Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Ophthalmology
Conjunctival melanoma (CM) is a rare ocular disease, accounting for about 2% of all ocular malignancies. Its incidence has been increasing in recent years, with 0.3–0.8 cases per million people in Western countries, mainly in Caucasian ethnicity, but can occur in African or in Afro-Americans as well. It most commonly appears in middle-aged or elderly white individuals. In the US, the incidence increased by 295% from 1973 to 1999.
- Conjunctival Melanoma
1. Introduction and General Overview
Usually, conjunctival melanoma (CM) is a rare ocular disease, accounting for about 2% of all ocular malignancies. Its incidence has been increasing in recent years, with 0.3–0.8 cases per million people in Western countries, mainly in Caucasian ethnicity, but can occur in African or in Afro-Americans as well. It most commonly appears in middle-aged or elderly white individuals. In the US, the incidence increased by 295% from 1973 to 1999 [1,2,3]. arises from primary acquired melanosis (PAM), in about 75% of cases, less frequently from a pre-existing conjunctival nevus or de novo. CM arising from PAM appears as a thickening lesion. Histopathologically, PAM with mild atypia has less likelihood of transformation to melanoma than PAM with severe atypia [4].
De novo melanomas carry a higher risk of metastasis and death. As in cutaneous melanoma, sun exposure is a high risk factor and plays an important role in the pathogenesis of CM.
Clinically, CM appears as a nodular or flat pigmented lesion, commonly located on the nasal or temporal bulbar conjunctiva (Figure 1), and, less frequently, as amelanotic tumors. It has a tendency to spread directly to any part of conjunctiva, to the cornea, globe, eyelid, orbit, sinus or central nervous system, and through lympho-vascular drainage to the laterocervical lymph nodes or distant organs, as reported in the AJCC eighth edition staging system [5,6]. Pathologic staging is based on vertical thickness and depth of invasion, and, consequently, it is classified as follows: ≤0.5, 0.5–1.5, and >1.5 mm. Breslow stages are not routinely used to classify CM.
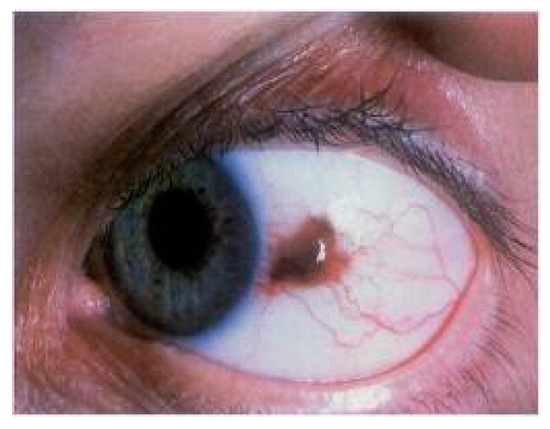
Figure 1. Slitlamp photograph of a conjuntival melanoma, histologically confirmed, with a tendency to invade the limbus and cornea.
Biologically, CM shows different behavior than uveal melanoma, while it is quite similar to that of its cutaneous counterpart. Molecular characterization studies, developed to understand the tumor biology and possibly to implement any new therapeutic approaches, have confirmed this similitude with cutaneous melanoma. In particular, Griewank et al. found BRAF (of which, 91% were V600E) mutations in 29% and NRAS in 18% of conjunctival melanoma analyzed, similar to cutaneous melanomas [7].
Diagnosis requires a complete examination of bulbar and tarsal conjunctiva, as well as orbital rim, due to the high rate of recurrence in that region. Opthalmic examination, slit-lamp photography with clinical drawing, in vivo confocal microscopy (IVCM), optical coherence tomography (OCT), and ultrasound biomicroscopy (UBM) are variably used for documenting the localization and size of the lesion and to study its local extension. These non-invasive imaging techniques could support the diagnosis, but the excisional biopsy of a clinically suspicious lesion is undoubtedly the standard of care.
