Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Propolis is a resinous mixture, made by the honeybees from substances collected from tree or other plant buds, plant exudates, or resins found in the stem, branches, or leaves of different plants.
- Poplar-Type Propolis
1. Introduction
Propolis is a bee product, made by the honeybees (Apis mellifera) from different resins, collected from plant leaves, buds, or exudates, mixed with bee saliva and wax [69]. This mixture is taken into the hive and used to protect the bee family from outside enemies, to bond the frames between them, to seal any hole in the hive, and to maintain a stable indoor temperature [21]. The color of propolis varies greatly with the botanical source and geographical origin. Poplar-type propolis color can vary from yellow orangish, to reddish and brown, or dark brown. Plant bud resins from Poplar species are primary sources for propolis from temperate zones (Europe, North America, and Asia), but also other species contribute to the chemistry of propolis from these areas (Betula sp., Acacia sp., Pinus sp., Salix, or Aesculus hippocastanum [70]. To the best of our knowledge, poplar type propolis have the widest spread along the globe, and its composition and properties are the most studied from all bee products, apart from honey.
Having such a large distribution over the temperate zones of the globe, poplar-type propolis is also very different in chemical compounds, although volatiles from the class of terpenoids and polyphenolic substances (phenolic acids and flavonoids) are the major compounds. A recent study by [71], identified a new type of propolis rich in flavonoids which exhibit also a very powerful antibacterial activity.
Different studies on the chemical composition of propolis have made possible the classification of propolis from different countries, knowing the fact that European propolis have as the main vegetal source, the exudates from different Populus species. It is generally accepted that propolis from temperate zones are rich in pinocembrin, pinobanksin, galangin, chrysin, caffeic, and ferulic acids; these are all phenolics reported in Poplar exudates. Miguel (2013) [53] reviewed the propolis type of countries from the Mediterranean basin. Italian propolis samples analysis revealed the presence of phenolic acids and flavonoids as the main components and concluded that poplar-type propolis was characteristic to Italy [72,73]. Other studies [74,75] have reported also phenolic acids and their esters and flavonoids in propolis samples from France. Hydroalcoholic extracts of propolis from Spain, revealed the presence of flavonoids as predominant components, demonstrating the poplar appurtenance [76,77]. Portuguese propolis characterization revealed methylated and/or esterified or hydroxylated derivatives of poplar flavonoids [53,78,79].
1.1. Chemical Composition and Analysis Methods
Propolis is known to be a very important natural antibiotic. Its properties were observed before its chemical composition was really analyzed. Before the development of separation and purification techniques to reveal chemical components of propolis, the existing studies focused mostly on bioactive properties and mainly attributed its entire composition to these properties. After the mentioned techniques were used more and more, the chemical composition of propolis was established, and its properties were attributed to different classes of compounds originating from different geographical areas [42,53,55,78,80,81,82].
Generally speaking, poplar-type propolis have about 50% resins, 30% beeswax, 10% aromatic oils, 5% pollen, and 5% other substances (minerals, vitamins, and amino acids) [18], and, so far, more than 350 compounds have been identified and quantified [83,84].
Different scientific studies have classified these components as phenolic acids and their esters, all classes of flavonoids (aglycones and glycosides), chalcones and dihydrochalcones, terpenes and hydrocarbons, alcohols and their esters, aldehydes, amino acids, fatty acids, sterols, sugars, and sugar alcohols [83]. The majority of these substances came from resins, plant exudates, but also from bee metabolism. Sugars and pollen came from cross contamination with nectar and the fatty component of propolis (fatty acids, esters, and glycerol) came from beeswax [83]. The major compounds of poplar-type propolis all over the world are presented in Table 1. As can be seen in the table, the majority of compounds belong to polyphenolic substances.
Table 1. Chemical composition of poplar-type propolis of the major producing poplar-type propolis of the world.
