Eggshell is a biocompatible grafting material, with osteoconduction proprieties. It forms new bone similar to Bio-Oss and demineralized freeze-dried bone matrix. It can be combined with other materials to enhance its proprieties.
- eggshell
- guided bone regeneration
1. Introduction
Guided bone regeneration (GBR) is the method used in oral surgery to increase the volume of available host bone in sites chosen for dental implant therapy [1]. The original concept that led to the biological principles of guided tissue regeneration were developed as a desire to regenerate lost periodontal tissues [2][3]. This principle has become golden standard in situations with an inadequate volume of bone where dental implants are planned [4].
Avian eggshell has been introduced for a while now in the maxillofacial reconstructive surgery due to its mineral composition similar to coral (95% CaCO3) [5]. Reports show issues with the healing, to be more exact fibrous union [5][6][7]. Some authors have tried to surface-modify the eggshell to enhance its proprieties [8]. It is expected that the use of a derived material from eggshell (a bioresorbable CaCO3) may have several advantages due to its availability and biodegradability [7][8].
2. Study Selection
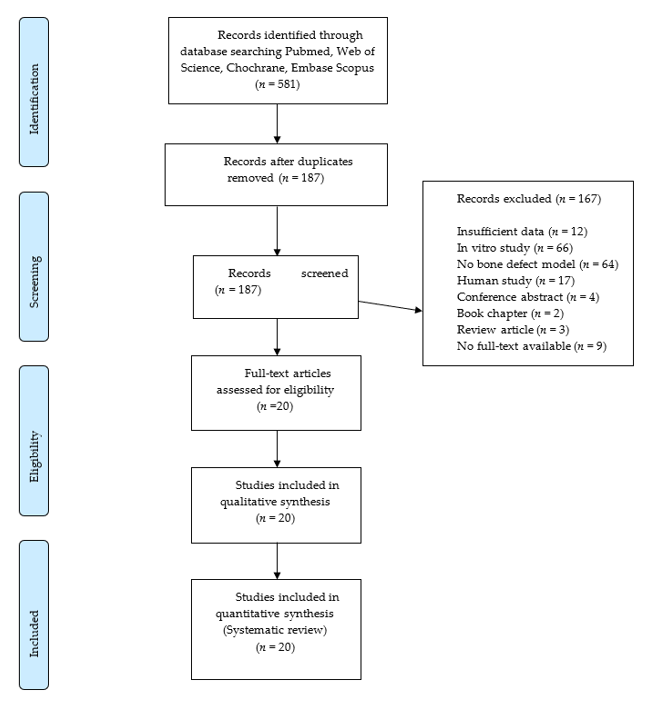
The electronic search provided 518 articles that were reduced to 187 after the duplicate removal. No further articles were identified by manual search. Screening of titles and abstracts led to the exclusion of 167 records. The full texts of the remaining 20 articles were obtained. These papers were analyzed systematically and quality-wise. The flow diagram of the search results is shown in Figure 1.
3. Study Characteristics
Only qualitative data was extracted from each study and it was synthetized in analytic tables. Table 1 and Table 2 summarize the definition of the critical sized defect in the selected studies. In eight of the included papers Wistar Rats were used [5][6][12][13][14][15][16][17], six studies used Sprague-Dawley rats [8][18][19][20][21][22] and seven New Zealand rabbits [6][23][24][25][26][27][28]. The calvaria critical-sized bone defect was used in 90% of the included papers to assess new bone formation (Table 1 and Table 2).
None of the studies included the use of eggshell scaffold with bone marrow-derived mesenchymal stem cells. Non-Resorbable membranes such as ePTFE (expanded polytetrafluoroethylene) were used for GBR in two studies [13][14]. Collagen resorbable membranes were used for guided bone regeneration in one study [17]. A paper [23] attempted to use eggshell membranes as a resorbable alternative in bone grafting. Histology was used in all studies to assess bone healing (n = 20), histomorphometry in 30% of papers (n = 6), microCT in 25% of papers (n = 5) and contact radiograph in 30% of papers (n = 6). Other less often used investigations include immunohistochemistry (n = 1) and fluorescent labeling (n = 1). Follow-ups ranged from 2 to 24 weeks. A single observation interval was reported for eight of the included studies [5][6][12][13][15][22][23][28], while the rest had multiple observation points.
Figure 1. Flowchart of the article selection procedure.
