AMY-101 treatment initiation, the patient was afebrile with no respiratory symptoms and was without need for oxygen support. This case report preliminarily demonstrates the safe and effective use of AMY-101 in the treatment of severe COVID-19 and reinforces the need for further clinical studies of C3 inhibition as anti-inflammatory therapy.
2.2. C5 Inhibition
C5 inhibition has been used clinically for 15 years in the setting of paroxysmal nocturnal hemoglobinuria (PNH), a rare blood disorder characterized by complement-dependent hemolysis [6][7]. The C5 inhibitors eculizumab (Soliris) and ravulizumab-cwvz (Ultomiris) have both been proposed in the treatment of ARDS in COVID-19 [8][9]. Eculizumab, a long-acting humanized monoclonal antibody against complement C5, is currently available for compassionate use in COVID-19 under an expanded access program [8]. Preliminary results of patients with severe COVID-19 treated with eculizumab yielded positive results. In a case report of four patients treated with eculizumab off-label, all patients successfully recovered after treatment with a noted drop in inflammatory markers [10]. The Eculizumab (Soliris) in COVID-19 Infected Patients (SOLID C-19) trial is an ongoing compassionate use study [11]. A preliminary case report demonstrates the safe and effective use of eculizumab in a 44-year-old female patient with severe COVID-19 pneumonia on invasive mechanical ventilation [11]. Upon treatment with eculizumab, the patient’s chest x-ray and clinical condition improved. The patient continued to improve with consecutive doses and was able to be extubated 14 days later after four doses of eculizumab. These preliminary results are promising until more data become available. Ravulizumab-cwvz is a long-acting C5 complement inhibitor with an upcoming phase III clinical trial for COVID-19 severe pneumonia [9][12]. Based on anecdotal information from compassionate use cases, the phase III controlled clinical trial will assess 28-day survival among patients with severe COVID-19 receiving ravulizumab with current best supportive care or best supportive care alone. The study is expected to have primary outcome data by May 2021 and to be completed by August 2021 [9].
Results of a recent exploratory study comparing the efficacy of eculizumab to AMY-101 in independent cohorts reported clinical improvement in both groups of patients [13]. Three patients with severe COVID-19 ARDS were given AMY-101 via continuous IV infusion, while 10 patients with COVID-19 enrolled in a phase I/II single-arm clinical trial were given eculizumab IV once weekly. All treated patients required oxygen support prior to treatment initiation. Both treatments resulted in sustained anti-inflammatory response marked by reductions in C-reactive protein and IL-6, improvement in lung function, and resolution of ARDS. C3 inhibition with AMY-101 resulted in further reduced neutrophil counts, serum LDH levels, and lymphocyte recovery. These early results demonstrate not only the role of complement in COVID-19, but the utility of C3 and C5 as therapeutic targets to improve patient outcomes.
Clinical trials have begun for monoclonal anti-human C5a antibody, IFX-1, in patients with COVID-19 with severe pneumonia [14]. IFX-1 is not thought to impact formation of the MAC. IFX-1 does not currently have any approved indications but is in development to treat inflammatory conditions. According to preclinical data, IFX-1 controls inflammatory-related tissue and organ damage [14]. Phase II/III trials investigating the use of IFX-1 in COVID-19 are currently underway with results expected in mid-2021 [15]. Preliminary results are reported to be positive, with IFX-1 treatment associated with a trend in lower 28-day all-cause mortality compared to best supportive care alone [16]. Treatment with IFX-1 has also shown trends of maintained kidney function, faster lymphocyte count normalization, and greater reduction in LDH in patients with severe COVID-19 [16].
Avdoralimab is a fully human monoclonal antibody that blocks the C5a receptor [17]. By preventing the binding of C5a to the receptor, avdoralimab has the potential to reduce the C5a mediated inflammatory response to SARS-CoV-2 in the lungs [17]. Double-blind, randomized phase II trials, named FOR COVID-19 Elimination (FORCE), began in April 2020 [18]. The trial compares avdoralimab against placebo in two cohorts of patients with COVID-19: those requiring supplemental oxygen and those requiring mechanical ventilation. Results of the FORCE trial are expected at the end of 2020.
2.3. Heparin
Heparin is a familiar anticoagulant with clinical use dating back to the 1920s [19]. It achieves anticoagulation through antithrombin dependent inactivation of thrombin and activated coagulation factor Xa [20]. It also prevents fibrin formation and inhibits thrombin-induced activation of platelets and other coagulation factors [20].
