Gynaecological cancers can be primary neoplasms, originating either from the reproductive tract or the products of conception, or secondary neoplasms, representative of metastatic disease. For some of these cancers, the exact causes are unknown; however, it is recognised that the precise aetiopathogeneses for most are multifactorial and include exogenous (such as diet) and endogenous factors (such as genetic predisposition), which mutually interact in a complex manner. One factor that has been recognised to be involved in the pathogenesis and progression of gynaecological cancers is the endocannabinoid system (ECS). The ECS consists of endocannabinoids (bioactive lipids), their receptors, and metabolic enzymes responsible for their synthesis and degradation.
- cannabinoids
- cervical cancer
- endocannabinoids
- endometrial cancer
- enzymes
- receptors
1. Cannabis and Endocannabinoids and the Discovery of the Endocannabinoid System
Cannabis is the botanical name of an annual herbaceous plant of the Cannabaceae family that is cultivated and distributed all over the world. This genus consists of three major species, C. sativa, C. indica, and C. ruderalis, which, through interbreeding, share similar genetic backgrounds and physical traits [1]. One distinctive trait of Cannabis plants is production of secondary compounds called “phytocannabinoids”, of which over 100 are produced by the female Cannabis inflorescence [2]. The first evidence for the medical use of Cannabis dates to the Han Dynasty in ancient China, where it was recommended for pain, constipation, agitation, hysteria, spasmodic cough, disorders of the female reproductive tract, and other less defined conditions [3]. Of the 100 or so phytocannabinoids, the most potent is Δ-9-tetrahydrocannabinol (THC), which was isolated and identified as a major psychoactive compound in the 1960s [4]. This was followed by the discovery of additional phytocannabinoids, such as cannabidiol (CBD), cannabinol (CBN), cannabichromene (CBC), cannabigerol (CBG), tetrahydrocannabivirin (THCV), and Δ-8-THC [5].
In the early 1990s, two different G-protein-coupled receptors able to interact with phytocannabinoids were discovered in the central nervous system and the spleen; these receptors are now called type 1 and type 2 cannabinoid receptors (CB1 and CB2), respectively [6][7]. Their discovery was shortly followed by that of their ligands—two specific endogenous bioactive lipids, N-arachidonoylethanolamine (also known as anandamide, AEA) and 2-arachidonoylglycerol (2-AG) from animal tissues [8][9]. Later, the metabolic enzymes that regulate the production and degradation of these endogenous cannabinoids (endocannabinoids; eCBs) were discovered, followed by ancillary ligands, receptors, and transporters. These altogether represent the “endocannabinoid system (ECS)”, which is ubiquitously distributed in the body [4][10][11], including both the male and female reproductive tissues [12][13].
2. ECS in Female Tissues and Reproductive Events
The main elements of the ECS are all expressed in human female reproductive tissues, such as the ovaries [14], Fallopian tubes (oviduct) [15], uterus [16], and placenta [17] (Figure 1). They have also been localised to areas of the hypothalamus responsible for producing hormones, which act through the hypothalamic–pituitary–gonadal (HPG) axis to control a great number of reproductive functions [18]. In the human ovaries, CB1 and CB2 have been shown to be expressed in the granulosa cells of primordial, primary, secondary, and tertiary follicles, as well as in theca cells of secondary and tertiary follicles (Figure 1), with the highest expression at the time of ovulation [14]. Additionally, both receptors are expressed in the corpus luteum and corpus albicans, even in the absence of pregnancy [14]. Moreover, FAAH has been shown to be present within theca cells, but NAPE-PLD appears only in the granulosa of secondary and tertiary follicles, the corpus luteum, and corpus albicans [14].
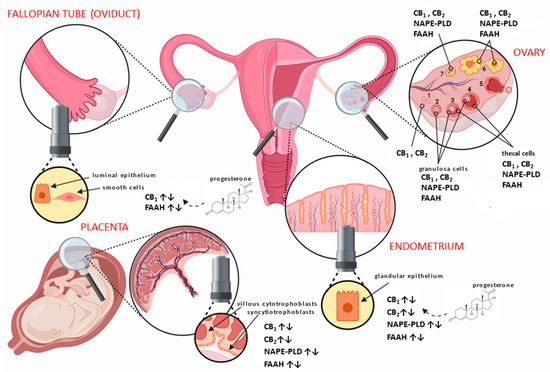
Figure 1. Distribution of the main endocannabinoid system (ECS) components in human female reproductive tissues. In the ovary, the different stages of follicular development from (1) primordial/primary, (2) secondary, (3) tertiary, (4) pre-ovulatory/Graafian, to (5) ovulating follicles are depicted. After ovulation is complete, the condensing granulosa and mural thecal cells form the corpus luteum (6), a structure that produces the progesterone required for continued early pregnancy. In the absence of pregnancy, the corpus luteum degenerates into the corpus albicans (7). Throughout the ovarian cycle, CB1 (type 1 cannabinoid receptor) and CB2 (type 2 cannabinoid receptor), fatty acid amide hydrolase (FAAH), and N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) are produced in the various cells of the developing follicle and corpus luteum, including the oocyte [14]. Similarly, CB1, CB2, FAAH, and NAPE-PLD are expressed in the Fallopian tube [19] and endometrium [16] throughout the menstrual cycle, where they are regulated by the actions of estradiol and progesterone. The cytotrophoblast and syncytiotrophoblast cells of the early placenta also express CB1, CB2, FAAH, and NAPE-PLD [17], where modulation of protein expression occurs when production of progesterone changes from the corpus luteum to the placenta.
In the Fallopian tube, CB1 is expressed primarily in the smooth muscle cells and in surrounding blood vessels, with lower expression in the cytoplasm of epithelial cells lining the lumen of the tube [15]. In the endometrium, CB1 mRNA and protein levels increase in the secretory phase, probably under the influence of progesterone [20], while CB2 expression is minimal at the beginning of the cycle and increases markedly during the late proliferate phase of the menstrual cycle [16]. Interestingly, CB1 mRNA is only present at low levels in both the Fallopian tube and the endometrium of women with an ectopic pregnancy [15].