Table 1. Summary of characteristics and main results of studies in rats (n = 14).
|
|
Ref |
Lot |
Defect |
Biomaterials |
Control |
Other Materials/Treatments |
Assessment Time, Method(s) Main Findings |
|
WISTAR RAT |
[5] |
10 |
Mandible angle 5 mm ⌀ full THK bilateral |
Eggshell powder |
Split mouth empty defect |
N/S |
8 weeks Contact Rx: grafts were dense with a uniform distribution in the bony defect Histology: grafts were surrounded by a thin fibrous layer |
|
[13] |
45 |
Calvaria 6 mm ⌀ full THK bilateral |
Eggshell powder Group 1: CMC Group 2: PP + CMC Group 3: ES + PP + CMC |
Empty defect |
ePTFE membrane for each defect |
6 weeks Contact Rx: centripetal bone regeneration in group I, II; in group III the implant was surrounded by radiolucent border Histology: in group 1 and 2 partial bone healing, in group 3 ES displayed marked resorption with fibrous interposition. |
|
|
[6] |
10 |
Calvaria 7 mm ⌀ full THK bilateral |
Ostrich eggshell implant |
Empty defect |
N/S |
12 weeks Contact Rx: the graft was dense but with a surrounding radiolucency. Histology: dense capsule lining the outer and inner surface of implant; interposition of fibrous tissue at bone-implant interface. |
|
|
[16] |
5 |
Periodontal defect 1.5 × 6 mm bilateral |
Eggshell powder Eggshell membrane |
Split mouth DMBM Collagen membrane |
N/S |
6.5 weeks Histology: new bone formation at sides of the defect but more bone formation was noted in the control group; more connective tissue was observed in the graft group. |
|
|
[17] |
18 |
Calvaria 4 mm ⌀ full THK bilateral |
Eggshell brushite cement |
Pure brushite cement Empty with collagen |
Collagen membrane on the graft |
6–12 weeks microCT: EB degrades faster than PB; new bone formation from boundaries in the EB group. Histology: woven bone was observed for the EB group; PB group surrounded by inflammatory cells. Immunohistochemistry: confirmed new bone formation by osteopontin for EB > PB. |
|
|
[12] |
15 |
Calvaria 5 mm ⌀ full THK bilateral |
Coated eggshell particles (a) CaCO3/MgO/CMC2/BMP2 (b) CaCO3/MgO/CMC2 |
(c) Negative control |
N/S |
8 weeks microCT: (a), (b) covered with bone completely. Fluorescent labeling: (a) has the best new bone forming capabilities Histology: new bone formation in group (a) and (b) |
|
|
[15] |
40 |
Calvaria 7 mm ⌀ full THK |
(c) Ostrich eggshell powder (d) Ostrich eggshell implant |
(a) Empty control group |
(b) DMBM |
24 weeks Histology: group (b) showed the most new bone formation. No difference between groups (c) and (d) regarding the new bone formation. |
|
|
[14] |
45 |
Calvaria 6 mm ⌀ full THK bilateral |
Eggshell powder (c) |
Empty (a) |
ePTFE membrane for GBR in each group Periosteal graft (b) |
2–4–12 weeks Contact Rx: group (a) compete closure in 60% of cases; (b) no mineralization; (c) no resorption of the implant. Histology: complete closure for 60% of cases (a), no closure for 80% of cases (b) and no closure for all cases in group (c). |
|
|
SPRAGUE-DAWLEY RAT |
[8] |
30 |
Calvaria 5 mm ⌀ full THK bilateral |
Eggshell particles (c) Surface modified eggshell particles (d) (ES/CaP-1, ES/CaP-2, ES/CaP-3) |
Empty (a) |
Bio-Oss (b) |
4–8 weeks Histology: (a) no defect was filled. (b) bone healing incomplete, graft particles surrounded by fibrous tissue, no resorption of graft. (c) none had complete bone bridging, but two defects showed complete bony closure. (d) completely bone bridging was seen more often than in (b) and (c) Histomorphometry: bone formation superior to (d). |
|
[22] |
14 |
Calvaria 4 mm ⌀ full THK bilateral |
Microroughened ostrich eggshell particles (c) CaP coated ostrich eggshell particles (d) |
Empty (a) |
BioCoral (b) |
4 weeks Histology: (a) mainly fibrous tissue and bone was formed at defect margins; - new bone formation around grafted particles in the middle area and defect margin for (b), (c), (d). |
|
|
[19] |
56 |
Calvaria 8 mm ⌀ full THK |
Eggshell nano-hydroxyapatite (b) |
Empty (a) |
Bio-Oss (c) EHA with CS (d) |
