Hemorheology, the study of cell deformation and blood flow, has been more focused on red blood cells (RBCs) rheology, relating the deformation and aggregation of RBCs, since erythrocytes comprise the major components in blood. Blood rheology can be used as an important clinical assay, correlating various aspects of blood rheology and associated changes in cell behavior and morphology to a wide range of diseases and health conditions.
- hemorheology
- blood diseases
- microfluidics
- single-cell analysis
- red blood cells deformability
- blood analogues
1. Introduction
Hemorheological alterations in the majority of metabolic diseases are always connected with blood rheology disturbances, such as the increase of blood and plasma viscosity, cell aggregation enhancement, and reduction of the red blood cells (RBCs) deformability. Blood and plasma viscosity are risk factors essentially, for example for atherosclerosis [1][2][3] and other studies exposed that RBCs rheological changes have been observed in patients with hypertension[1][4][3] and diabetes mellitus[5][6][7][8], which are diseases more often associated with obesity[3][9]. Obesity-related blood rheological disturbances are currently being investigated as one of the risk factors for several co-morbid pathologies because they play a significant role in microcirculation blood flow [3]. Lee et al., 2019 [10] have performed a review with the most recent clinical studies of diabetic kidney disease associated with hemorheological parameters, demonstrating that critical shear-rate and –stress, measured by a microfluidic aggregometry[10], aggregation index and RBC deformability elongation index, measured by a microfluidic ektacytometry[11], must be combined as a tool for a successful diagnosis of disease stage and possible derivate complications. Additionally, Caprari et al. (2019) [12] have demonstrated that blood viscosity and RBC aggregation increase with the decreasing of the RBCs deformability, by using blood samples from subjects with sickle cell anemia. Likewise, the electrical blood behavior can be used to help in the isolation of plasma and populations of blood cells and to quantify hemorheological properties[13][14]. Note that the electrical properties of blood tend to change due to hemorheological variations of the RBCs behavior and blood plasma. Several impedance measurements can be related to RBCs behavior, for example, the electrical impedance of blood flow increases at low shear rates because of RBC aggregation. A clear example that the electrical behavior of blood can help in the clinical diagnosis is the work developed by Yeom et al., (2015)[13]. In this work, a simple speckle analysis based on a microfluidic measurement method to detect the hyperaggregation caused by diabetes was used. By this method, they have demonstrated the potential of evaluating the differences of the biophysical properties of cardiovascular diseases’ blood samples [15]. However, the measurements were restricted on superficial vessels, and ultrasonic signal requires calibration as a function of the flow speed under steady flow conditions.
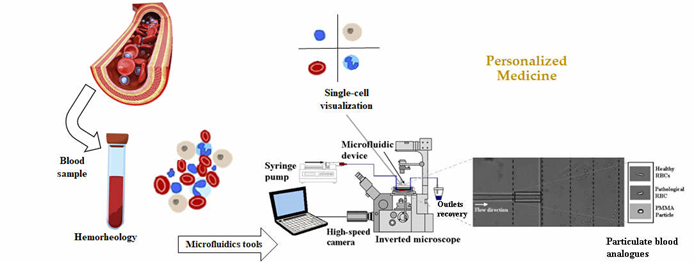
Therefore, the progress on hemorheology, regarding the phenomena associated with blood components, their interactions and impact on blood properties, and blood flow, i.e., the hemorheological profile of the patients, strongly dependent on the interactions and mechanical properties of blood cells and in particular with the behavior of the RBCs, can bring further insight into the human health state and it can be an important parameter in clinical diagnosis (Figure 1). Implementation of rapid test technologies in clinical environments leads to minimal user intervention during operation; user-friendly, easy-to-use, and simple detection platforms; high diagnostic sensitivity and specificity; immediate clinical assessment; and low manufacturing and consumables costs. However, it should be noted that it essential to achieve high specificity in the detection and read of biomarkers. Microfluidics has potential to provide all that solutions since it enables the processing of samples that are not available in large quantities (e.g., cells from patient biopsies), reduce cost, provides a high level of automation, and allows the set-up of complex models (for example for cancer studies). Another advantage more closely related to the rapid tests is the possibility of using small amounts of different kind’s body fluids such as blood, urine, saliva, and sweat.