3. The Endocannabinoid System in Relation to Normal Gynaecological Tissues
There have been several reviews [21] on this, and these are summarised in Table 1. All the components of the ECS are present and active in all parts of the female reproductive tract. Here, they play roles that include oocyte production [14][22][23][24][25], oviductal transport [25][26][27], and blastocyst maturity and implantation [28], as well as in preparing the endometrium for implantation [16][29][30][31][32]. When the ECS is dysfunctional or interfered with by, for example, cannabinoids [33][34], human fertility may be impaired (reviewed in [12][28][35][36]) and there may be associated reproductive-tissue-dependent pathologies, such as endometriosis, miscarriage, ectopic pregnancy, or pre-eclampsia [37][28][35][38][39][40]. Two recent reviews [12][18] on the ECS in the female reproductive tract summarise what is known on this topic, but crucially, these omit some important details on the main ECS components in gynaecological cancers, which we hope to address in this review. In this context, the ECS has been studied the most in the human ovary [14][22][41][42][43][44][45][46], cervix [47][48][49][50][51], and endometrium [16][20][52][53][54][55][56][57][58][59][60], the most common gynaecological cancers ([21][61][62]); however, other female cancers have not been studied, as shown in Figure 2. Although the presence and actions of the most commonly studied endogenous ligands (AEA, 2-AG, OEA, and PEA) in human reproductive tissues have been demonstrated, the presence and actions of others, such as SEA, virodhamine, stearamide, and monoolein [12][63][18][14][19][37][64][65][54][66][21][28][32][35][40][67][68][69][70][71][72][73][74] have not. Studies on receptor expression and function are few, and although there are some published studies on NAPE-PLD and FAAH expression and action in the female reproductive tract, many other (endo)cannabinoid metabolising enzymes have yet to be investigated, either in the normal female reproductive tract or in their related neoplasms (Figure 1 and Figure 2).
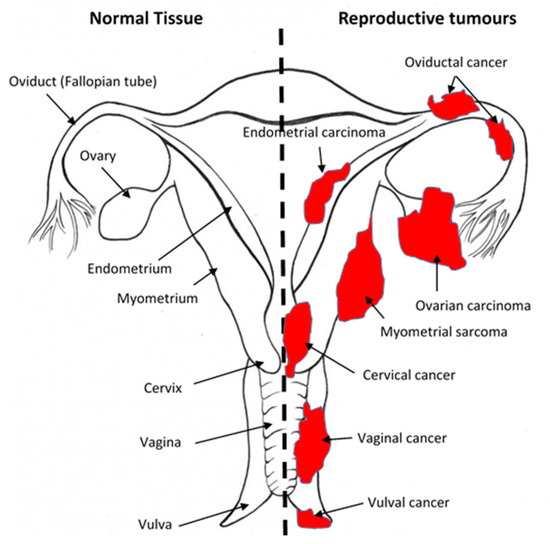
Figure 2. Sites of gynaecological cancers in the female reproductive tract. The diagram indicates the names of the normal tissues of the female reproductive tract (left side) and the sites and names of the cancers (right side) for the corresponding normal tissues. Please add copyright if necessary.
4. The Effects of Cannabinoids on Gynaecological Cancers
The main cannabinoids that are considered to have pharmaceutical promise in the treatment of cancer are the potent psychoactive and the commonly investigated non-psychoactive components of the Cannabis species, tetrahydrocannabinol (THC) and cannabidiol (CBD). Although there is scant evidence for their effectiveness in the treatment of gynaecological cancers, they are often promoted on medical cannabis production and distribution sites as having proven effectiveness [75][76][77][78][79][80]. Here, we examine the evidence in the scientific and clinical literature to support the current and future use of such compounds in the treatment of gynaecological cancers. These data are summarised in Table 1.
4.1. Cannabinoids and Ovarian Cancer
Among gynaecological cancers, those of the ovary have the highest morbidity and mortality rates [81]. In an attempt to establish if there is a possible role for the ECS in ovarian pathophysiology, we [14] studied the expression levels of different components of the ECS [20], and demonstrated expression of CB1, CB2, and the NAE-modulating enzymes NAPE-PLD and FAAH in normal human ovaries using immunohistochemistry [14]. Additionally, AEA concentrations in follicular fluid after ovarian stimulation by hormones (following an in vitro fertilisation protocol that caused an increase in follicle size) were directly correlated with follicle size, suggesting that AEA is indeed involved in the hormonal maturation of follicles and oocytes [14][20]. Furthermore, data exist to indicate that AEA, OEA, and PEA are all elevated in follicular fluids of ovarian cancer patients and women with ovarian cysts [22].
Bagavandoss and colleagues demonstrated CB1 and FAAH expression in ovarian surface epithelium, the site from which some ovarian cancers often arise, providing another clue for a possible involvement of the ECS in ovarian cancer [23]. Regarding the expression of CB1 in ovarian cancer, Messalli and coworkers [82] showed that CB1 expression was moderate in benign and borderline epithelial rat ovarian tumours, but was increased in invasive ovarian tumours, suggesting a correlation between the extent of expression of the ECS components and the prognosis for patients with more aggressive ovarian cancer [82]. The levels of lysophospholipids such as lysophosphatidylinositol (an endogenous GPR55 agonist) in blood and ascitic fluids were also found to be elevated in ovarian cancer patients compared to healthy controls, a finding associated with proliferation and the metastatic potential of ovarian cancer cells [83]. Hofman and colleagues [84] more recently found that elevated lysophosphatidylinositol levels in the ovarian cancer cell lines OVCAR-3, OVCAR-5, and COV-362 resulted in GPR55-dependent angiogenesis. Their conclusion was based on experiments where pharmacological inhibition and genetic deletion of GPR55 reduced the pro-angiogenic potential of lysophosphatidylinositol in these cell lines. Additionally, they demonstrated that the mitogen-activated protein kinase pathway triggered via GPR55 by phosphorylation of ERK1/2 and p38, which are signalling molecules known to be involved in proliferative and migratory responses, could be curtailed by chemical interventions [84]. This observation suggests that some ovarian cancers might be amenable to pharmaceutical intercession. In addition, other components of the endocannabinoid system are important here. For example, the 2-AG degrading enzyme MAGL has been shown to be upregulated in aggressive human ovary cancer cells [45], and it is also thought to be involved in oncogenic signalling and, hence, in increased migration, invasion, and survival of many other cancer cell types [85]. These data suggest that identification of an effective drug that targets the ECS to treat ovarian cancer may have applications in the treatment of other cancers too. The application of such therapies would need to be timely, because MAGL overexpression in non-aggressive cancer cells often results in tumours that subsequently exhibit an increased pathogenic phenotype [45]. Moreover, the application of an MAGL inhibitor led to a reversion of the enhanced pathogenicity [45]. Thus, the involvement of the ECS, and especially the 2-AG signalling pathways in ovarian cancer, may fuel expectations on new therapeutics to combat this and other types of cancer. Some preliminary evidence suggests that OEA and its structural analogues may also have a beneficial effect on inhibiting ovarian cancer growth, but these data need to be confirmed in vivo [41]. There is little evidence that plant-derived (phyto)cannabinoids have any effect on ovarian biology or ovarian cancer development or progression, a concept that came from a study where SKOV-3-derived tumours were grown on the chorioallantoic membrane of fertilised chicken eggs [86], and then were treated with CBD-containing nanoparticles. The data indicated that CBD caused a 1.35- to 1.50-fold reduction in tumour size depending on the type of CBD formulation used [86]. The authors indicated that these nanoparticle preparations might be useful in the treatment of peritoneal metastases of ovarian cancer, possibly with lower adverse drug effects [86]. Furthermore, the preparations also reduced SKOV-3 ovarian cancer cell numbers in vitro, to almost zero within 48 h, possibly making this a good candidate for a randomised clinical trial. Of course, many additional studies are required before any candidate CBD formulation can be used in such clinical trials.