6–12 weeks Histology: (a) new bone formation at the margins of the defect. (b) increased bone formation compared to (c) and (d). (c) bone formed from the defect margin with few bony islands in the center (d) new bone formation similar to Bio-Oss. |
|
|
[20] |
30 |
Calvaria 8 mm ⌀ full THK |
Eggshell hydroxyapatite (b) |
Empty (a) |
sHA (c) |
4–8 weeks Histology: new bone around de graft particles. (b) (c)—many foreign body multinucleated giant cells were observed surrounding the graft particles. MicroCT: bone volume was significantly higher in (b) |
|
|
[18] |
16 |
Calvaria 5mm ⌀ full THK bilateral |
Deproteinized ES (a) Hydrothermally treated ES (b) |
Empty |
Bio-Oss (c) |
4–8 weeks Histology: (c) new bone was shown in direct apposition to graft particles (b) almost complete bone healing at dura mater side. Histomorphometry: (b) showed significantly greater new bone formation than (c) |
|
|
[21] |
30 |
4 mm ⌀ half THK 4 defects: -two mandible -two maxilla |
ES + carrageenan gel (a) ES + xanthan gel (b) ES powder (c) |
Empty |
N/S |
2–4–6.5 weeks Histology: No inflammation at the end of study period. Complete defect healing occurred for group (a). |
⌀ = diameter; Contact Rx = contact radiograph; Rx = radiograph, REF. = reference, THK = thickness, N/S = not specified, ePTFE = expanded polytetrafluoroethylene, DMBM = demineralized freeze-dried bone matrix, GBR = guided bone regeneration, microCT = microcomputer tomography, EB = eggshell brushite, BMP 2 = bone morphogenetic protein 2, MgO = magnesium oxide, CaCO3 = calcium carbonate, CMC2 = Carboxymethyl chitosan, CT = computer tomography, ESP = eggshell particles, CaP = calcium phosphate, EHA = eggshell hydroxyapatite, CS = calcium sulfate, eHA = eggshell hydroxyapatite, sHA = synthetic hydroxyapatite, ES = eggshell, CMC = carboxymethyl cellulose, PP = pentosan polysulphate, EB = Eggshell brushite cement, PB = Pure brushite cement, ES = eggshell, CaP = Calcium Phosphate, (a), (b), (c), (d) = study groups in the selected study.
Table 2. Summary of characteristics and main results of studies in rabbits (n = 7).
|
Ref. |
Lot |
Defect |
Biomaterials(s) |
Control |
Other Materials/Treatments |
Analysis |
Assessment Method(s) AND Main Findings |
|
[23] |
18 |
Calvaria 6 mm ⌀ ½ THK (total of 3 defects) |
Ostrich eggshell powder |
Empty defect (n = 1 per rabbit) |
Outer shell membrane Inner shell membrane |
13 |
Contact Rx: membrane group displayed partial bone healing. In the grafted membrane advanced bone regeneration was present. Histology: bone regeneration was seen in the margins of the defect. No statistical difference in bone regeneration between grafted group with membrane or eggshell powder. |
|
[6] |
5 |
Calvaria 15 mm ⌀ full THK
|
Eggshell implant (interposition graft) |
No control |
No fixation or osteosynthesis |
24 |
Contact Rx: grafts were delineated from the surrounding bone by radiolucency. Histology: Similar to Rx, bone condensation was higher at bone-implant interface. There were no signs of remodeling. |
|
[24] |
18 |
Calvaria 6 mm ⌀ ½ THK (n = 6 defects in total per rabbit)
|
Ostrich eggshell particles of different size (grade 1, 2, 3, 4) – each rabbit was grafted with a material per defect (n = 5) |
Empty defect (n = 1 per rabbit) |
DBM |
4 12 24 |
Contact Rx: bone regeneration at the periphery of the empty defect; small eggshell particles were resorbed faster than larger ones. Higher grade and DBM groups show advanced bone regeneration at 24 weeks. Histology: No inflammatory reaction. DBM was completely resorbed and lamellar bone occupied the defect. Smaller eggshell particles were completely resorbed with lamellar bone surrounding it. Connective tissue infiltrated into larger particles of eggshell. Histomorphometry: DBM had the largest osseous area. Grade 3 particles followed closely. The empty defect had the least osseous area. Resorption rate of DBM was the highest and the eggshell was resorbed in a size-dependent manner. |
|
[25] |
16 |
Calvaria 8 mm ⌀ Full THK (n = 2 defects per rabbit) |
Eggshell nanohydroxyapatite |
Empty defect (n = 1 per rabbit) |
nHA+ silk fibroin |
4 8 |