Figure 1. Hemorheology combined with microfluidic technology can bring further advances in personalized medicine for new treatments and diagnosis. From J. Pers. Med. 2020, 10(4), 249; https://doi.org/10.3390/jpm10040249.
2. Microfluidics Tools: Single-Cell Approach
Owing to recent developments in microfluidic technology, several hemorheological point-of-care devices have been designed that allow the possibility of conducting extensive clinical studies using hemorheological measurements[10][16]. The field of microfluidics technology is characterized by the study and manipulation of fluids in microstructures at the submillimeter length scale and it has emerged as an important tool to be applied in many engineering and biomedical fields[17]. The cost reduction and the performance enhancement were appellatives for the biomedical research community to create novel strategies for applications in the diagnostics and/or therapy of several diseases, providing new sets of solutions to overcome the shortcomings of conventional detection and treatment methods available in clinics and hospitals [18].
Microfluidic technologies can be used to obtain a variety of interesting applications, such as cell manipulation and separation[19][20][21], cell patterning[22] and single-cell analysis [23][24][25], and measurements such as fluid viscosity[26][27] of the blood samples and its constituents. Separation of cells/molecules or other fluid elements plays also an important role in sample preparation for biological, biochemical, and pharmaceutical applications [28] and need to be carried out precisely to develop microfluidic systems as an accurate tool with high detection and quantification efficiency, leading to an efficient single-cell analysis [29]. Different kinds of single-cell analysis can be performed depending on what we are observing, e.g., single-cell immunology, single-cell biology, single-cell systems biology, single-cell pharmacology, and single-cell toxicology. For instance, single-cell studies require cells capture/isolation using different microfluidic methods, such as hydrodynamic, electrical, optical, magnetic, and acoustic methods [29], and various detection methods, such as fluorescence microscopy, fluorometry and mass spectroscopy, optical or electrochemical[29] that can be combined with passive [19][30][31], active [32][33][34] or/and hybrid isolation methods [35]. As for either single-cell manipulation or single-cell analysis, it is hard to obtain a comprehensive result by merely using one method [29]. Therefore, two or more methods are usually combined into a microfluidic system comprising several single-cell testing modules [36].
RBCs’ deformability (the ability to change their shape and pass through small capillaries and splenic sinuses), a single-cell measurement, has been considered a potential biomarker for blood disorders, such as diabetes [37], obesity [38], and malaria [39][40][41]. Depending on the diseases, several alterations can occur in the viscoelasticity of the membrane of the cell or in the cytoplasmic viscosity, or even both. For example, nonenzymatic glycation of several proteins, especially red cell-membrane glycoproteins and hemoglobin, has been found in patients with diabetes, and such a biochemical modification of the erythrocyte is one factor that may account for altered rheological properties of human erythrocytes in diabetes [15]. Reduction in cancer-cell elasticity and stiffness-sensing ability could cause the loss of cancer cells to response to microenvironmental changes and it was suggested as important biomarkers of a cancer-cell phenotype, mechanosensation, or mechanotransduction [13]. Other diseases that include changes in RBCs stiffness due to cytoskeletal modifications such as spherocytosis, increased cell deformability of invasive cancer cells compared to benign or normal cells of the same origin, and changes in stiffness of leukocytes in response to activation with antigens or other signals. An increased deformability has also been identified as a potential biomarker for pluripotent stem cells.
Primarily, the available methods to measure the biomechanical properties of RBCs, such as the conventional rotational viscometer, ektacytometer, and micro-pore filtration assay, use high sample concentrations, since they have been used to measure the blood viscosity and other rheological properties, but they are generally expensive, labor-intensive, and do not provide a direct and detailed source of information on the mechanical properties of the RBCs. As single-cell techniques, micropipette aspiration, and optical tweezers, are also extremely popular for measuring the mechanical properties of the RBC membrane. However, these techniques also have several drawbacks, such as a low-throughput, labor-intensive, and static process. Additionally, it is argued that these methods do not represent the actual RBC deformability that happens during microcirculation [42][43]. For this reason, the deformability of RBCs, by using microfluidic devices to measure the deformation of blood cells, has gained great attention.