4.2. Cannabinoids and Fallopian Tube Cancer
Fallopian tube cancer is a relatively rare gynaecological cancer (Figure 2). It is often categorised as being part of ovarian cancer (especially as there is emerging evidence that most surface epithelial ovarian cancers maybe of fimbrial origin), but it is important to study it as a separate entity. Just like other parts of the female reproductive tract, the oviduct (Fallopian tube) expresses all the components of the ECS, with CB1 and FAAH expression intimately associated with proper oviductal function [19][25][26]. When dysfunctional, the risk of ectopic pregnancy is markedly increased [15][19][26][27][87]. There is little evidence on the effect of cannabinoids on human oviductal function, but in the murine oviduct [38], THC reduces fertility because of the increased number of ectopically implanting embryos. In the bovine oviduct, there is gradation of AEA, OEA, and PEA concentrations in the oviductal epithelial cells with low levels in the isthmus and significantly higher levels of OEA and PEA (but not AEA) in the ampulla at the same point of the oestrous cycle [88]. These levels significantly fluctuated during the oestrous cycle [88], as they do in the human oviduct (Fallopian tube) during the menstrual cycle and along its length [19], with OEA causing a reduction in epithelial cell cilia beat frequencies [87], an effect that is likely to prevent timely movement of fertilised oocytes and precipitate ectopic pregnancy [19]. Although possible relationships between the ECS, cannabinoids, and oviductal cancer currently do not exist (Table 1), the fact that dysregulation of the ECS in the fallopian tube is related to the development of ectopic pregnancy makes us speculate that there could be a role for the ECS in oviductal cancer, and that such a possibility deserves to be investigated.
4.3. Cannabinoids and Endometrial Cancer
Endometrial cancer, which is classified into type 1 and 2 [89], is the fourth most common cancer in women [90] and the most common gynaecological cancer. Various therapies exist depending on the disease grade and stage. Prognosis is poor, especially in those women with late presentation/detection [89]. Guida and coworkers [58] reported an upregulation of CB2 expression in endometrial cancer, whereby immunostaining was only successful in transformed malignant cells, while being completely absent in normal endometrial tissue. Furthermore, 2-AG levels were increased, but MAGL expression was decreased in comparison to controls, while AEA levels and FAAH expression were unaffected [58]. Similarly, Jove and colleagues [68] demonstrated that CB1 and CB2 were expressed at higher levels in stage III and IV endometrial carcinoma that has a poor prognosis. Unlike Guida and coworkers, the latter researchers found, by immunohistochemistry, an increase in CB1 expression, but no change in CB2 expression in stage 1 endometrial carcinoma tissue compared to normal endometrial tissue [68]. These observations were at odds with those of Risinger and coworkers, who found a decrease in CB1 receptor at the transcriptional level in stage 1 tissue [59]. These contradictory observations prompted us to investigate the ECS in endometrial cancer, using more than a single technique to interrogate CB1 and CB2 expression in endometrial cancer [55][56][57]. Our data indicated that CB1 and CB2 expression are decreased not only at the transcript level, but also at the protein level in both types 1 and 2 (stage 1) endometrial cancers (Table 1; Figure 3). We concluded that the discrepancy between these and previous studies was due to technical issues in the different methodologies used, including tissue sampling [56][57]. Furthermore, we examined the concentrations of plasma and tumour levels of AEA, OEA, and PEA in women with and without endometrial cancer, and showed that although the levels of all three N-acylethanolamines were increased in the tumours and in blood, only AEA and PEA were significantly higher in the plasma of such patients [91][53]. These data suggest that the differential catabolism of these three N-acylethanolamines might explain the different patterns of expression in endometrial cancer and plasma. We subsequently discovered that the apparent discrepancy between the tissue levels and plasma concentrations of OEA in the sample patient cohort was due to a decrease in the expression of FAAH in the tumour [66], without any change in the expression of NAPE-PLD (Table 1; Figure 3). The latter study also allowed us to define cut-off values for plasma AEA, OEA, and PEA concentrations (>1.36, >4.97, and 27.5 nM, respectively) that could be used in the prediction of endometrial cancer in symptomatic women [92], an observation that awaits confirmation in a larger, multicentre trial.
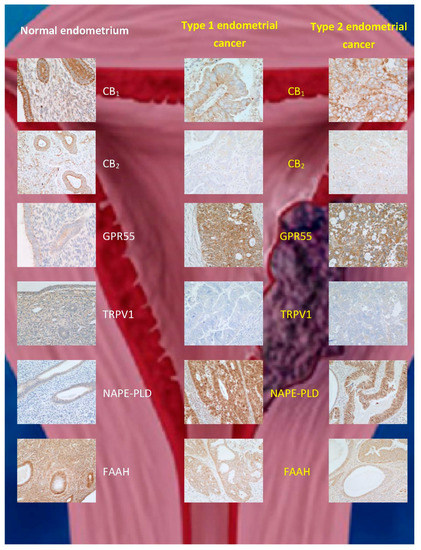
Figure 3. Immunohistochemical staining patterns for ECS proteins in normal endometrium and (type 1 and type 2) endometrial cancer. The data are taken from [58], where validation for the commercial antibodies and techniques used can be found. Note the reduction in CB1, CB2, TRPV1, and FAAH protein staining and increases for GPR55 and N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) expression in both types of endometrial cancer when compared to that of normal tissue.