microCT: there is a statistically significant difference between the control group and the grafted groups. The nHA+ silk fibroin showed more bone formation than the nHA group. Histomorphometry: nHA group showed good bone formation with well-organized lamellar bony islands. The space formed by silk degradation was replaced by new bone but the most area was occupied by poorly degraded biomaterial. |
|
[28] |
6 |
Calvaria 5 × 10 × 1 mm Full THK (n = 2 defects per rabbit) |
Membrell’s Bonehealth Plus (n = 3 rabbits with 6 defects) |
Empty defect (n = 3 rabbits with 6 defects) |
N/S |
2 |
Histology: deposition of osteoid newly formed bone trabeculae. No inflammatory cell infiltrate was present. The defects were free from any graft traces. Histomorphometry: larger area of newly formed bone was found in the experimental group |
|
[26] |
16 |
Calvaria 8 mm ⌀ Full THK (n = 2 defects per rabbit) |
Eggshell hydroxyapatite (assigned randomly with the sHA) |
Empty defect (n = 1 per rabbit) |
sHA |
4 8 |
Histomorphometry: no difference between the sHA and eHA groups regarding new bone formation. Both had low inflammatory response. |
|
[27] |
16 |
Calvaria 8 mm ⌀ Full THK (n = 2 defects per rabbit) |
Eggshell hydroxyapatite |
Empty defect |
N/S |
4 8 |
microCT: bone mineral content, bone mineral density, tissue mineral content, tissue mineral density were higher for experimental than control Histology: statistically significant more bone formation for the experimental group |
THK = thickness, N/S = not specified, EP = eggshell powder, EP = eggshell powder, Rx = radiograph, ESM = eggshell membrane, OSM/ISM = outer/inner shell membrane, DBM = demineralized bone matrix, sHA = synthetic hydroxyapatite, nHA = nano hydroxyapatite, eHA = eggshell hydroxyapatite, microCT = microcomputer tomograph.
Because chemical composition and processing technology are considered important factors for determining the benefit of using the biomaterial, they were analyzed and summarized in Table 3 for the hen and ostrich eggshell. The most employed methods of assessing the materials are scanning electron microscopy (55%), x-ray diffractometry (40%), Fourier-transform infrared spectroscopy (30%) and energy-dispersive x-ray spectroscopy (10%).
Table 3. Eggshell originating from Gallus gallus domesticus (Hen) and Struthio camelus (Ostrich) production method and main proprieties.
|
Ref. |
Biomaterial |
Production Method and Sterilization |
PROPRIETIES |
|||
|
Eggshell Originating from Gallus Gallus Domesticus (Hen) |
||||||
|
[5] |
Eggshell powder |
Eggshell crushed to powder (400–600 µm). Sterilization: Ethylene oxide |
- |
|||
|
[13] |
Eggshell powder |
Eggshell was cleaned, grounded to powder (100–200 µm), and bleached in 6% NaClO 24 h, after which it was washed. Sterilization: Autoclaving |
- |
|||
|
[14] |
Eggshell powder |
Eggshell stripped of membranes, then grounded to powder (400–600 µm). This was bleached in 6% NaClO 24 h. Sterilization: Autoclaving |
- |
|||
|
[18] |
Deproteinized eggshell (ES-1) Hydrothermally treated eggshell (ES-2, ES-3) |
(a) Particulate eggshell was prepared (300 µm), immersed in NaOCl and then HT 300 °C 24 h (ES-1). (b) Hydrothermally treated in phosphate buffered saline at 80 °C (ES-2) (c) Hydrothermally treated in di-ammonium phosphate solution at 150 °C 24 h (ES-2)
|
SEM: ES-2,3 showed microporous surface composed of platelet-like or rod like surface EDS: Ca/P atomic ratio Bio-Oss = 1.57 ± 0.41 ES-2 = 1.51 ± 0.20 ES-3 = 1.34 ± 0.09 XDR: Calcite peak of CaCO3 appeared in ES-1 FT-IR: in ES-2 and ES-3 showed sharp splitting of phosphate specific band |
|||
|
[8] |
Surface modified natural calcium carbonate eggshell |
(a) Fragmented ES were milled (300–500 µm), immersed in 5% NaClO, washed in deionized water and heat treated at 300 °C 24 h (ES). (b) Further treated with phosphate buffer saline (PBS) at 80 °C (ES/CaP-1). Soaked in di-ammonium phosphate solution at 150 °C 24 h (ES/CaP-2) (c) Soaked in a phosphate containing solution at 80 °C (ES/CaP-3). Sterilization: Gamma irradiation |