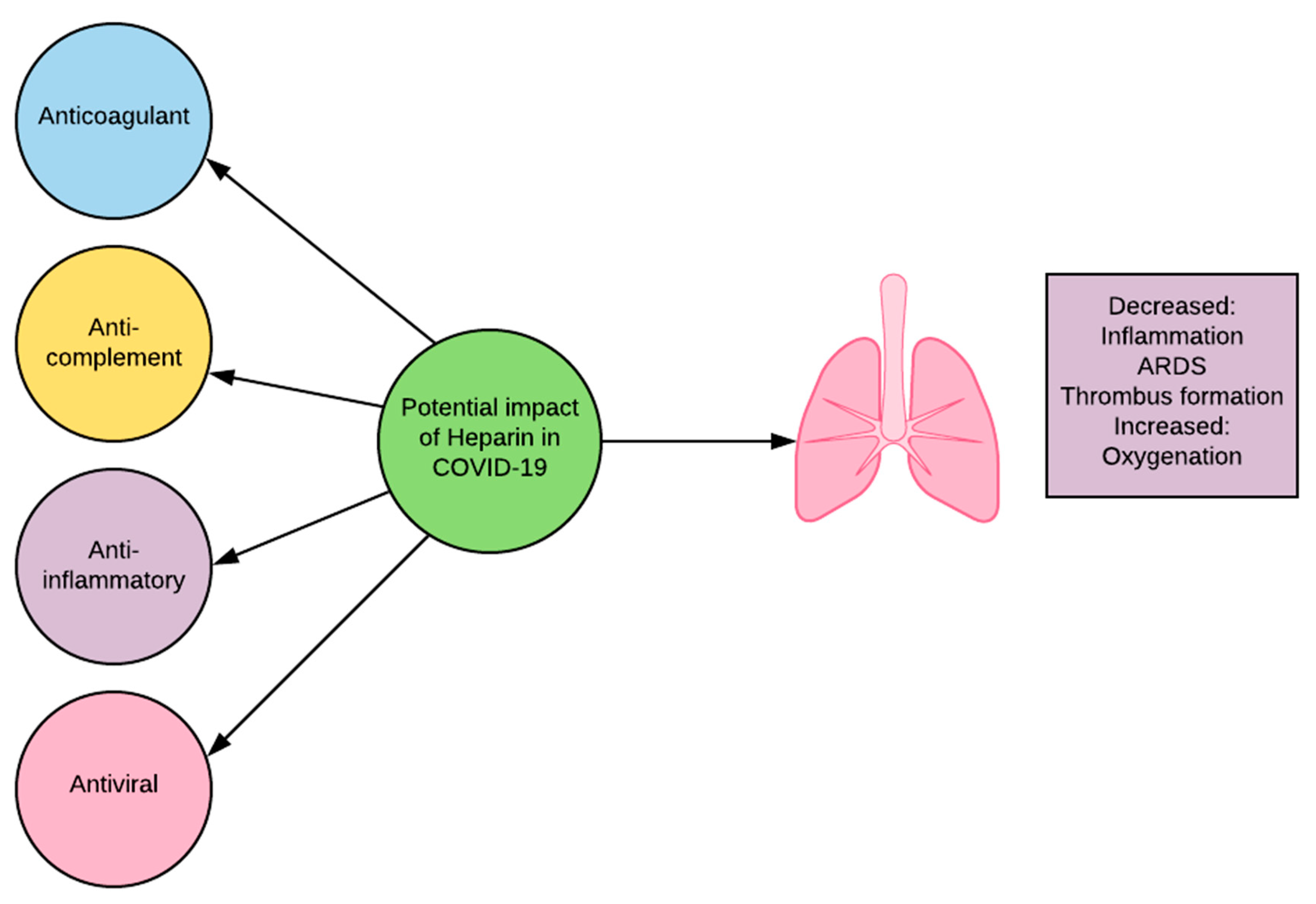
The utility of heparin in COVID-19 is not limited to its anticoagulant activity (Figure 1). Heparin has been shown to also have anti-complement, anti-inflammatory, and anti-viral properties. Heparin interferes with C3 convertases and with the function of late components of the complement system [21]. It can do this independently of antithrombin binding activity [21]. The inhibition of C3 convertase blocks the cleavage of C3, preventing the creation of detrimental fragments C3a and downstream C5a. Heparin inhibits inflammation through alteration of pro-inflammatory protein activity and prevention of adhesion and influx of inflammatory cells in affected areas [22]. Heparin interacts directly with viral particles and has been shown to bind to the SARS-CoV-2 S1 Spike RBD, causing significant structural change and alteration of function [23]. Coronavirus surface proteins are known to interact with heparan sulfate, a glycosaminoglycans (GAG) closely related to heparin, in order to attach to target cells [23][24]. Heparin may act as a competitive inhibitor for these sites, decreasing viral attachment to host cells [25]. The ability of heparin to decrease complement activation, inflammation, and viral infiltration makes it a promising candidate for clinical review in COVID-19.