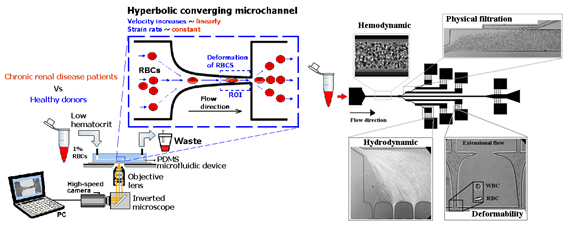
Lima’s research group have applied a hyperbolic-shaped microchannel [44][45][46]to study the RBCs deformability demonstrating that single RBCs deformability analysis can provide more precise and detailed information about blood disorders. In a more recent study performed by Faustino et al., 2018 [47], based in the work of Pinho et al., 2013 [48] and Rodrigues et al., 2015 [49], they have combined several passive sorting methods. In this work, separation by the biomechanical cells properties, hydrodynamic phenomena and hemodynamic cells behavior were fully integrated in a single microfluidic system to achieve single RBC visualizations and respective mechanical properties of cells (i.e., deformability) analysis.
Figure 2. Microfluidic devices to assess of motions and deformations of red blood cells (RBCs) from healthy donors and pathological patients, e.g., chronic renal disease and diabetes mellitus [50]. From J. Pers. Med. 2020, 10(4), 249; https://doi.org/10.3390/jpm10040249.
3. Blood Analogues — Particulate Blood Approaches
Human blood is a multiphase complex fluid that consists of a concentrated suspension of formed cellular elements (RBCs, WBCs, and platelets) in plasma [51]. However, the manipulation of real whole blood in vitro is difficult, not only because of ethical, economical, and safety problems but also because the rheological properties of blood vary with temperature, and it is difficult to control [52][53]. For these reasons, the development of reliable blood analogues to represent and reproduce the properties of human blood, is of great importance.
In the literature, there are several proposed blood analogue fluids; however, they are mostly Newtonian fluids using mixtures of water/glycerol [54] and water/dimethylsulfoxide(DMSO) [83], or non-Newtonian analogue fluids based on aqueous solutions of xanthan gum (XG) and polyacrylamide (PAA) [55], in which the addition of glycerin is used to obtain the blood rheology at different hematocrit levels [55][56]. Vlastos et al., 1997 [57] compared the rheology of PAA and XG solutions at different concentrations for the preparation of blood analogue solutions and performed a combination of steady and oscillatory shear tests and found that the PAA solution displayed a viscoelastic behavior close to that of blood at a concentration of 125 ppm and the XG at 500 ppm, at low shear regions. Following that, Calejo et al., 2016 [58] and Pinho et al., 2017 [59] performed a study in which particulate Newtonian and non-Newtonian blood analogues were successfully developed, able to form the cell-free layer (CFL) downstream of a microfluidic hyperbolic contraction and to mimic the viscosity behavior of RBCs. Both solutions were composed of dextran 40 with rigid spheres particles of PMMA, and one of them exhibited a viscoelastic behavior due to the addition of XG. Nevertheless, the use of rigid particles limits the physiological realism of these studies. To overcome this limitation, over the years, several researchers have investigated other alternatives such as, polydimethylsiloxane (PDMS) microparticles [60][61][62] and hydrogel microparticles [63]. An example of the suitability of PDMS microparticle was presented by Pinho et al., 2019 [62]. They developed flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids and these showed a great potential to mimic the structural and rheological properties of RBCs suspensions and consequently to develop blood analogue fluids similar to real blood. The authors visualized the flow through a hyperbolic-shaped contraction and observed that all PDMS microparticles present some ability to elongate when passing through the smallest dimension of the microchannel. Figure 3 shows the deformation behavior of PDMS particles in comparison to RBCs and rigid particles of PMMA. They also verified that the final working fluid, formed of transparent PDMS microparticles, can reproduce the viscosity curve of human RBCs suspensions, showing an intermediate shear-thinning degree between healthy and pathological RBCs. In addition, the development of blood analogue fluids capable of mimic the RBCs behavior and the blood microcirculation phenomena at in vitro conditions is crucial. These analogue fluids are extremely important for experimental work since they minimize the use of real blood and the ethical issues. Lima and his colleagues have proposed a blood analogue fluid composed of Brij L4 [64] surfactant micelles suspended in pure water. This analogue is extremely easy to be produced and is able to investigate different kinds of microscale blood flow phenomena in simple and complex geometries. In addition, by using this analogue it is possible to track the flexible micelles avoiding sedimentation, aggregation, clogging and blockage difficulties that researchers have been pointed out by using rigid microparticles.