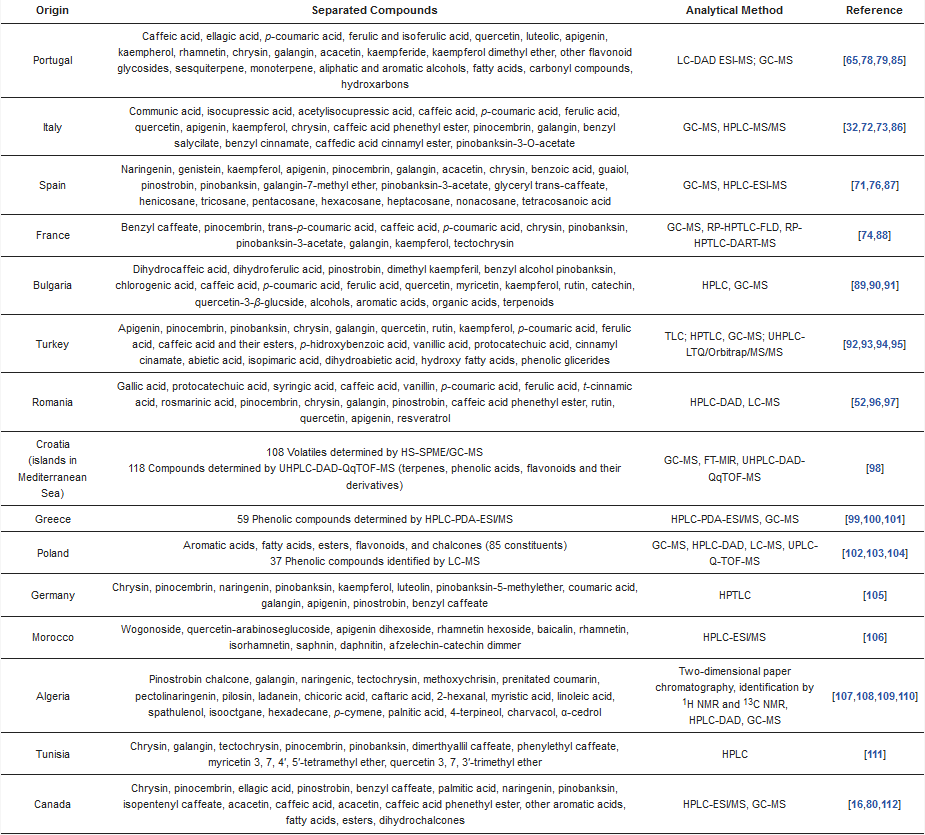
Over time, the methods used for propolis analysis have evolved significantly. Due to the nature of the main components of propolis, spectrophotometric and chromatographic (liquid and gas) methods have been used. Two different types of extraction are used in propolis analysis, i.e., extraction for the nonvolatile metabolites and the extraction for volatiles analysis. The first class of compounds are obtained by simple extraction with ethanol or methanol of different concentrations, extraction time, and temperatures [22]. Because no international regulations are available for propolis analysis, different conditions are used for these extractions. Generally, phenolic compounds are determined by liquid chromatography with different detections. A study on Portuguese propolis [79] used liquid chromatography with diode-array detection coupled to electrospray ionization tandem mass spectrometry (LC-DAD-ESI-MS) and characterized the phenolic compounds by comparing UV spectra, retention time, and MS information (m/z values) with reference compounds.
The most recent study on propolis phenolics and volatiles [98] used ultrahigh-performance liquid chromatography with diode array detector and quadrupole time-of-flight mass spectrometry (UHPLC-DAD-QqTOF-MS), and identified a high number of compounds (118 phenolics), suggesting that equipment and methods that are more elaborate and up-to-date can identify and quantify more compounds.
Propolis volatiles are responsible for the aroma and smells of the product, although they are found in small concentrations. Volatiles may give important information regarding plant sources, and thus the origin of propolis. Volatiles that present as the most abundant compounds in poplar-type propolis, include monoterpenes, sesquiterpenes, and organic compounds [10,43,85,101].
The most important criteria in gas chromatography mass spectrometry analysis is the computed match factor of the spectrum and the respective one in the existing library [100]. The identification of the compounds is generally done by computer searches in available libraries. In GC analysis, in some cases, unidentified compounds remain, because their spectra are not found in the respective libraries. In these cases, only the structural type of the compound is proposed, based on the fragmentation spectrum of the query compound.