CT and MRI of the orbit, maxillofacial region and brain are useful when an orbital, nasal and paranasal sinus or central nervous system involvement is suspected. Whole body PET/CT scan or MRI of the neck can reveal lymphatic spread to proximal nodes, and metastatic disease to regional lymph nodes is accessed using sentinel lymph node biopsy (SLNB) [8].
Surgery is the mainstay of treatment. Shields introduced in 1997 the “no touch” technique, describing surgical guidelines to allow a complete en-bloc tumor removal with wide margins, avoiding direct tumor manipulation [9]. This technique is widely accepted and applied, in order to minimise cell seeding and reduce the likelihood of recurrence. Incisional biopsy is discouraged, as well as narrow-margin resection; a margin of at least 2 mm is generally advised, though some surgeons suggest 5 mm when possible. In case of suspect involvement of the Tenon capsule, or when there is any evidence of scleral adhesion or pigment, a local dissection to these structures should also be performed.
Less frequently, CM presents as extensive infiltrating tumor, requiring enucleation or orbital exenteration. This is the case of eyes with limbal lesion, tumors underwent multiple resections, painful eyes and unacceptable cosmesis.
Intraoperative adjuvant treatments are often performed to the exposed scleral base and surrounding conjunctival margins to destroy possible remaining tumor cells. These include a variable combination of different adjuvant treatments: absolute alcohol application, Mitomycin C (MMC) or Interferon-alpha-2B instillation, double freeze–thaw cryotherapy. Cryotherapy works by freezing the cells and then producing ischemia from the disruption of the microvasculature; it showed to be superior in preventing the tumor recurrence than surgical excision alone, with a recurrence rate of 18% vs. 52% [10]. Topical chemotherapy treats the entire ocular surface in eyes with poorly defined tumor margins, which allows for the treatment of diffuse or multifocal lesions, or occult areas. Recurrence rates after treatment with adjuvant MMC range from 33 to 50% [11].
Lymphatic spread to the neck lymphnodes can be detected with 18F-FDG-PET or, when microscopic, with SLNB.
Despite surgical and intraoperative adjuvant treatments, CM have a high incidence of local recurrence, about 50–60% at 5 years. The recurrence rate is lower with the use of combined radiotherapy treatment [12]. The use of adjuvant radiotherapy is widely increasing, mainly by means of brachytherapy. Application of external-beam conventional X-rays techniques, stereotactic and radiosurgery, or proton-beam radiotherapy is less frequent and long-term results are awaited. Here, we describe the different radiotherapy techniques, focusing on the benefits and application difficulties of each.
1. Introduction and General Overview
Conjunctival melanoma (CM) is a rare ocular disease, accounting for about 2% of all ocular malignancies. Its incidence has been increasing in recent years, with 0.3–0.8 cases per million people in Western countries, mainly in Caucasian ethnicity, but can occur in African or in Afro-Americans as well. It most commonly appears in middle-aged or elderly white individuals. In the US, the incidence increased by 295% from 1973 to 1999 [1,2,3].
Usually, it arises from primary acquired melanosis (PAM), in about 75% of cases, less frequently from a pre-existing conjunctival nevus or de novo. CM arising from PAM appears as a thickening lesion. Histopathologically, PAM with mild atypia has less likelihood of transformation to melanoma than PAM with severe atypia [4].
De novo melanomas carry a higher risk of metastasis and death. As in cutaneous melanoma, sun exposure is a high risk factor and plays an important role in the pathogenesis of CM.
Clinically, CM appears as a nodular or flat pigmented lesion, commonly located on the nasal or temporal bulbar conjunctiva (Figure 1), and, less frequently, as amelanotic tumors. It has a tendency to spread directly to any part of conjunctiva, to the cornea, globe, eyelid, orbit, sinus or central nervous system, and through lympho-vascular drainage to the laterocervical lymph nodes or distant organs, as reported in the AJCC eighth edition staging system [5,6]. Pathologic staging is based on vertical thickness and depth of invasion, and, consequently, it is classified as follows: ≤0.5, 0.5–1.5, and >1.5 mm. Breslow stages are not routinely used to classify CM.