SEM: hydrothermally treated eggshell showed different surface morphology—platelet-like, needle-like or rod-like microstructure EDXA: atomic ratio of Ca/P showed lower values in the ES/CaP groups than in Bio-Oss XDR and FT-IR: indicates partial conversion of the calcite into hydroxyapatite MTT based assay: osteoblast cultured with surface modified ES showed significantly higher absorbance compared to Bio-Oss |
|||
|
[19] |
Nanohydroxyapatite derived from hen eggshell |
Fragmented eggshell (300 µm) was immersed in NaClO. Further they are treated with di-ammonium phosphate solution at 180 °C to make N-HA. prepare a bone substitute composed of outer HA layer and inner CaCO3 core. Sterilization: Gamma irradiation |
XDR and FT-IR: indicate that eggshell (CaCO3 in calcite form) converted partially to HA. XDR shows strong peaks of HA and weak peaks of original calcite in N-HA. FT-IR: phosphate band resulted from the newly formed HA structure These results indicate that N-HA in composed of outer HA and inner CaCO3. |
|||
|
[25] |
Nanohydroxyapatite derived from eggshells with or without silk fibroin scaffolds |
Raw eggshells underwent calcination at 900 °C 3 h where were crushed and treated with H3PO4. The powder was milled 10 h in ethanol and pressed at 220 MPa. Then it was sintered at 900 °C 2 h. A Silk fibroin sponge was dipped into a supersaturated solution of N-HA for 1 h. |
SEM: particles of N-HA showed rectangular shape, some were aggregated; The silk fibroin scaffold was web shaped, with highly porous structures with a round shape at the end. When the N-HA was precipitated into the silk fibroin, the particles were evenly distributed to the surface of the scaffold. |
|||
|
[26] |
Hydroxyapatite derived from eggshells |
- |
SEM: sHA had consistent particle size of < 1 µm whereas eHA had > 1 µm The surface roughness of sHA > eHA. Particle size sHA > eHA FT-IR: sHA characteristic vibrational model for PO43− eHA PO43− were still detected as a major component and the absorbed water was largely reduced compared to sHA XDR: patterns of the sHA and eHA samples matched well the characteristic hexagonal phase of HA. eHA has impure phases of CaO and Ca(OH)2. |
|||
|
[21] |
Eggshell derived calcium carbonate |
Eggshells were cleaned, were crushed to 1 mm porosity of 75%. They were autoclaved. Eggshell derived calcium carbonate combined with carrageenan gel Eggshell derived calcium carbonate combined with xanthan gum gel Eggshell derived calcium carbonate powder |
- |
|||
|
[27] |
Eggshell derived hydroxyapatite |
Eggshells were cleaned and heat treated at 900 °C. The shells were crushed and milled to synthesize calcium phosphate powders. |
SEM: grain size of 100–200 nm TEM: heat treated HA powder consists of two different nanograins: globular (200 nm) and “nanostructured” (70 nm) XDR: the main phase is hydroxyapatite. The minor phase of HA powder after milling is hydrogen phosphate, monetite and calcite. |
|||
|
[20] |
Hydroxyapatite from eggshells |
Eggshells are washed and calcinated at 900 °C for 3 h. Then they are crushed and milled. They are then reacted with phosphoric acid. These mixtures were milled again and sintered at 900 °C. |
SEM: similar structure with the seashell HA. Average particle size is 0.8 µm × 0.5 µm. XDR: the main phase in seashell and eggshell HA was identified as HA. FT-IR: characteristic absorption of HA was observed in both samples ICP-OES: sodium and strontium contents were higher for the seashell HA. A higher magnesium content was found in eggshell HA. |
|||
|
[17] |
Eggshell derived brushite cement (EB) |
Eggshells were cleaned, rinsed, dried at 100 °C overnight and powdered. A mixture of eggshell powder (94% CaCO3) 1:2 was heated for 12 h to synthesize eggshell derived β-tricalcium phosphate (ETCP). Powder component of pure brushite (PB) cement was made by mixing pure β-tricalcium phosphate (PTCP) with mono calcium phosphate monohydrate (MCPM) 1:1. Powder component of EB was made by mixing ETCP and MCPM. |