Figure 3. Behavior of healthy RBCs, flexible particles (PDMS) and rigid particles (PMMA) when flowing through a constriction in a microchannel. Adapted from [58][62].
4. Future Perspectives[3]
The main idea of personalized medicine is the concept of a patient-centered care. By combining the unique features of the hemorheology and microfluidic technology for single-cell analysis, valuable advances in personalized medicine for new treatments and diagnosis approach can be achieved. The visualization and measurements of blood cells, flowing in microfluidic devices have been proven to be important in providing not only essential information about hydrodynamic characteristics of the blood, but also vital information to diagnose early symptoms of diseases during clinical investigations. For instance, point-of-care devices combined with flow visualization techniques, can be used to assess the cardiac risk profile of a specific patient and help the medical staff to decide the appropriate treatment and consequently to increase the chances of survival of this patient. Moreover, the new generation of blood analogue fluids, with the ability of reproducing healthy or pathological conditions, have been demonstrated to be a promising approach to improve the knowledge about several blood diseases. Ideally, particulate solutions having flexible particles that mimic key structural attributes of RBCs including size, shape, and mechanical properties would be an excellent candidate to reproduce multiphase effects of the blood flow in microcirculation.
This entry is adapted from the peer-reviewed paper 10.3390/jpm10040249
References
- Wiewiora, M.; Piecuch, J.; Gluck, M.; Slowinska-Lozynska, L.; Sosada, K. The effects of weight loss surgery on blood rheology in severely obese patients. Surg. Obes. Relat. Dis. 2015, 11, 1307–1314.
- Senen, K.; Topal, E.; Kilinc, E.; ten Cate, H.; Tek, I.; Karakoc, Y.; Yetkin, E. Plasma viscosity and mean platelet volume in patients undergoing coronary angiography. Clin. Hemorheol. Microcirc. 2010, 44, 35–41.
- Baskurt, O.K.; Hardeman, M.R.; Rampling, M.W. Handbook of Hemorheology and Hemodynamics; IOS Press: Amsterdam, The Netherlands, 2007
- Cicco, G.; Pirrelli, A. Red blood cell (RBC) deformability, RBC aggregability and tissue oxygenation in hypertension. Clin. Hemorheol. Microcirc. 1999, 21, 169–177
- Bessonov, N.; Sequeira, A.; Simakov, S.; Vassilevskii, Y.; Volpert, V. Methods of Blood Flow Modelling. Math. Model. Nat. Phenom. 2016, 11, 1–25.
- Babu, N.; Singh, M. Influence of hyperglycemia on aggregation, deformability and shape parameters of erythrocytes. Clin. Hemorheol. Microcirc. 2004, 31, 273–280.
- Peng, W.K.; Chen, L.; Boehm, B.O.; Han, J.; Loh, T.P. Molecular Phenotyping of Oxidative Stress in Diabetes Mellitus with Point-of-care NMR system. bioRxiv 2019, 565325.
- Loh, T.P.; Peng, W.K.; Chen, L.; Sethi, S.K. Application of smoothed continuous labile haemoglobin A1c reference intervals for identification of potentially spurious HbA1c results. J. Clin. Pathol. 2014, 67, 712–716.
- Ritchie, S.A.; Connell, J.M.C. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326.
- Lee, H.; Na, W.; Lee, S.B.; Ahn, C.W.; Moon, J.S.; Won, K.C.; Shin, S. Potential Diagnostic Hemorheological Indexes for Chronic Kidney Disease in Patients With Type 2 Diabetes. Front. Physiol. 2019, 10.