Another method of propolis composition analysis is fourier transform infrared attenuated total reflectance (FTIR-ATR) [98]. The complexity of the propolis spectrum measured by FTIR, give its’ overall chemical composition, and the identification of every signal represents a demanding task. Trained specialists can distinguish different signals corresponding to particular organic compounds, based on the literature data of propolis composition and different spectral data of FTIR libraries. The mentioned study is among the few studies existing on propolis analysis.
Over the last decades, the old method of TLC has been improved, and coupled with high-performance liquid chromatography, for direct identification of the antioxidant compounds of poplar propolis and other natural matrices, using also antioxidant radical 2, 2-diphenyl-1-picrylhidrazyl (DPPH) [105,113]. The method is based on the separation of bioactive constituents from the polyphenolic class using high-performance thin layer chromatography, visualization of the compounds being made using DPPH as the derivatizing reagent. Overall, the most used analysis methods for chemical composition of propolis extracts remains liquid and gas chromatography.
1.2. Main Bioactive Properties of Propolis
Regarding the bioactive properties of propolis, there are many demonstrated activities such as antioxidant [114,115,116,117], anti-inflammatory [118], antibacterial [119,120,121,122], antifungal [123,124,125], anticancer [126,127,128,129], immunosuppressant [118], and antiviral activity [130,131,132,133,134,135]. Antioxidant activity of propolis extracts have been evaluated over time using different spectrophotometric methods in vitro. The simplest method used for antioxidant activity determination for different natural extracts, including plants and in our case propolis, is radical scavenging activity (RSA) using 2, 2-diphenyl-1-picrylhidrazyl (DPPH) assay. DPPH is a stable radical which reacts with bioactive compounds present in the extract and is expressed as a % of inhibition [136,137]. Combining HPTLC and DPPH, a new method has been developed that is simple and accurate, which facilitates explorative work by testing different natural matrices with complex chemical composition [113]. The method is regarded as a novel analytical quality control tool that can be applied to different complex natural matrices. Ferric reducing ability power (FRAP) assay is based on the redox reaction between the bioactive compounds contained in the extract and the Fe3+-TPTZ complex (FRAP reagent) and is expressed as the potential of the antioxidants to reduce Fe3+ to Fe2+, which is spectrophotometrically measured at 593 nm [138].
Miguel et al. [115] demonstrated that there were no statistical difference between the antioxidant activities of brown propolis harvested in different seasons of the year. According to their results, the ABTS (2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) value was between 0.019 mg/mL in spring collected propolis and 0.020 mg/mL in those harvested during the winter period. The DPPH values ranged between 0.027 mg/mL and 0.031 mg/mL, respectively.
A more recent study by Seibert et al., 2019 [117] reported that the concentration required to obtain a 50% antioxidant effect of propolis (EC50) using the DPPH method was 25.04 for ethanolic extracts and 3.14% when the ABTS method was applied. For hexanic extracts and etyl acetate extracts, with both methods, the values were superior.
Svečnjak et al. (2020) [98] analyzed seven raw propolis coming from the Croatian Islands and stated that the highest activity was observed for the samples of Populus spp. origin. The antioxidant potential of these samples, determined by DPPH ranging from 2.6 to 81.6 mg GAE/g and by FRAP assay values ranging from 0.1 to 0.8 mmol Fe2+/g were registered.
Generally, in propolis research observations, the antimicrobial activity of the extracts has been higher in Gram-positive as compared with Gram-negative bacteria, where limited effects have been observed [139,140,141]. Gram-negative bacteria have a species-specific structure of the outer membrane and produce a hydrolytic enzyme which breaks down the active ingredients of propolis [142]. The antibacterial activity of propolis is due to its bioactive compounds (aromatic compounds and polyphenols). Interactions among different classes of chemical compounds have an important role, which has also been demonstrated against Paenibacillus larvae (a honeybee pathogen) [143]. Flavone/flavonols and flavanone/dihydroflavonols are the two main classes of phenolics in propolis. The mentioned study developed a statistical model to detect a potential interaction between the two classes of flavonoids and the inhibition activity of different propolis extracts (10 mg/mL) originating in different geographical origins from Romania on Paenibacillus larvae. The inhibitory effect of different propolis extracts was statistically significant. The content of these compounds influences the strength of antibacterial effects, and the significant interaction effect between flavonoids should also be taken into consideration. How does propolis acts as bactericidal agent? It has a direct action on the microorganism and another indirect activity by stimulating the immune system of the bees for activating natural defense of the organism against different bacterial diseases. The process stops the division of bacterial cells, destroying the cell wall and bacterial cytoplasm, and thus stopping the bacterial protein synthesis, as described in different scientific studies [144,145,146,147].