Figure 1. Slitlamp photograph of a conjuntival melanoma, histologically confirmed, with a tendency to invade the limbus and cornea.
Biologically, CM shows different behavior than uveal melanoma, while it is quite similar to that of its cutaneous counterpart. Molecular characterization studies, developed to understand the tumor biology and possibly to implement any new therapeutic approaches, have confirmed this similitude with cutaneous melanoma. In particular, Griewank et al. found BRAF (of which, 91% were V600E) mutations in 29% and NRAS in 18% of conjunctival melanoma analyzed, similar to cutaneous melanomas [7].
Diagnosis requires a complete examination of bulbar and tarsal conjunctiva, as well as orbital rim, due to the high rate of recurrence in that region. Opthalmic examination, slit-lamp photography with clinical drawing, in vivo confocal microscopy (IVCM), optical coherence tomography (OCT), and ultrasound biomicroscopy (UBM) are variably used for documenting the localization and size of the lesion and to study its local extension. These non-invasive imaging techniques could support the diagnosis, but the excisional biopsy of a clinically suspicious lesion is undoubtedly the standard of care.
CT and MRI of the orbit, maxillofacial region and brain are useful when an orbital, nasal and paranasal sinus or central nervous system involvement is suspected. Whole body PET/CT scan or MRI of the neck can reveal lymphatic spread to proximal nodes, and metastatic disease to regional lymph nodes is accessed using sentinel lymph node biopsy (SLNB) [8].
Surgery is the mainstay of treatment. Shields introduced in 1997 the “no touch” technique, describing surgical guidelines to allow a complete en-bloc tumor removal with wide margins, avoiding direct tumor manipulation [9]. This technique is widely accepted and applied, in order to minimise cell seeding and reduce the likelihood of recurrence. Incisional biopsy is discouraged, as well as narrow-margin resection; a margin of at least 2 mm is generally advised, though some surgeons suggest 5 mm when possible. In case of suspect involvement of the Tenon capsule, or when there is any evidence of scleral adhesion or pigment, a local dissection to these structures should also be performed.
Less frequently, CM presents as extensive infiltrating tumor, requiring enucleation or orbital exenteration. This is the case of eyes with limbal lesion, tumors underwent multiple resections, painful eyes and unacceptable cosmesis.
Intraoperative adjuvant treatments are often performed to the exposed scleral base and surrounding conjunctival margins to destroy possible remaining tumor cells. These include a variable combination of different adjuvant treatments: absolute alcohol application, Mitomycin C (MMC) or Interferon-alpha-2B instillation, double freeze–thaw cryotherapy. Cryotherapy works by freezing the cells and then producing ischemia from the disruption of the microvasculature; it showed to be superior in preventing the tumor recurrence than surgical excision alone, with a recurrence rate of 18% vs. 52% [10]. Topical chemotherapy treats the entire ocular surface in eyes with poorly defined tumor margins, which allows for the treatment of diffuse or multifocal lesions, or occult areas. Recurrence rates after treatment with adjuvant MMC range from 33 to 50% [11].
Lymphatic spread to the neck lymphnodes can be detected with 18F-FDG-PET or, when microscopic, with SLNB.
Despite surgical and intraoperative adjuvant treatments, CM have a high incidence of local recurrence, about 50–60% at 5 years. The recurrence rate is lower with the use of combined radiotherapy treatment [12]. The use of adjuvant radiotherapy is widely increasing, mainly by means of brachytherapy. Application of external-beam conventional X-rays techniques, stereotactic and radiosurgery, or proton-beam radiotherapy is less frequent and long-term results are awaited. Here, we describe the different radiotherapy techniques, focusing on the benefits and application difficulties of each.