4.4. Cannabinoids and Cervical Cancer
Cervical cancer is the second leading cause of malignancy-related deaths in women worldwide due to the lack of customisable and effective treatments (especially in low- and middle-income countries), with more than 250,000 deaths being reported annually [93]. A possible role of the ECS in the development of cervical cancer has been elucidated in recent years. Contassot and coworkers [48] reported a strong expression pattern of CB1 and CB2, as well as of TRPV1, in cervical carcinoma cell lines and biopsies. In addition, it was shown that AEA had a pro-apoptotic effect on cervical carcinoma cell lines (HeLa and Caski) [48], which were not inhibited, but were instead enhanced by CB1 and CB2 antagonists. On the other hand, the TRPV1 selective antagonist capsazepine protected the cell lines from AEA-induced apoptosis, indicating an important role of the TRPV1 channel in the pro-apoptotic action of AEA [94]. Additionally, it was demonstrated by Ramer and collaborators [95] that CBD decreased the invasiveness of cancer cells in a concentration-dependent manner. This effect was observed in the cervical cancer cell lines HeLa and C33A, as well as in the lung cancer cell line A549, and seemed to be mediated by the upregulation of TIMP-1 via CB1/CB2 and TRPV1. TIMP-1 is an inhibitor of matrix metalloproteinases, and as such, it prevents the movement of cells out of the tissue and, hence, a metastatic disease, as has been observed in a patient with ovarian cancer treated with CBD [96].
The activation of p38 and p42/44 mitogen-activated protein kinases was identified as an upstream event in TIMP-1 upregulation [95]. In agreement with these findings, it was reported that treatment of different cervical cancer cell lines (HeLa, SiHa, ME-180) with CBD led to a decrease in cell proliferation [97]. Furthermore, CBD induced cell death by the accumulation of cells in the sub-G0 phase (cell death phase) of the cell cycle, a finding that was most likely caspase-dependent because caspase-9 as well as caspase-3 were upregulated upon CBD treatment [97]. Hence, CBD may be an additional therapeutic tool for the treatment of cervical cancer, yet additional in vivo studies, similar to that performed on a single ovarian cancer patient [96], will be needed to clarify the impact of CBD on cervical cancer.
4.5. Cannabinoids and Vaginal Cancer
Vaginal cancer is uncommon, and the American Cancer Society estimated that >6000 women will be diagnosed with it in 2020. The estimated lifetime risk is 1 in 1100 (i.e., less than 0.1%). Of the 6000 USA women expected to be diagnosed with vaginal cancer in 2020, 1450 will die because they have this disease [98]. The role of the ECS in vaginal cancer has not been fully examined. We [47] have demonstrated that CB1 and FAAH are expressed in the normal vagina; however, there are no data on the expression of other components of the ECS (Table 1), nor on what their normal function might be. What happens to the expression of these factors or what effects cannabinoid and eCB ligands might have on the vagina or on cells of vaginal tumours is uncertain/unclear (Table 1). The internet is one source of information, and for the vagina, it is reported that some women experience a “vaginal high” when using cannabinoids, especially as a topical application [76]. The problem with these data is that only 40% of women experience this “psychological” effect [76]. Nevertheless, these statements have led some internet sites to suggest that different cannabis-containing preparations might be useful for the treatment of some of the symptoms associated with vaginal cancer [75][99]. Obviously, a lot more information is needed on the role of cannabinoids and eCBs in the human vagina, and especially in vaginal cancer.
4.6. Cannabinoids and Vulvar Cancer
Vulvar cancer is a less common gynaecological cancer [100]. The vulva is very similar to normal thin skin and is known to express CB1 and FAAH [47], but it is not known if it contains all the main components of the ECS (Table 1). The only existing evidence that cannabinoids have an effect on the vulva comes from a less-than-reliable internet source [78]. A C. sativa ethanolic extract and a purified CBD preparation had anti-inflammatory effects on keratinocytes and skin fibroblasts in vitro, suggesting that CBD was the main active ingredient that would be effective in wound injury [101].
This seems important because women with vulvar cancer often undergo radical surgery to remove their malignancy, which causes disfiguration of the female external genitalia, and causes significant long-term emotional and physical instability [102]. Indeed, the use of the CBD derivative VCE-004.3 on skin fibrosis and inflammation [103] demonstrated a CB2/PPARγ-dependent effect, and suggested that similar compounds might be beneficial for patients with vulvar cancer who have undergone surgery and need topical treatment for the pruritus; the latter is associated with skin fibrosis and inflammation, especially as VCE-004.3 appears to inhibit mast cell degranulation [103]. The toxicity profile of such topical administrations remains to be determined; however, ethanolic extracts of THC, CBD, and other cannabinoids appear in the blood shortly after administration; thus, some caution is advised, also in the light of the pleiotropic effects of these compounds [104]. Obviously, more detailed analysis of the role of the ECS and of plant-derived cannabinoids in the treatment of vulvar cancer is warranted.
4.7. Cannabinoids and Choriocarcinoma
The function of the female reproductive tract is to support the embryo and fetus during its development into an independent offspring (Figure 1). In order to do this, the coordinated actions of many interacting factors need to take place, of which the ECS is an integral part [28][35][39][105][106][107][108]. A key tissue in human reproduction is the fetoplacental unit. The entire ECS is present in the placenta [108] (see also Table 1), and modifications of its components result in obstetrical problems, such as miscarriage [109][65][46][110], babies that are small for gestational age [111][112], and pre-eclampsia [113]. In addition, dysregulated N-acylethanolamine levels may be responsible for preterm delivery [72][74]. The placenta can also undergo neoplastic changes into two clinically relevant conditions, hydatiform mole (a non-malignant transformation) and choriocarcinoma (a malignant transformation), which appear noteworthy. Currently, there are no data on the expression of the ECS in either of these tumours; there is, however, evidence that AEA and THC both affect a model for choriocarcinoma, like BeWo cells [114][115][116][117], and a model for normal trophoblast, like TCL-1 cells [118], where cell growth is affected mainly through a CB2-dependent mechanism [116][117][118]. These observations, coupled with evidence that THC decreases STAT3 signalling in mice with reduced fetus numbers and placental weights [111], support the view that cannabinoid use in human pregnancy is likely to affect the placenta in a similarly dangerous manner [119]. The increased use of CBD in pregnancy as an anti-emetic [120][121] is thus of great concern because the toxicity profile of CBD in pregnancy is not fully known [120][122][123], and especially as CBD can inactivate both placental CB1 and CB2 receptors in vitro [124].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13010037
References
- Schilling, S.; Melzer, R.; McCabe, P.F. Cannabis sativa. Curr. Biol. 2020, 30, R8–R9.