A comprehensive review was published recently [148] that characterized the latest studies on the antibacterial activity of propolis on Gram-positive (Staphylococcus aureus, S. epidermidis, Streptococcus mutans, S. viridians, S. pyogenes, S. pneumoniae, S. oralis, S. agalactiae, S. sobrinus, Enterococcus spp. Micrococcus luteus, Bacillus subtilis, and Clostridium dificile) and Gram-negative bacteria (Escherichia coli, Salmonella spp., Klebsiella spp., Yersinia enterocolitica, Proteus mirabilis, Shigella flexneri, Enterobacter cloacae, Enterobacter aerogenes, Pseudomonas aeruginosa, Acinetobacter baumannii, Haemophilus influenza, Campylobacter jejuni, Bacteroides fragilis, and Burkholderia cepacia). Propolis antibacterial activity was most often tested on E. coli, S. aureus, Salmonella spp, and P. aeruginosa [148]. More than 600 bacterial strains were tested, according to literature studies, and the efficacy of propolis on Gram-positive over Gram-negative bacteria was confirmed, the first class presenting lower minimum inhibitory concentrations (MIC) over the second class [148].
Cancer is one of the most severe and often deadly diseases in our times. Treatment methods include surgery, but also chemotherapy, radiotherapy, or immunotherapy according to individual characteristics of the patient. Chemotherapy and radiotherapy have different toxic effects and, nowadays, different antioxidant substances are used as enhancers of the immune system and reduce the toxic effects on patients. Propolis is a very powerful antioxidant and its antiproliferative activity has been tested either in vitro on different cancer cells or in vivo on animal models, where reduction of the tumor was observed. In 2003, Orsolic and Basic [149] observed an anti-metastatic activity of a water-soluble propolis derivative upon a CBA mouse mammary carcinoma tumor. The propolis derivative reduced the metastases in mice lung and also changed several immunological parameters of mice. Other different malignant cells (ME45 malignant melanoma, HTC 116, Caco-2, DLD-1, HT-29 human colorectal carcinoma, A549 and H23 lung cancer cells, MCF-7 hormone dependent and MDA-MB-468 human breast cell lines, LN18, and U87 glioblastoma cell lines), were treated in vitro with propolis extracts and an antitumor activity was observed, dependent on the cell lines. At the same time, L-929 normal fibroblast cells were not affected by propolis at a concentration of 1 μg/mL [103]. A recently published study [150] used propolis and a new designed product (chitosan-coated nano-propolis NP) to reduce the side effects of cisplatin, a drug widely used in cancer treatment. The in vivo study used Wistar rats divided into seven groups with different treatment schemes. The experimental groups treated with propolis and NP ameliorated the cisplatin effect and protected liver and kidney tissue from the toxicity induced by the drug.
Another important property of propolis extracts is exerted in oral cavity diseases [139]. Dental caries can be caused by different bacteria (Streptococcus mutans, S. sobrinus, different Actinomyces, and Lactobacillus). Propolis extract have antimicrobial activity against L. fermentum isolated from cavities of patients diagnosed with dental caries [120]. A comprehensive review on the potential uses of propolis in oral health was published in 2010 by Parolia et al. [144]. Different beneficial properties of propolis were mentioned, which included dental surgical wound healing [151], new storage media following avulsion [152,153,154], pulp capping agent [155], as an intracanal irrigant [156], as a mouth rinse [153,157], for dentinal hypersensitivity [158,159], for treatment of perodontitis [160], for treatment of denture stomatitis [161], as an intra-canal medicament [162], an effect on recurrent aphthous stomatitis [163], and an effect on Candida albicans [164]. A conclusion of the review was that propolis can be used in all these pathologies, but cautions must be taken due to some allergic reactions in some patients. Propolis extracts can also be used in the composition of mouthwashes and toothpastes, to enhance the prevention of microbial infection and treatment of gums inflammation [147].