1. Introduction and General Overview
Conjunctival melanoma (CM) is a rare ocular disease, accounting for about 2% of all ocular malignancies. Its incidence has been increasing in recent years, with 0.3–0.8 cases per million people in Western countries, mainly in Caucasian ethnicity, but can occur in African or in Afro-Americans as well. It most commonly appears in middle-aged or elderly white individuals. In the US, the incidence increased by 295% from 1973 to 1999 [1,2,3].
Usually, it arises from primary acquired melanosis (PAM), in about 75% of cases, less frequently from a pre-existing conjunctival nevus or de novo. CM arising from PAM appears as a thickening lesion. Histopathologically, PAM with mild atypia has less likelihood of transformation to melanoma than PAM with severe atypia [4].
De novo melanomas carry a higher risk of metastasis and death. As in cutaneous melanoma, sun exposure is a high risk factor and plays an important role in the pathogenesis of CM.
Clinically, CM appears as a nodular or flat pigmented lesion, commonly located on the nasal or temporal bulbar conjunctiva (Figure 1), and, less frequently, as amelanotic tumors. It has a tendency to spread directly to any part of conjunctiva, to the cornea, globe, eyelid, orbit, sinus or central nervous system, and through lympho-vascular drainage to the laterocervical lymph nodes or distant organs, as reported in the AJCC eighth edition staging system [5,6]. Pathologic staging is based on vertical thickness and depth of invasion, and, consequently, it is classified as follows: ≤0.5, 0.5–1.5, and >1.5 mm. Breslow stages are not routinely used to classify CM.

Figure 1. Slitlamp photograph of a conjuntival melanoma, histologically confirmed, with a tendency to invade the limbus and cornea.
Biologically, CM shows different behavior than uveal melanoma, while it is quite similar to that of its cutaneous counterpart. Molecular characterization studies, developed to understand the tumor biology and possibly to implement any new therapeutic approaches, have confirmed this similitude with cutaneous melanoma. In particular, Griewank et al. found BRAF (of which, 91% were V600E) mutations in 29% and NRAS in 18% of conjunctival melanoma analyzed, similar to cutaneous melanomas [7].
Diagnosis requires a complete examination of bulbar and tarsal conjunctiva, as well as orbital rim, due to the high rate of recurrence in that region. Opthalmic examination, slit-lamp photography with clinical drawing, in vivo confocal microscopy (IVCM), optical coherence tomography (OCT), and ultrasound biomicroscopy (UBM) are variably used for documenting the localization and size of the lesion and to study its local extension. These non-invasive imaging techniques could support the diagnosis, but the excisional biopsy of a clinically suspicious lesion is undoubtedly the standard of care.
CT and MRI of the orbit, maxillofacial region and brain are useful when an orbital, nasal and paranasal sinus or central nervous system involvement is suspected. Whole body PET/CT scan or MRI of the neck can reveal lymphatic spread to proximal nodes, and metastatic disease to regional lymph nodes is accessed using sentinel lymph node biopsy (SLNB) [8].
Surgery is the mainstay of treatment. Shields introduced in 1997 the “no touch” technique, describing surgical guidelines to allow a complete en-bloc tumor removal with wide margins, avoiding direct tumor manipulation [9]. This technique is widely accepted and applied, in order to minimise cell seeding and reduce the likelihood of recurrence. Incisional biopsy is discouraged, as well as narrow-margin resection; a margin of at least 2 mm is generally advised, though some surgeons suggest 5 mm when possible. In case of suspect involvement of the Tenon capsule, or when there is any evidence of scleral adhesion or pigment, a local dissection to these structures should also be performed.
Less frequently, CM presents as extensive infiltrating tumor, requiring enucleation or orbital exenteration. This is the case of eyes with limbal lesion, tumors underwent multiple resections, painful eyes and unacceptable cosmesis.