XDR: results confirm the formation of the brushite phase FT-IR: presence of the characteristic peaks of brushite ICP-AES: it found trace levels of magnesium and sodium other than Ca and P elements. SEM: formation of large thin plate-like brushite crystals in the pure brushite cement. In the EB the crystals were found to be smaller in size with irregular morphology. |
|||
|
[16] |
Eggshell powder |
Eggshells were washed and rinsed. They were crushed to 1 mm diameter. After they were rinsed at 370 °C. Sterilization: Autoclaving |
- |
|||
|
[28] |
Membrell’s Bonehealth Plus |
It is an eggshell based commercially available over the counter dietary supplement for bone health. |
TEM: particle size < 50 nm |
|||
|
[12] |
CaCO3/MgO powder |
Eggshell powder and magnesium acetate were dissolved and stirred for 3 h and then calcinated at 600 °C for 3 h. |
XDR: pure phase of MgO and CaCO3. The MgO nanoparticles were conjugated well on the surface of eggshell. SEM: 10–100 µm particles and good dispersion proprieties. Porous structure of individual particles of 200 to 400 nm. Successful uniform loading of MgO particles (5 nm). TEM: successful loading of MgO particles on the surface of the eggshell. |
|||
|
CaCO3/MgO/CMC/BMP2 scaffold |
Carboxymethyl chitosan (CMC) was dissolved in CaCO3/MgO solution. BMP2 was added to the mixture. |
FTIR: amide bone is persistent in the scaffold, existence of carboxymethyl groups, CaCO3 SEM: interconnected porous architecture, pore size of 50–80 µm ELISA: BMP2 could sustainably release from chemically crosslinked scaffold in 4 weeks. Compression load test: compression strength higher than CMC scaffold |
||||
|
Eggshell Originating from Struthio Camelus (Ostrich) |
||||||
|
[6] |
Block 2 mm thick |
The shell was immersed in 10% sodium hypochlorite for 24 h. They were washed and autoclaved. |
- |
|||
|
[23] |
375 |
Eggshell was ground, washed, dried, and sterilized using ethylene oxide. |
- |
|||
|
[22] |
300–500 |
Particles were obtained using mill and sieve, followed by immersion in sodium hypochlorite solution for 48 h. After it was washed. Treatment: Alkaline etching (microroughened-OES) Immersion in supersaturated calcification solution (CaP coated OES) All particles were sterilized using gamma irradiation. |
SEM: alkaline etching increased surface area; calcium phosphate coating showed platelet-like morphology of crystals. |
|||
|
[24] |
700–1500 |
Small pieces of eggshells were put into Petri dish with glutaraldehyde for 24 h. After they were washed and ground. They were sterilized using ethylene oxide. |
- |
|||
|
[15] |
20–150
or
Block 7 mm Ø
|
Inner and outer membranes were removed. The eggshells were broken into small pieces of 7 mm diameter. Powder was then prepared with electrical burr. Sterilization was done with ethylene oxide. |
- SEM: outer surface of ostrich eggshell resembles compact bone and inner surface resembles trabecular bone. |
|||
REF = reference, HT = heat treatment, ES-1 = Deproteinized eggshell, ES-2/ES-3 = Hydrothermally treated eggshell, NaClO = sodium hypochlorite, NaOH = sodium hydroxide, H3PO4 = phosphoric acid, SEM = scanning electron microscopy, TEM = transmission electron microscopy, EDS = energy dispersive spectroscopy, EDXA = energy-dispersive X-ray analysis, XDR = X-ray Diffractometry, FT-IT = Fourier-transform infrared absorbance spectra, ICP-OES = plasma optical emission spectroscopy, ICP-AES = inductively coupled plasma atomic emission spectroscopy, Membrell’s Bonehealth Plus = commercially available eggshell supplement, Ø = diameter, CaCO3 = calcium carbonate, MgO = magnesium oxide, CMC = carboxymethyl chitosan, BMP2 = bone morphogenetic proteins 2, PBS = phosphate buffer saline, CaP = calcium phosphate, ES = eggshell, Ca = calcium, P = phosphate, N-HA = nanohydroxyapatite, HA = hydroxyapatite, eHA = eggshell derived hydroxyapatite, sHA = synthetic hydroxyapatite, Ca(OH)2 = calcium hydroxide, EB = Eggshell derived brushite cement, ETCP = eggshell derived β-tricalcium phosphate, PB = pure brushite, PTCP = pure β-tricalcium phosphate, MCPM = mono calcium phosphate monohydrate, CMC = Carboxymethyl chitosan, OES = ostrich eggshell.