- Shin, S.; Hou, J.X.; Suh, J.S.; Singh, M. Validation and application of a microfluidic ektacytometer (RheoScan-D) in measuring erythrocyte deformability. Clin. Hemorheol. Microcirc. 2007, 37, 319–328.
- Caprari, P.; Massimi, S.; Diana, L.; Sorrentino, F.; Maffei, L.; Materazzi, S.; Risoluti, R. Hemorheological Alterations and Oxidative Damage in Sickle Cell Anemia. Front. Mol. Biosci. 2019, 6, 142.
- Yeom, E.; Lee, S.J. Microfluidic-based speckle analysis for sensitive measurement of erythrocyte aggregation: A comparison of four methods for detection of elevated erythrocyte aggregation in diabetic rat blood. Biomicrofluidics 2015, 9, 024110.
- Zeng, H.; Zhao, Y. Rheological analysis of non-Newtonian blood flow using a microfluidic device. Sensors Actuators A Phys. 2011, 166, 207–213.
- Brown, C.D.; Ghali, H.S.; Zhao, Z.; Thomas, L.L.; Friedman, E.L.I.A. Association of reduced red blood cell deformability and diabetic nephropathy. Kidney Int. 2005, 67, 295–300.
- Mohammadi Aria, M.; Erten, A.; Yalcin, O. Technology Advancements in Blood Coagulation Measurements for Point-of-Care Diagnostic Testing. Front. Bioeng. Biotechnol. 2019, 7, 395.
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189.
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60
- Rodrigues, R.O.; Pinho, D.; Faustino, V.; Lima, R. A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 2015, 17, 108.
- Yan, S.; Zhang, J.; Yuan, D.; Li, W. Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 2016, 38, 238–249.
- Jackson, E.L.; Lu, H. Advances in microfluidic cell separation and manipulation. Curr. Opin. Chem. Eng. 2013, 2, 398–404.
- u, C.; Huang, B.; Zhou, J.; Liang, Y.; Tian, J.; Ji, L.; Liang, X.; Ye, X. A Microfluidic Chip for Cell Patterning Utilizing Paired Microwells and Protein Patterns. Micromachines 2016, 8, 1.
- Wu, H.; Zhu, J.; Huang, Y.; Wu, D.; Sun, J. Microfluidic-Based Single-Cell Study: Current Status and Future Perspective. Molecules 2018, 23, 2347
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A Microfluidic Deformability Assessment of Pathological Red Blood Cells Flowing in a Hyperbolic Converging Microchannel. Micromachines 2019
- Bento, D.; Rodrigues, R.O.; Faustino, V.; Pinho, D.; Fernandes, C.S.; Pereira, A.I.; Garcia, V.; Miranda, J.M.; Lima, R. Deformation of red blood cells, air bubbles, and droplets in microfluidic devices: Flow visualizations and measurements. Micromachines 2018, 9, 151.
- Tzeng, B.-B.; Sun, Y.-S. Design and Fabrication of a Microfluidic Viscometer Based on Electrofluidic Circuits. Micromachines 2018, 9, 375.
- André, E.; Pannacci, N.; Dalmazzone, C.; Colin, A. A new way to measure viscosity in droplet-based microfluidics for high throughput analysis. Soft Matter 2019, 15, 504–514.
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluidics 2014, 17, 1–52.
- Nguyen, N.-T.; Hejazian, M.; Ooi, H.C.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186.
- Siddhartha, T.; Kumar, Y.V.B.V.; Amit, P.; Suhas, S.J.; Amit, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromechan. Microeng. 2015, 25, 083001.
- Tripathi, S.; Kumar, Y.V.; Agrawal, A.; Prabhakar, A.; Joshi, S.S. Microdevice for plasma separation from whole human blood using bio-physical and geometrical effects. Sci. Rep. 2016, 6, 26749.
- Yu, Z.T.F.; Yong, K.M.A.; Fu, J. Microfluidic Blood Cell Preparation: Now and Beyond. Small 2014, 10, 1687–1703.
- Zheng, Y.; Nguyen, J.; Wei, Y.; Sun, Y. Recent advances in microfluidic techniques for single-cell biophysical characterization. Lab Chip 2013, 13, 2464–2483.