- Mechoulam, R.; Hanus, L. A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids 2000, 108, 1–13.
- Brand, E.J.; Zhao, Z. Cannabis in Chinese medicine: Are some traditional indications referenced in ancient literature related to cannabinoids? Front. Pharmacol. 2017, 8, 108.
- Maccarrone, M.; Bab, I.; Biro, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296.
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. Biomed. Res. Int. 2018, 2018, 1691428.
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564.
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65.
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949.
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90.
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. 2009, 60, 85–92.
- Bab, I.; Ofek, O.; Tam, J.; Rehnelt, J.; Zimmer, A. Endocannabinoids and the regulation of bone metabolism. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 69–74.
- Cecconi, S.; Rapino, C.; Di Nisio, V.; Rossi, G.; Maccarrone, M. The (endo)cannabinoid signaling in female reproduction: What are the latest advances? Prog. Lipid. Res. 2020, 77, 101019.
- Silver, R.J. The endocannabinoid system of animals. Animals 2019, 9, 686.
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and function of the endocannabinoid system in the human ovary. PLoS ONE 2009, 4, e4579.
- Horne, A.W.; Phillips, J.A., 3rd; Kane, N.; Lourenco, P.C.; McDonald, S.E.; Williams, A.R.; Simon, C.; Dey, S.K.; Critchley, H.O. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE 2008, 3, e3969.
- Taylor, A.H.; Abbas, M.S.; Habiba, M.A.; Konje, J.C. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem. Cell Biol. 2010, 133, 557–565.
- Taylor, A.H.; Finney, M.; Lam, P.M.; Konje, J.C. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod. Biol. Endocrinol. 2011, 9, 152.
- Walker, O.S.; Holloway, A.C.; Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019, 12, 3.
- Gebeh, A.K.; Willets, J.M.; Marczylo, E.L.; Taylor, A.H.; Konje, J.C. Ectopic pregnancy is associated with high anandamide levels and aberrant expression of FAAH and CB1 in fallopian tubes. J. Clin. Endocrinol. Metab. 2012, 97, 2827–2835.
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010, 93, 1989–1996.
- Ayakannu, T.; Taylor, A.H.; Willets, J.M.; Konje, J.C. The evolving role of the endocannabinoid system in gynaecological cancer. Hum. Reprod. Update 2015, 21, 517–535.
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227.
- Bagavandoss, P.; Grimshaw, S. Temporal and spatial distribution of the cannabinoid receptors (CB1, CB2) and fatty acid amide hydroxylase in the rat ovary. Anat. Rec. (Hoboken) 2010, 293, 1425–1432.
- Cui, N.; Feng, X.; Zhao, Z.; Zhang, J.; Xu, Y.; Wang, L.; Hao, G. Restored plasma anandamide and endometrial expression of fatty acid amide hydrolase in women with polycystic ovary syndrome by the combination use of Diane-35 and metformin. Clin. Ther. 2017, 39, 751–758.
- Pirone, A.; Lenzi, C.; Briganti, A.; Abbate, F.; Levanti, M.; Abramo, F.; Miragliotta, V. Spatial distribution of cannabinoid receptor 1 and fatty acid amide hydrolase in the cat ovary and oviduct. Acta Histochem. 2017, 119, 417–422.
- Schuel, H. Tuning the oviduct to the anandamide tone. J. Clin. Investig. 2006, 116, 2087–2090.
- Wang, H.; Guo, Y.; Wang, D.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; DuBois, R.N.; Dey, S.K. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat. Med. 2004, 10, 1074–1080.
- Bambang, K.N.; Karasu, T.; Gebeh, A.; Taylor, A.H.; Marczylo, T.H.; Lam, P.; Willets, J.M.; Konje, J.C. From fertilisation to implantation in mammalian pregnancy-modulation of early human reproduction by the endocannabinoid system. Pharmaceuticals 2010, 3, 2910–2929.
- Abolghasemi, A.; Dirandeh, E.; Ansari Pirsaraei, Z.; Shohreh, B. Dietary conjugated linoleic acid supplementation alters the expression of genes involved in the endocannabinoid system in the bovine endometrium and increases plasma progesterone concentrations. Theriogenology 2016, 86, 1453–1459.
- Dirandeh, E.; Ghaffari, J. Effects of feeding a source of omega-3 fatty acid during the early postpartum period on the endocannabinoid system in the bovine endometrium. Theriogenology 2018, 121, 141–146.
- Scotchie, J.G.; Savaris, R.F.; Martin, C.E.; Young, S.L. Endocannabinoid regulation in human endometrium across the menstrual cycle. Reprod. Sci. 2015, 22, 113–123.
- Shen, X.; Duan, H.; Wang, S.; Gan, L.; Xu, Q.; Li, J.J. Decreased expression of cannabinoid receptors in the eutopic and ectopic endometrium of patients with adenomyosis. Biomed. Res. Int. 2019, 2019, 5468954.
- Neradugomma, N.K.; Drafton, K.; Mor, G.G.; Mao, Q. Marijuana-derived cannabinoids inhibit uterine endometrial stromal cell decidualization and compromise trophoblast-endometrium cross-talk. Reprod. Toxicol. 2019, 87, 100–107.
- Neradugomma, N.K.; Drafton, K.; O’Day, D.R.; Liao, M.Z.; Han, L.W.; Glass, I.A.; Mao, Q. Marijuana use differentially affects cannabinoid receptor expression in early gestational human endometrium and placenta. Placenta 2018, 66, 36–39.
- Taylor, A.H.; Amoako, A.A.; Bambang, K.; Karasu, T.; Gebeh, A.; Lam, P.M.; Marzcylo, T.H.; Konje, J.C. Endocannabinoids and pregnancy. Clin. Chim. Acta 2010, 411, 921–930.
- Rapino, C.; Battista, N.; Bari, M.; Maccarrone, M. Endocannabinoids as biomarkers of human reproduction. Hum. Reprod. Update 2014, 20, 501–516.