A variety of production methods are described for the eggshell grafts, starting with eggshell blocks [6][15]. 75% of the included studies produced the biomaterial by crushing and milling eggshell into a powder. Calcination is being used in 35% of the cases to treat eggshell to improve its bone regeneration capabilities (n = 7). Hydrothermally treating the grafts greatly improves the porosity and structure of the eggshell [8][18]. A study [25] designed the use of silk fibroin scaffolds with eggshell to try to increase the proprieties of the biomaterial. Others [21] used a combination of carrageenan gel, xanthan gum gel to manufacture new biomaterials. Pure brushite cement was used [17] to find the best cement as bone augmentation material.
One study employed a commercially available nutritional supplement derived from eggshells used to promote healthy bones [28]. One study used a scaffold with carboxymethyl chitosan and BMP2 to enhance bone regeneration [12]. The ostrich eggshells were used in particles ranging from 20 to 1500 µm size. Sterilization of the products was done by either autoclaving, gamma irradiation or ethylene oxide.
This entry is adapted from the peer-reviewed paper 10.3390/biology9120476
References
- Buser, D.; Dula, K.; Belser, U.C.; Hirt, H.P.; Berthold, H. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. J. Periodontics Restor. Dent. 1995, 15, 10–29.
- Hämmerle, C.H.F.; Karring, T. Guided bone regeneration at oral implant sites. 2000 1998, 17, 151–175, doi:10.1111/j.1600-0757.1998.tb00132.x.
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Oral Implants Res. 2010, 21, 567–576, doi:10.1111/j.1600-0501.2010.01922.x.
- Benic, G.I.; Hämmerle, C.H.F. Horizontal bone augmentation by means of guided bone regeneration. 2000 2014, 66, 13–40, doi:10.1111/prd.12039.
- Dupoirieux, L.; Pourquier, D.; Souyris, F.F.; Surgery, M.; Prof, H.; Surgery, E.; Prof, H. Powdered eggshell: A pilot study on a new bone substitute for use in maxillofacial surgery. Cranio-Maxillofac. Surg. 1995, 23, 187–194, doi:10.1016/S1010-5182(05)80009-5.
- Dupoirieux, L. Ostrich eggshell as a bone substitute: A preliminary report of its biological behaviour in animals--a possibility in facial reconstructive surgery. J. Oral Maxillofac. Surg. 1999, 37, 467–71, doi:10.1054/bjom.1999.0041.
- Opris, H.; Bran, S.; Dinu, C.; Baciut, M.; Prodan, D.A.; Mester, A.; Baciut, G. Clinical applications of avian eggshell-derived hydroxyapatite. J. Basic Med. Sci. 2020, 20, 430–437, doi:10.17305/bjbms.2020.4888.
- Park, J.-W.; Bae, S.-R.; Suh, J.-Y.; Lee, D.-H.; Kim, S.-H.; Kim, H.; Lee, C.-S. Evaluation of bone healing with eggshell-derived bone graft substitutes in rat calvaria: A pilot study. Biomed. Mater. Res. Part A 2008, 87A, 203–214, doi:10.1002/jbm.a.31768.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097, doi:10.1371/journal.pmed.1000097.
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. J. Pharmacol. 2010, 160, 1577–1579, doi:10.1111/j.1476-5381.2010.00872.x.
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, doi:10.1186/1471-2288-14-43.
- Huang, Y.; Ji, Y.; Kang, Z.; Li, F.; Ge, S.; Yang, D.-P.; Ruan, J.; Fan, X. Integrating eggshell-derived CaCO3/MgO nanocomposites and chitosan into a biomimetic scaffold for bone regeneration. Eng. J. 2020, 395, 125098, doi:10.1016/j.cej.2020.125098.
- Dupoirieux, L.; Pourquier, D.; Picot, M.-C.C.; Neves, M. The effect of pentosan polysulphate on bone healing of rat cranial defects. Craniomaxillofac. Surg. 1999, 27, 314–320, doi:10.1054/jcms.1999.0901.
- Dupoirieux, L.; Neves, M.; Pourquier, D. Comparison of pericranium and eggshell as space fillers used in combination with guided bone regeneration: An experimental study. Oral Maxillofac. Surg. 2000, 58, 40–6, discussion 47–48, doi:10.1016/s0278-2391(00)80013-0.