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031.
- Chen, H.; Zhang, Z.; Liu, H.; Zhang, Z.; Lin, C.; Wang, B. Hybrid magnetic and deformability based isolation of circulating tumor cells using microfluidics. AIP Adv. 2019, 9, 025023.
- Yasukawa, T.; Nagamine, K.; Horiguchi, Y.; Shiku, H.; Koide, M.; Itayama, T.; Shiraishi, F.; Matsue, T. Electrophoretic Cell Manipulation and Electrochemical Gene-Function Analysis Based on a Yeast Two-Hybrid System in a Microfluidic Device. Anal. Chem. 2008, 80, 3722–3727.
- Agrawal, R.; Smart, T.; Nobre-Cardoso, J.; Richards, C.; Bhatnagar, R.; Tufail, A.; Shima, D.; Jones, P.H.; Pavesio, C. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci. Rep. 2016, 6, 15873.
- Zeng, N.F.; Mancuso, J.E.; Zivkovic, A.M.; Smilowitz, J.T.; Ristenpart, W.D. Red Blood Cells from Individuals with Abdominal Obesity or Metabolic Abnormalities Exhibit Less Deformability upon Entering a Constriction. PLoS ONE 2016, 11, e0156070.
- Barber, B.E.; Russell, B.; Grigg, M.J.; Zhang, R.; William, T.; Amir, A.; Lau, Y.L.; Chatfield, M.D.; Dondorp, A.M.; Anstey, N.M.; et al. Reduced red blood cell deformability in Plasmodium knowlesi malaria. Blood Adv. 2018, 2, 433–443.
- Rey, J.; Buffet, P.A.; Ciceron, L.; Milon, G.; Mercereau-Puijalon, O.; Safeukui, I. Reduced erythrocyte deformability associated with hypoargininemia during Plasmodiumfalciparum malaria. Sci. Rep. 2014, 4, 3767.
- Kong, T.F.; Ye, W.; Peng, W.K.; Hou, H.W.; Preiser, P.R.; Nguyen, N.-T.; Han, J. Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection. Sci. Rep. 2015, 5, 11425.
- Tomaiuolo, G. Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics 2014, 8, 051501.
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A Microfluidic Deformability Assessment of Pathological Red Blood Cells Flowing in a Hyperbolic Converging Microchannel. Micromachines 2019, 10, 645.
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A Microfluidic Deformability Assessment of Pathological Red Blood Cells Flowing in a Hyperbolic Converging Microchannel. Micromachines 2019, 10, 645.
- Rodrigues, R.O.; Bañobre-López, M.; Gallo, J.; Tavares, P.B.; Silva, A.M.T.; Lima, R.; Gomes, H.T. Haemocompatibility of iron oxide nanoparticles synthesized for theranostic applications: A high-sensitivity microfluidic tool. J. Nanoparticle Res. 2016, 18, 194.
- Boas, L.V.; Faustino, V.; Lima, R.; Miranda, J.M.; Minas, G.; Fernandes, C.S.V.; Catarino, S.O. Assessment of the Deformability and Velocity of Healthy and Artificially Impaired Red Blood Cells in Narrow Polydimethylsiloxane (PDMS) Microchannels. Micromachines 2018, 9, 384.
- Faustino, V.; Catarino, S.O.; Pinho, D.; Lima, R.A.; Minas, G. A Passive Microfluidic Device Based on Crossflow Filtration for Cell Separation Measurements: A Spectrophotometric Characterization. Biosensors 2018, 8, 125.
- Pinho, D.; Yaginuma, T.; Lima, R. A microfluidic device for partial cell separation and deformability assessment. BioChip J. 2013, 7, 367–374.
- Rodrigues, R.O.; Pinho, D.; Faustino, V.; Lima, R. A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 2015, 17, 108.
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A Microfluidic Deformability Assessment of Pathological Red Blood Cells Flowing in a Hyperbolic Converging Microchannel. Micromachines 2019, 10, 645.
- 7—Microfluidic Devices Based on Biomechanics. In Integrated Nano-Biomechanics; Yamaguchi, T.; Ishikawa, T.; Imai, Y. (Eds.) Elsevier: Boston, MA, USA, 2018; pp. 217–263.