- Maia, J.; Fonseca, B.M.; Teixeira, N.; Correia-da-Silva, G. The fundamental role of the endocannabinoid system in endometrium and placenta: Implications in pathophysiological aspects of uterine and pregnancy disorders. Hum. Reprod. Update 2020.
- Maccarrone, M. Endocannabinoids: Friends and foes of reproduction. Prog. Lipid Res. 2009, 48, 344–354.
- Maccarrone, M. Endocannabinoids and reproductive biology. Hum. Reprod. 2009, 24, 1771.
- Wang, H.; Dey, S.K.; Maccarrone, M. Jekyll and hyde: Two faces of cannabinoid signaling in male and female fertility. Endocr. Rev. 2006, 27, 427–448.
- Kisgeropoulis, E. Inhibition of Ovarian Cancer Cell Proliferation by Oleoylethanolamide and Its Metabolically Stable Analog AM3102. Ohio State, Researchgate.net. 2013. Available online: https://www.researchgate.net/publication/278025965_Inhibition_of_Ovarian_Cancer_Cell_Proliferation_by_Oleoylethanolamide_and_its_Metabolically_Stable_Analog_AM3102 (accessed on 23 December 2020).
- Bradshaw, H.B.; Allard, C. Endogenous cannabinoid production in the rat female reproductive tract is regulated by changes in the hormonal milieu. Pharmaceuticals 2011, 4, 933–949.
- Han, G.H.; Chay, D.B.; Nam, S.; Cho, H.; Chung, J.Y.; Kim, J.H. Prognostic significance of transient receptor potential vanilloid type 1 (TRPV1) and phosphatase and tension homolog (PTEN) in epithelial ovarian cancer. Cancer Genom. Proteom. 2020, 17, 309–319.
- Pineiro, R.; Maffucci, T.; Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 2011, 30, 142–152.
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.W.; Cravatt, B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010, 140, 49–61.
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. Fluctuation in anandamide levels from ovulation to early pregnancy in in-vitro fertilization-embryo transfer women, and its hormonal regulation. Hum. Reprod. 2009, 24, 1989–1998.
- Habayeb, O.; Taylor, A.; Bharkhada, R.; Taylor, D.; Bell, S.; Konje, J. Immunohistochemical localisation of cannabinoid receptor CB1 and fatty acid amide hydrolase (FAAH) in maternal and fetal tissues. Proceedings of British Congress of Obstetrics & Gynaecology Abstracts Book; Royal College of Obstetricians and Gynecologists: London, UK, 2004; p. 10.
- Contassot, E.; Tenan, M.; Schnuriger, V.; Pelte, M.F.; Dietrich, P.Y. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol. Oncol. 2004, 93, 182–188.
- Eichele, K.; Ramer, R.; Hinz, B. R(+)-methanandamide-induced apoptosis of human cervical carcinoma cells involves a cyclooxygenase-2-dependent pathway. Pharm Res. 2009, 26, 346–355.
- Yan, L.; Li, J.; Zhao, T.; Wang, H.; Lai, G. [Over-expression of cannabinoid receptor 2 induces the apoptosis of cervical carcinoma Caski cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015, 31, 758–762.
- Han, G.H.; Chay, D.B.; Nam, S.; Cho, H.; Chung, J.Y.; Kim, J.H. The combination of transient receptor potential vanilloid type 1 (TRPV1) and phosphatase and tension homolog (PTEN) is an effective prognostic biomarker in cervical cancer. Int. J. Gynecol. Pathol. 2020.
- Fonseca, B.M.; Cunha, S.C.; Goncalves, D.; Mendes, A.; Braga, J.; Correia-da-Silva, G.; Teixeira, N.A. Decidual NK cell-derived conditioned medium from miscarriages affects endometrial stromal cell decidualisation: Endocannabinoid anandamide and tumour necrosis factor-alpha crosstalk. Hum. Reprod. 2020, 35, 265–274.
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Maccarrone, M.; Konje, J.C. Identification of novel predictive biomarkers for endometrial malignancies: N-Acylethanolamines. Front. Oncol. 2019, 9, 430.
- Ayakannu, T.; Taylor, A.; Willets, J.; Marczylo, T.; Brown, L.; Davies, Q.; Moss, E.; Konje, J. Effect of anandamide on endometrial adenocarcinoma (Ishikawa) cell numbers: Implications for endometrial cancer therapy. Lancet 2015, 385 (Suppl. 1), S20.
- Ayakannu, T.; Taylor, A.H.; Davies, Q.; Moss, E.L.; Konje, J.C. Optimisation of uniplex and duplex reactions is not required for real-time PCR amplification of target genes in endometrial cancer. Insights Obstet. Gynaecol. 2017, 1, 5.1. Available online: https://www.researchgate.net/publication/347910796 (accessed on 24 December 2020).
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Cannabinoid receptor expression in estrogen-dependent and estrogen-independent endometrial cancer. J. Recept. Signal. Transduct. Res. 2018, 38, 385–392.
- Ayakannu, T.; Taylor, A.H.; Willets, J.M.; Brown, L.; Lambert, D.G.; McDonald, J.; Davies, Q.; Moss, E.L.; Konje, J.C. Validation of endogenous control reference genes for normalizing gene expression studies in endometrial carcinoma. Mol. Hum. Reprod 2015, 21, 723–735.
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928.
- Risinger, J.I.; Maxwell, G.L.; Chandramouli, G.V.; Jazaeri, A.; Aprelikova, O.; Patterson, T.; Berchuck, A.; Barrett, J.C. Microarray analysis reveals distinct gene expression profiles among different histologic types of endometrial cancer. Cancer Res. 2003, 63, 6–11.
- Wang, H.; Xie, H.; Sun, X.; Kingsley, P.J.; Marnett, L.J.; Cravatt, B.F.; Dey, S.K. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins Other Lipid Mediat. 2007, 83, 62–74.
- Sankaranarayanan, R.; Ferlay, J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 207–225.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Konje, J.C. New insights of uterine leiomyoma pathogenesis: Endocannabinoid system. Med. Sci. Monit. Basic Res. 2019, 25, 76–87.
- Fugedi, G.; Molnar, M.; Rigo, J., Jr.; Schonleber, J.; Kovalszky, I.; Molvarec, A. Increased placental expression of cannabinoid receptor 1 in preeclampsia: An observational study. BMC Pregnancy Childbirth 2014, 14, 395.
- Maccarrone, M.; Valensise, H.; Bari, M.; Lazzarin, N.; Romanini, C.; Finazzi-Agro, A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet 2000, 355, 1326–1329.