Heparin use in clinical trials has been divided into prophylactic or therapeutic dosing in the treatment of COVID-19 associated thrombosis. A retrospective study by Tang et al. reviewed data from 449 patients with severe COVID-19 [26]. All patients received antiviral therapy with supportive care and 99 patients also received low molecular weight heparin (LMWH) at prophylactic doses. Although no difference was found in 28-day mortality overall between the groups, heparin receivers did have lower 28-day mortality if they had a sepsis-induced coagulopathy (SIC) score ≥4 or D-dimer greater than six times the upper limit of normal. It was concluded that LMWH at prophylactic doses may be associated with improved prognosis in patients with severe COVID-19 meeting SIC criteria or with markedly elevated D-dimer.
There is limited data available for the outcomes of patients with COVID-19 receiving therapeutic doses of heparin. One cohort study of 2,773 patients found that of patients on invasive mechanical ventilation (n = 395), anticoagulation was associated with an in-hospital mortality of 29.1% [27]. Patients who did not receive anticoagulation (n = 1987) had an in-hospital mortality of 62.7%. The study also found that patients were more likely to require invasive mechanical ventilation if they received anticoagulation [27]. A retrospective, observational study of 2,075 patients with COVID-19 compared mortality between patients who received heparin (n = 1734) versus those who did not (n = 341) [28]. The data were adjusted for age, gender, signs of increasing severity (temperature greater than 30° C or oxygen saturation less than 90%), and other COVID-19 treatments. Heparin was associated with lower mortality when the model was adjusted for age and gender and remained significant when additionally adjusted for severity and then other treatments. These promising observational results highlight the need for further, controlled trials of heparin use in COVID-19.
Although promising, the use of heparin in COVID-19 is not without safety concerns. Therapeutic dosing of heparin is associated with a 10–15% risk of significant bleeding [22]. Another concern is the rare risk for heparin-induced thrombocytopenia. Although excess coagulation can cause many complications, coagulation in the alveoli and airways is protective to isolate pulmonary pathogens and prevent infection. Heparin also has effects on various growth factors that may result in protection or harm to organs [22]. More information is needed before a recommendation may be made one way or the other on the use of heparin in patients with COVID-19.
3. Conclusions
While there are many promising treatment options being investigated, the need for further discovery and understanding of drugs to treat COVID-19 is paramount. Until the infection can be effectively prevented, proper diagnosis and drug therapy remain on the forefront of treatment. Diagnostic testing and clinical presentation should be used to guide treatment so patients are receiving the best therapy, offering them the best chance at survival and recovery. The complement system is key in the body’s immune response to invading pathogens, its unchecked activation may lead to serious host damage and organ dysfunction. Targeting complement has been demonstrated to be effective in the treatment of other inflammatory conditions and may be the key to relieving immune-mediated lung inflammation and damage in COVID-19. Preliminary data suggest the successful use of several anti-complement drugs in patients with severe COVID-19 pneumonia. Regulating complement activation in response to SARS-CoV-2 has the potential to alleviate respiratory symptoms and prevent disease progression. This has the power to improve patient outcomes and save lives.
Beyond COVID-19, complement is a possible target for many immune-mediated inflammatory conditions. Complement plays a contributory role in many auto-immune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and antiphospholipid antibody syndrome [29]. Further demonstrated use of anti-complement therapies in COVID-19 may open the door for their investigation in additional settings.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9010011
References
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 17 July 2020).
- World Health Organization. Clinical Management of COVID-19: Intermin Guidance. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 18 June 2020).
- Coronavirus Cases Worldometer. Available online: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdUOA?Si (accessed on 4 September 2020).
- Ricklin, D.; Lambris, J.D. Compstatin: A complement inhibitor on its way to clinical application. In Current Topics in Complement II.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 262–281.
- Mastaglio, S.; Ruggeri, A.; Risitano, A.M.; Angelillo, P.; Yancopoulou, D.; Mastellos, D.C.; Huber-Lang, M.; Piemontese, S.; Assanelli, A.; Garlanda, C.; et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020, 215, 108450.
- The Sidney Kimmel Comprehensive Cancer Center. Paroxysmal nocturnal hemoglobinuria (PNH). Available online: https://www.hopkinsmedicine.org/kimmel_cancer_center/types_cancer/paroxysmal_nocturnal_hemoglobinuria_PNH.html (accessed on 17 July 2020).