- Carvalho, V.; Maia, I.; Souza, A.; Ribeiro, J.; Costa, P.; Puga, H.; Teixeira, S.F.C.F.; Lima, R.A. In vitro stenotic arteries to perform blood analogues flow visualizations and measurements: A Review. Open Biomed. Eng. J. 2020, in press.
- Campo-Deaño, L.; Dullens, R.P.A.; Aarts, D.G.A.L.; Pinho, F.T.; Oliveira, M.S.N. Viscoelasticity of blood and viscoelastic blood analogues for use in polydymethylsiloxane in vitro models of the circulatory system. Biomicrofluidics 2013, 7, 034102.
- Lieber, B.B.; Sadasivan, C.; Hao, Q.; Seong, J.; Cesar, L. The mixability of angiographic contrast with arterial blood. Med. Phys. 2009, 36, 5064–5078.
- Sousa, P.C.; Pinho, F.T.; Oliveira, M.S.N.; Alves, M.A. Extensional flow of blood analog solutions in microfluidic devices. Biomicrofluidics 2011, 5, 014108.
- Broek, C.N. Medium with Blood-Analog Mechanical Properties for Cardiovascular Tissue Culturing; IOS Press: Eindhoven, The Netherlands, 2008; pp. 651–661. [Google Scholar]
- Vlastos, G.; Lerche, D.; Koch, B.; Samba, O.; Pohl, M. The effect of parallel combined steady and oscillatory shear flows on blood and polymer solutions. Rheol. Acta 1997, 36, 160–172.
- Calejo, J.; Pinho, D.; Galindo-Rosales, F.; Lima, R.; Campo-Deaño, L. Particulate Blood Analogues Reproducing the Erythrocytes Cell-Free Layer in a Microfluidic Device Containing a Hyperbolic Contraction. Micromachines 2016, 7, 4.
- Pinho, D.; Muñoz-Sánchez, B.N.; Vega, E.J.; Lima, R.; Pinho, F.T. Rheological behaviour of dextran suspensions of PDMS microbeads flowing through a hyperbolic microchannel. In Proceedings of the 7th Portuguese Congress on Biomecahnics, Guimarães, Portugal, 10–11 February 2017.
- Muñoz-Sánchez, B.N.; Silva, S.F.; Pinho, D.; Vega, E.J.; Lima, R. Generation of micro-sized PDMS particles by a flow focusing technique for biomicrofluidics applications. Biomicrofluidics 2016, 10, 014122.
- Choi, J.; Hyun, J.-C.; Yang, S. On-chip Extraction of Intracellular Molecules in White Blood Cells from Whole Blood. Sci. Rep. 2015, 5, 15167.
- Pinho, D.; Muñoz-Sánchez, B.N.; Anes, C.F.; Vega, E.J.; Lima, R. Flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids. Mech. Res. Commun. 2019, 100, 103399.
- Nguyen, T.T.; Mongrain, R.; Prakash, S.; Tardif, J.C. Development of a Blood Analog for the Hernodynamic Efficiency Evaluation of Cardiovascular Devices. In Proceedings of the Canadian Design Engineering Network Conference, Montreal, QC, Canada, 29–30 July 2004.
- Lima, R.; Vega, E.J.; Moita, A.S.; Miranda, J.M.; Pinho, D.; Moreira, A.L.N. Fast, flexible and low-cost multiphase blood analogue for biomedical and energy applications. Exp. Fluids 2020, 61, 231.
- Nguyen, T.T.; Mongrain, R.; Prakash, S.; Tardif, J.C. Development of a Blood Analog for the Hernodynamic Efficiency Evaluation of Cardiovascular Devices. In Proceedings of the Canadian Design Engineering Network Conference, Montreal, QC, Canada, 29–30 July 2004.
- Lima, R.; Vega, E.J.; Moita, A.S.; Miranda, J.M.; Pinho, D.; Moreira, A.L.N. Fast, flexible and low-cost multiphase blood analogue for biomedical and energy applications. Exp. Fluids 2020, 61, 231.