- Ayakannu, T.; Taylor, A.H.; Bari, M.; Mastrangelo, N.; Maccarrone, M.; Konje, J.C. Expression and function of the endocannabinoid modulating enzymes fatty acid amide hydrolase and N-acylphosphatidylethanolamine-specific phospholipase D in endometrial carcinoma. Front. Oncol. 2019, 9, 1363.
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB(1) and CB(2). Pharmacol. Rev. 2010, 62, 588–631.
- Jove, M.; Gatius, S.; Yeramian, A.; Portero-Otin, M.; Eritja, N.; Santacana, M.; Colas, E.; Ruiz, M.; Pamplona, R.; Matias-Guiu, X. Metabotyping human endometrioid endometrial adenocarcinoma reveals an implication of endocannabinoid metabolism. Oncotarget 2016, 7, 52364–52374.
- Leconte, M.; Nicco, C.; Ngo, C.; Arkwright, S.; Chereau, C.; Guibourdenche, J.; Weill, B.; Chapron, C.; Dousset, B.; Batteux, F. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am. J. Pathol. 2010, 177, 2963–2970.
- Sun, X.; Dey, S.K. Endocannabinoid signaling in female reproduction. ACS Chem. Neurosci. 2012, 3, 349–355.
- Wang, H.; Xie, H.; Dey, S.K. Endocannabinoid signaling directs periimplantation events. AAPS J. 2006, 8, E425–E432.
- Bachkangi, P.; Taylor, A.H.; Bari, M.; Maccarrone, M.; Konje, J.C. Prediction of preterm labour from a single blood test: The role of the endocannabinoid system in predicting preterm birth in high-risk women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 1–6.
- Bari, M.; Battista, N.; Pirazzi, V.; Maccarrone, M. The manifold actions of endocannabinoids on female and male reproductive events. Front. Biosci. (Landmark Ed.) 2011, 16, 498–516.
- Habayeb, O.M.; Taylor, A.H.; Evans, M.D.; Cooke, M.S.; Taylor, D.J.; Bell, S.C.; Konje, J.C. Plasma levels of the endocannabinoid anandamide in women—A potential role in pregnancy maintenance and labor? J. Clin. Endocrinol. Metab. 2004, 89, 5482–5487.
- Green, M. Cannabis and Your Vagina: Here’s the 411 on Cannabis Suppositories. Available online: https://www.byrdie.com/how-to-use-cannabis-suppositories-4771875 (accessed on 26 June 2020).
- Harper-Gold, C. How to Get Your Vagina High. Available online: https://greenrushdaily.com/women/get-vagina-high/ (accessed on 26 June 2020).
- Lowry, J. Cannabinoids Can Cause Cell Death in Endometrial Cancer. Available online: https://www.cannahealth.org/cannabinoids-can-cause-cell-death-in-endometrial-cancer/ (accessed on 26 June 2020).
- Moore, G.R. Menopause: Cannabinoids & Sexual Health. Available online: https://www.foriawellness.com/blogs/learn/cbd-thc-sexual-health-menopause (accessed on 26 June 2020).
- Smith, D. Cannabis for Endometrial Cancer. Available online: https://cannabis.net/blog/medical/cannabis-for-endometrial-cancer (accessed on 26 June 2020).
- Trassoff-Jilg, V. Cannabinoids and Endometrial Cancer. Available online: https://waayb.com/cannabinoids-and-endometrial-cancer/ (accessed on 26 June 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30.
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 211, P234.E1–P234.E6.
- Xu, Y.; Xiao, Y.J.; Baudhuin, L.M.; Schwartz, B.M. The role and clinical applications of bioactive lysolipids in ovarian cancer. J. Soc. Gynecol. Investig. 2001, 8, 1–13.
- Hofmann, N.A.; Yang, J.; Trauger, S.A.; Nakayama, H.; Huang, L.; Strunk, D.; Moses, M.A.; Klagsbrun, M.; Bischoff, J.; Graier, W.F. The GPR 55 agonist, L-alpha-lysophosphatidylinositol, mediates ovarian carcinoma cell-induced angiogenesis. Br. J. Pharmacol. 2015, 172, 4107–4118.
- Deng, H.; Li, W. Monoacylglycerol lipase inhibitors: Modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders. Acta Pharm. Sin. B 2020, 10, 582–602.
- Fraguas-Sanchez, A.I.; Torres-Suarez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernandez-Carballido, A. PLGA nanoparticles for the intraperitoneal administration of CBD in the treatment of ovarian cancer: In Vitro and In Ovo assessment. Pharmaceutics 2020, 12, 439.
- Gebeh, A.K.; Willets, J.M.; Bari, M.; Hirst, R.A.; Marczylo, T.H.; Taylor, A.H.; Maccarrone, M.; Konje, J.C. Elevated anandamide and related N-acylethanolamine levels occur in the peripheral blood of women with ectopic pregnancy and are mirrored by changes in peripheral fatty acid amide hydrolase activity. J. Clin Endocrinol. Metab. 2013, 98, 1226–1234.
- Gervasi, M.G.; Marczylo, T.H.; Lam, P.M.; Rana, S.; Franchi, A.M.; Konje, J.C.; Perez-Martinez, S. Anandamide levels fluctuate in the bovine oviduct during the oestrous cycle. PLoS ONE 2013, 8, e72521.
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17.
- Leslie, K.K.; Thiel, K.W.; Goodheart, M.J.; De Geest, K.; Jia, Y.; Yang, S. Endometrial cancer. Obstet. Gynecol. Clin. N. Am. 2012, 39, 255–268.
- Tingaker, B.K.; Ekman-Ordeberg, G.; Facer, P.; Irestedt, L.; Anand, P. Influence of pregnancy and labor on the occurrence of nerve fibers expressing the capsaicin receptor TRPV1 in human corpus and cervix uteri. Reprod. Biol. Endocrinol. 2008, 6, 8.
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910.
- International Agency for Research on Cancer [Internet]. GLOBOCAN 2012 v1.0 (2013): IARC Publications Website—Cancer Today (powered by GLOBOCAN 2018)–IARC CancerBase No. 15. Available online: https://publications.iarc.fr/577 (accessed on 24 December 2020).
- Fonseca, B.M.; Teixeira, N.A.; Almada, M.; Taylor, A.H.; Konje, J.C.; Correia-da-Silva, G. Modulation of the novel cannabinoid receptor-GPR55-during rat fetoplacental development. Placenta 2011, 32, 462–469.