Intraoperative adjuvant treatments are often performed to the exposed scleral base and surrounding conjunctival margins to destroy possible remaining tumor cells. These include a variable combination of different adjuvant treatments: absolute alcohol application, Mitomycin C (MMC) or Interferon-alpha-2B instillation, double freeze–thaw cryotherapy. Cryotherapy works by freezing the cells and then producing ischemia from the disruption of the microvasculature; it showed to be superior in preventing the tumor recurrence than surgical excision alone, with a recurrence rate of 18% vs. 52% [10]. Topical chemotherapy treats the entire ocular surface in eyes with poorly defined tumor margins, which allows for the treatment of diffuse or multifocal lesions, or occult areas. Recurrence rates after treatment with adjuvant MMC range from 33 to 50% [11].
Lymphatic spread to the neck lymphnodes can be detected with 18F-FDG-PET or, when microscopic, with SLNB.
Despite surgical and intraoperative adjuvant treatments, CM have a high incidence of local recurrence, about 50–60% at 5 years. The recurrence rate is lower with the use of combined radiotherapy treatment [12]. The use of adjuvant radiotherapy is widely increasing, mainly by means of brachytherapy. Application of external-beam conventional X-rays techniques, stereotactic and radiosurgery, or proton-beam radiotherapy is less frequent and long-term results are awaited. Here, we describe the different radiotherapy techniques, focusing on the benefits and application difficulties of each.
2. Brachytherapy
The standard treatment of primary conjunctival melanoma (CM) is local excision followed by adjuvant local therapy or only brachytherapy. This treatment modality is increasingly safe, effective and ever-expanding, although most of the experiences reported in the literature refer to its use for uveal melanoma in which it is considered a standard treatment for small or medium-sized tumors, ≤10 mm in apical height and ≤16 mm in diameter [13,14].
In CM, adjuvant treatment is important to improve tumor control and patient survival, especially when surgical margins are positive. Adjuvant therapy includes brachytherapy, cryotherapy, topical mitomycin C, proton beam radiotherapy, or alpha 2b interferon. Size and localization are important parameters of choice of these treatments to use [15].
Eye brachytherapy can use radionuclides emitting low-energy photons (125I, 103Pd, or 131Cs) or beta rays (106Ru/106Rh or 90Sr) [15,16]. Radionuclides emit several low-energy, in the X-ray range (20–35 keV), photons with different intensities. In this case, the photoelectric effect, in which photons transfer energy to electrons, is the predominant effect.
The choice of devices depends on the operator’s experience, properties of the specific radio-isotope, size and the method of use [16,17]. Custom plaque design can reduce unnecessary radiation to nearby healthy structures, reduce treatment time and increase its effectiveness. The 131Cs source provides a slightly more uniform dose distribution than the other sources, but the DVHs of these plaques (I, Pd) show a similar trend. Palladium-103 has a biologically effective dose better than I-125. Due to the rarity of the disease, there is not numerous data on the various ways of delivering brachytherapy [17].
Currently, there is no standardized dose protocol for the irradiation of intraocular tumors with 106Ru eye plaques; in fact, there are two different methods of delivery (high dose: 290–320 Gy or low dose: 100 Gy), while, for I125 eye plaques, the minimum dose required for tumor control should be at least 85 Gy [15,16,17,18,19]. Iodine plaques are usually used to treat tarsal conjuntival melanoma after primary excision. Even for Sr-90 there is not a standardized regime, but, on average, the total dose is 36–60 Gy and fraction size is typically 10 Gy, and it is preferable for lesions of the bulbar conjunctiva for the shape and the size of the applicator. The technical characteristics of the applicators may constitute a limitation for their use in cases where the ocular anatomy does not allow it. Strontium treatment using a hand-held applicator, and treatment is used in the Academic Medical Center (Amsterdam, the Netherlands) or Catharina Hospital (Eindhoven, the Netherlands) as in those centers a Sr-90 applicator was available. In these centers is dispensed a dose of 60 Gy in 6 fractions, 10 Gy at a time, at the conjunctival surface. The application lasts 60–90 s. While Ru-106 brachytherapy is used in Leiden University Medical Center (LUMC, Leiden, the Netherlands): 100 Gy in a single dose are dispensed in this center, 2 mm deep because all the lesions had been removed. Treatment time was variable and there were different types of plaques of different shapes and sizes. From 2012 onwards, in patients with primary acquired melanosis (PAM) in addition to CM, the topical use of mitomycin and brachytherapeutic treatment with Ru-106 [10,18].