- Dubois, E.A.; Cohen, A.F. Eculizumab. Br. J. Clin. Pharmacol. 2009, 68, 318–319.
- Eculizumab (Soliris) in Covid-19 Infected Patients (SOLID-C19). Available online: https://clinicaltrials.gov/ct2/show/NCT04288713?term=complement&cond=COVID-19&draw=2 (accessed on 4 September 2020).
- Efficacy and Safety Study of IV Ravulizumab in Patients with COVID-19 Severe Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04369469 (accessed on 17 July 2020).
- Diurno, F.; Numis, F.G.; Porta, G.; Cirillo, F.; Maddaluno, S.; Ragozzino, A.; De Negri, P.; Di Gennaro, C.; Pagano, A.; Allegorico, E.; et al. Eculizumab treatment in patients with COVID-19: Preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4040–4047.
- Pitts, T. A Preliminary Update to the Soliris to Stop Immune Mediated Death in Covid-19 (SOLID-C19) Compassionate Use Study. Available online: https://hudsonmedical.com/articles/soliris-stop-death-covid-19/ (accessed on 18 June 2020).
- Clinical Trial Arena. Alexion to Study Ultomiris for Covid-19 Treatment. Available online: https://www.clinicaltrialsarena.com/news/alexion-ultomiris-covid-19-trial/ (accessed on 18 June 2020).
- Mastellos, D.C.; Pires da Silva, B.G.P.; Fonseca, B.A.L.; Fonseca, N.P.; Auxiliadora-Martins, M.; Mastaglio, S.; Ruggeri, A.; Sironi, M.; Radermacher, P.; Chrysanthopoulou, A.; et al. Complement C3 vs c5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020, 220, 108598.
- Clincal Trials Arena. Inflarx Starts Dosing Covid-19 Patients in Europe. Available online: https://www.clinicaltrialsarena.com/news/inflarx-covid-19-dosing-europe/ (accessed on 18 June 2020).
- Open-Label, Randomized Study of IFX-1 in Patients with Severe COVID-19 Pneumonia (PANAMO). Available online: https://clinicaltrials.gov/ct2/show/NCT04333420 (accessed on 18 June 2020).
- Inflarx Reports Encouraging Topline Results from the Exploratory Phase II Part of the Adaptive Randomized Phase II/III Trial of IFX-1 in COVID-19. Available online: https://www.inflarx.de/Home/Investors/Press-Releases/06-2020-InflaRx-Reports-Encouraging-Topline-Results-from-the-Exploratory-Phase-II-Part-of-the-Adaptive-Randomized-Phase-II-III-Trial-of-IFX-1-in-COVID-19.html (accessed on 23 July 2020).
- Innate Pharma. Avdoralimab/IPH5401: Anti-C5AR MAB. Available online: Innate-pharma.com/en/pipeline/avdoralimab/iph5401-anti-c5ar-mab (accessed on 17 December 2020).
- Avdoralimab an anti-C5aR Antibody, in Patients with COVID-19 Severe Pneumonia (FORCE). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04371367 (accessed on 17 December 2020).
- Barrowcliffe, T. History of heparin. In Heparin-a Century of Progress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22.
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1094–1096.
- Kazatchkine, M.D.; Fearon, D.T.; Metcalfe, D.D.; Rosenberg, R.D.; Austen, K.F. Structural determinants of the capacity of heparin to inhibit the formation of the human amplification C3 convertase. J. Clin. Investig. 1981, 67, 223–228.
- Hippensteel, J.A.; LaRiviere, W.B.; Colbert, J.F.; Langouet-Astrie, C.J.; Schmidt, E.P. Heparin as a therapy for COVID-19: Current evidence and future possibilities. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L211–L217.
- Mycroft-West, C.J.; Su, D.; Elli, S.; Li, Y.; Guimond, S.E.; Miller, G.J.; Turnbull, J.E.; Yates, E.A.; Guerrini, M.; Fernig, D.G. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. BioRxiv 2020.
- Milewska, A.; Zarebski, M.; Nowak, P.; Stozek, K.; Potempa, J.; Pyrc, K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014, 88, 13221–13230.
- de Haan, C.A.; Te Lintelo, E.; Li, Z.; Raaben, M.; Wurdinger, T.; Bosch, B.J.; Rottier, P.J. Cooperative involvement of the S1 and S2 subunits of the murine coronavirus spike protein in receptor binding and extended host range. J. Virol. 2006, 80, 10909–10918.
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099.
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124.
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis 2020, 50, 298–301.
- Ricklin, D.; Lambris, J.D. Complement in immune and inflammatory disorders: Pathophysiological mechanisms. J. Immunol. 2013, 190, 3831–3838.