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966.
- Barrie, A.M.; Gushue, A.C.; Eskander, R.N. Dramatic response to Laetrile and cannabidiol (CBD) oil in a patient with metastatic low grade serous ovarian carcinoma. Gynecol. Oncol. Rep. 2019, 29, 10–12.
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 335.
- American Cancer Society. Key Statistics for Vaginal Cancer. Available online: https://www.cancer.org/cancer/vaginal-cancer/about/key-statistics.html (accessed on 26 June 2020).
- Rosado, J. Vaginal Cancer. Available online: https://www.marijuanadoctors.com/conditions/vaginal-cancer/ (accessed on 26 June 2020).
- Rogers, L.J.; Cuello, M.A. Cancer of the vulva. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2) (Suppl. 2), 4–13.
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093.
- Stabile, C.; Gunn, A.; Sonoda, Y.; Carter, J. Emotional and sexual concerns in women undergoing pelvic surgery and associated treatment for gynecologic cancer. Transl. Androl. Urol. 2015, 4, 169–185.
- Del Rio, C.; Cantarero, I.; Palomares, B.; Gomez-Canas, M.; Fernandez-Ruiz, J.; Pavicic, C.; Garcia-Martin, A.; Luz Bellido, M.; Ortega-Castro, R.; Perez-Sanchez, C.; et al. VCE-004.3, a cannabidiol aminoquinone derivative, prevents bleomycin-induced skin fibrosis and inflammation through PPARgamma- and CB2 receptor-dependent pathways. Br. J. Pharmacol. 2018, 175, 3813–3831.
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human skin permeation of Delta8-tetrahydrocannabinol, cannabidiol and cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297.
- Fonseca, B.M.; Correia-da-Silva, G.; Taylor, A.H.; Lam, P.M.; Marczylo, T.H.; Konje, J.C.; Teixeira, N.A. Characterisation of the endocannabinoid system in rat haemochorial placenta. Reprod. Toxicol. 2012, 34, 347–356.
- Battista, N.; Bari, M.; Rapino, C.; Trasatti, F.; D’Agostino, A.; Maccarrone, M. Regulation of female fertility by the endocannabinoid system. Hum. Fertil. (Camb.) 2007, 10, 207–216.
- Battista, N.; Pasquariello, N.; Di Tommaso, M.; Maccarrone, M. Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 82–89.
- Maccarrone, M. Endocannabinoids and reproductive endocrinology. Curr. Opin. Investig. Drugs 2009, 10, 305–310.
- Habayeb, O.M.; Taylor, A.H.; Finney, M.; Evans, M.D.; Konje, J.C. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA 2008, 299, 1135–1136.
- Lockwood, C.J. Prediction of pregnancy loss. Lancet 2000, 355, 1292–1293.
- Costa, M.A.; Fonseca, B.M.; Marques, F.; Teixeira, N.A.; Correia-da-Silva, G. The psychoactive compound of Cannabis sativa, Delta(9)-tetrahydrocannabinol (THC) inhibits the human trophoblast cell turnover. Toxicology 2015, 334, 94–103.
- Frank, D.A.; Bauchner, H.; Parker, S.; Huber, A.M.; Kyei-Aboagye, K.; Cabral, H.; Zuckerman, B. Neonatal body proportionality and body composition after in utero exposure to cocaine and marijuana. J. Pediatrics 1990, 117, 622–626.
- Sidney, S. Cardiovascular consequences of marijuana use. J. Clin. Pharmacol. 2002, 42, 64S–70S.
- Habayeb, O.M.; Taylor, A.H.; Bell, S.C.; Taylor, D.J.; Konje, J.C. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology 2008, 149, 5052–5060.
- Fonseca, B.M.; Correia-da-Silva, G.; Taylor, A.H.; Lam, P.M.; Marczylo, T.H.; Bell, S.C.; Konje, J.C.; Teixeira, N.A. The endocannabinoid 2-arachidonoylglycerol (2-AG) and metabolizing enzymes during rat fetoplacental development: A role in uterine remodelling. Int. J. Biochem. Cell Biol. 2010, 42, 1884–1892.
- Costa, M.A.; Fonseca, B.M.; Keating, E.; Teixeira, N.A.; Correia-da-Silva, G. 2-arachidonoylglycerol effects in cytotrophoblasts: Metabolic enzymes expression and apoptosis in BeWo cells. Reproduction 2014, 147, 301–311.
- Taylor, A.H.; Abbas, M.S.; Bell, S.C.; Konje, J.C. The inhibitory effect of delta9-tetrahydrocannabinol on trophoblast cell proliferation and transcription is mediated via the CB-2 receptor. Br. J. Obstet. Gynaecol. 2006, 114, 1040.
- Taylor, A.H.; Abbas, M.S.; Bell, S.C.; Konje, J.C. The inhibitory effect of delta9-tetrahydrocannabinol on trophoblast cell proliferation and transcription is mainly mediated via the CB-2 receptor. Reprod. Sci. 2007, 14, 720.
- Maia, J.; Midao, L.; Cunha, S.C.; Almada, M.; Fonseca, B.M.; Braga, J.; Goncalves, D.; Teixeira, N.; Correia-da-Silva, G. Effects of cannabis tetrahydrocannabinol on endocannabinoid homeostasis in human placenta. Arch. Toxicol. 2019, 93, 649–658.
- Sarrafpour, S.; Urits, I.; Powell, J.; Nguyen, D.; Callan, J.; Orhurhu, V.; Simopoulos, T.; Viswanath, O.; Kaye, A.D.; Kaye, R.J.; et al. Considerations and implications of cannabidiol use during pregnancy. Curr. Pain Headache Rep. 2020, 24, 38.
- Taylor, B.N.; Mueller, M.; Sauls, R.S. Cannaboinoid antiemetic therapy. In StatPearls; Statpearls Publishing: Treasure Island, FL, USA, 2020.
- Almada, M.; Amaral, C.; Oliveira, A.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N. Cannabidiol (CBD) but not tetrahydrocannabinol (THC) dysregulate in vitro decidualization of human endometrial stromal cells by disruption of estrogen signaling. Reprod. Toxicol. 2020, 93, 75–82.
- Feinshtein, V.; Erez, O.; Ben-Zvi, Z.; Erez, N.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Huleihel, M.; Holcberg, G. Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. PeerJ 2013, 1, e153.
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623.