2. Plant Sources for Poplar-Type Propolis
Propolis is a resinous mixture, made by the honeybees from substances collected from tree or other plant buds, plant exudates, or resins found in the stem, branches, or leaves of different plants. These materials are generally lipophilic, such as mucilage, gums, and resins [9]. The list presented in this monograph includes numerous plant sources for propolis in different parts of the world. Two different approaches are used to determine the plant origin of propolis, i.e., observations of bee behavior or the chemical analysis of propolis and also plant materials [10]. Definitively, the second approach is more appropriate and correct, because it is scientifically proven. In early 1980s (40 years ago), scientific papers were published to evidence the similarity of plant species and propolis in different geographical regions [11,12,13,14,15,16,17].
In temperate zones, exudates from buds of the Populus species are the main source of resins for bees. In Europe, North America, and even New Zealand and the continental part of Australia, it has been reported that Populus was the main plant sources [12,13,16,17,18], although other plant resins are reported as precursors of propolis in the temperate zones of Europe and North America, including pine (Pinus sp.), alder (Alnus glutinosa), horse chestnut (Aesculus hippocastanum), elm (Ulmus sp.), ash (Fraxinus sp.), oak (Quercus sp.), and beech (Fagus sp.) [7,17,19,20,21]. In northern parts of Russia, aspen and silver birch buds (Betula veruucosa) supply bees with resins for propolis production [3,11,22,23].
Poplars (Populus spp., Salicaceae) include about 100 species and a lot of hybrids. These plants are the fastest growing species with very deep root systems (up to 20 m) and five-year-old trees are capable of uptaking up to 200 L of water per day [24]. Poplar hybrids, growing up to 3 m per year, are free of competition with weeds, even during the beginning of plantation.
Birches (Betula L.) are an essential ecological component in northern temperate and boreal forests [25]. In Europe, two important trees occur naturally, i.e., silver birch (Betula pendula Roth) and downy birch (Betula pubescens Ehrh.). These trees differ regarding the morphology of their leaves, twigs, branches, bark, seeds, and catkin scales, as well as cell size and wood anatomy, and they can reach a height of 20–30 m [26].
Generally, the genus Salix is very diverse, representing over 300 species [27] growing in the form of trees, shrubs, or dwarf shrubs with procumbent stems. Among the flavonoids most characteristic for poplars are flavanones, especially pinocembrin and pinostrobin. These compounds have shown antioxidant and anti-inflammatory effects in many in vitro tests and may play an important role in the pharmacological activity of Populus [28,29,30].
Furthermore, propolis has been used by humans as a traditional folk medicine to maintain good health since ancient times, due to many beneficial properties [31] including antioxidant, anti-inflammatory, immunomodulatory, antimicrobial, antitumor, anticancer, cardioprotective, neuroprotective, and many more [32].
Chemical determinations of propolis composition have led to the conclusion that more than one plant resin has been found in propolis [33,34,35,36] and the question raised has been if the bees show selectivity when collecting the resins in areas where multiple plant sources are found and what is the reason for this. Different studies have been published [37,38,39] that have shown that bees collect resins discriminately, due to proximity, availability, or even toxicity.
Differences in the chemical composition of poplar buds may be from different phenolic compounds such as terpenoids, flavonoid aglycones, and their chalcones, as well as phenolic acids and their esters [40], and therefore it is important to control the quality of plant material in terms of qualitative and quantitative profiles. A study conducted by de Marco et al. (2017) [4] compared the bioactive compounds of poplar buds and Italian propolis. The authors quantified the total flavonoids, chrysin, galangin, pinocembrine, and caffeic acid phenethyl ester (CAPE) that were responsible for the antioxidant activity of these matrices. The results obtained were in the range of 1.40% and 24.18% for poplar buds freeze-dried extract and 1.52% to 28.78% for Italian propolis freeze-dried extract.
Therefore, plants are the main source of bioactive compounds of propolis and bees intervene only with different enzymes to finalize the chemical composition of propolis.
This entry is adapted from the peer-reviewed paper 10.3390/plants10010022
This entry is offline, you can click here to edit this entry!