- Uygur, S.; Ozmen, S.; Kandal, S.; Lortlar, N.; Omeroglu, S.; Arac, M.; Cenetoglu, S. Reconstruction of cranial bone defects using Struthio camelus eggshell. Craniofac. Surg. 2011, 22, 1843–1846, doi:10.1097/SCS.0b013e31822e83e4.
- Kavarthapu, A.; Malaiappan, S. Comparative evaluation of demineralized bone matrix and type II collagen membrane versus eggshell powder as a graft material and membrane in rat model. Indian J. Dent. Res. 2019, 30, 877–880, doi:10.4103/ijdr.IJDR_489_17.
- Jayasree, R.; Kumar, T.S.S.; Venkateswari, R.; Nankar, R.P.; Doble, M. Eggshell derived brushite bone cement with minimal inflammatory response and higher osteoconductive potential. Mater. Sci. Mater. Med. 2019, 30, 113, doi:10.1007/s10856-019-6315-x.
- Bae, S.R.; Park, J.W.; Ahn, C.H.; Suh, J.Y. In Vivo Evaluation of Hydrothermally Converted Hydroxyapatite as a Bone Graft Substitute. Key Eng. Mater. 2007, 330–332, 135–138, doi:10.4028/www.scientific.net/KEM.330-332.135.
- Park, J.-W.; Jang, J.; Bae, S.-R.; An, C.-H.; Suh, J.-Y. Bone formation with various bone graft substitutes in critical-sized rat calvarial defect. Oral Implants Res. 2009, 20, 372–378, doi:10.1111/j.1600-0501.2008.01602.x.
- Lee, S.-W.; Balázsi, C.; Balázsi, K.; Seo, D.; Kim, H.S.; Kim, C.-H.; Kim, S.-G. Comparative Study of hydroxyapatite prepared from seashells and eggshells as a bone graft material. Tissue Eng. Regen. Med. 2014, 11, 113–120, doi:10.1007/s13770-014-0056-1.
- Uraz, A.; Gultekin, S.E.S.E.; Senguven, B.; Karaduman, B.; Sofuoglu, I.P.I.P.; Pehlivan, S.; Capan, Y.; Eren, K. Histologic and Histomorphometric Assessment of Eggshell-Derived Bone Graft Substitutes on Bone Healing in Rats. Clin. Exp. Dent. 2013, 5, doi:10.4317/jced.50968.
- Park, J.; Lee, C.; Choi, B.; Suh, J. Evaluation of natural calcium carbonate with surface modification as a bone graft substitute. Key Eng. Mater. 2006, 309-311 I, 183–186.
- Durmuş, E.; Celik, I.; Ozturk, A.; Ozkan, Y.; Aydin, M.F. Evaluation of the potential beneficial effects of ostrich eggshell combined with eggshell membranes in healing of cranial defects in rabbits. Int. Med. Res. 2003, 31, 223–30, doi:10.1177/147323000303100309.
- Durmuş, E.; Çelik, İ.; Aydın, M.F.; Yıldırım, G.; Sur, E. Evaluation of the biocompatibility and osteoproductive activity of ostrich eggshell powder in experimentally induced calvarial defects in rabbits. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86B, 82–89, doi:10.1002/jbm.b.30990.
- Kweon, H.; Lee, K.-G.; Chae, C.-H.; Balázsi, C.; Min, S.-K.; Kim, J.-Y.; Choi, J.-Y.; Kim, S.G. Development of nano-hydroxyapatite graft with silk fibroin scaffold as a new bone substitute. Oral Maxillofac. Surg. 2011, 69, 1578–1586, doi:10.1016/j.joms.2010.07.062.
- Lee, S.-W.; Kim, S.-G.; Balázsi, C.; Chae, W.-S.; Lee, H.-O. Comparative study of hydroxyapatite from eggshells and synthetic hydroxyapatite for bone regeneration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 348–355, doi:10.1016/j.tripleo.2011.03.033.
- Balázsi, C.; Gergely, G.; Balázsi, K.; Chae, C.H.; Sim, H.Y.; Choi, J.Y.; Kim, S.G. Bone Formation with Nano-Hydroxyapatite from Eggshell. Sci. Forum 2012, 729, 25–30, doi:10.4028/www.scientific.net/MSF.729.25.
- Salama, R.; Khashaba, M.; El Rouby, D.; El, D. Histomorphometric evaluation of a nano-sized eggshell-containing supplement as a natural alloplast: An animal study. Saudi Dent. J. 2019, 31, 375–381, doi:10.1016/j.sdentj.2019.03.011.