There are no statistically significant differences in the development of relapses, metastases or deaths between the two treatments; according to Wong, even the treatment with I-125 plaque does not differ much from the results with Ru-106 and Sr-90, but Ru-106 reduces local toxicity compared to Sr-90 [15,16,17,18]. Moreover, the adjuvant treatment with Ru106 had the same total recurrence rate as the treatment performed by Damato, who applied the same dose at a depth of 1 mm [10]. Obviously, we compare different doses because the biological effect of radiation depends not only on the total dose, but also on the dose rate, on the fractionation and on the total treatment time. As Sr-90 is applied in short sessions, the dose rate is much higher compared with that of Ru-106 (103.2 Gy/h for Sr-90 versus 4.0 Gy/h for Ru-106). Another difference between Sr-90 and Ru-106 is that while Sr-90 is outpatient, Ru-106 needs hospitalization. The half-life of the Sr-90 is much longer than that of the Ru-106 (28.8 years versus 374 days) [9,10,11,12,13,14,15,16,17,18]. To date, Sr90 applicators are out of production, so it is pretty hard to find them; there is only one lobby that produces them.
However, studies between the various isotopes are unreliable due to the different size of the tumor, localization and surgical technical variable. Brachyterapy offers better results in terms of quality of life (eye preservation) and is comparable in tumor control over enucleation, but it is not without side effects, for example cataract (45%) (Maximum Doses-Dmax 25 Gy), telangiectasia (40%) (Dmax 104 Gy), episcleritis (5%) (Dmax 125 Gy), descemetocele (5%) and secondly pain, clouding of the lens, dry eye complaints or corneal erosions, symblepharon, ptosis, corneal ulcers and scleral necrosis [13,14,15,16,17,18]. No patient developed a new brachytherapy-induced tumor.
Radiotherapy Sr-90 appears to be safer and more effective than other adjuvant treatments (Mytomicin C) for melanoma conjunctival. So, the most frequent contraindication in radiotherapy is cataract, although vision can be restored with surgery. Another side effect of radiation therapy is permanent vision loss due to damage to the macula or optic disc; retinopathy is also an important risk factor for vision loss. In fact, the most radiosensitive structures of the eye are the lens, followed by the cornea, retina and optic nerve [19,20]. Local excision with adjuvant brachytherapy provides good tumour control with excellent visual outcome and mild side effects in patients with limited conjunctival melanoma. Results after Sr-90 or Ru-106 were comparable; a choice for either treatment may be based on experience of the clinician and availability of materials.
3. External Photon-Beam Radiotherapy
The rarity of conjunctival and iris melanoma and the wider use of brachytherapy and proton therapy have limited the use of external photon-beam radiotherapy.
In a recent meta-analysis on the role of radiotherapy in ocular melanomas [21], it is underlined that stereotactic radiation therapy (SRT) and stereotactic radiosurgery (SRS), mainly administered with gamma knife and cyber knife, represent an alternative therapy. Authors concluded that, for the small number of studies available on this topic and applied methodology, are not able to determine what the most effective radiotherapy technique is.
For small and medium-sized lesions, external photon-beam radiotherapy has shown similar efficacy and side effects to proton therapy [21]. Stereotactic radiosurgery with charged-particle beams (carbon ions, protons) is effective in larger lesions with irregular margins, causing less adverse effect and less damages to surrounding organs at risk [22].
External beam radiation is also proposed as palliative treatments for nonresponsive, recalcitrant carcinomas for patients in whom conventional therapy has not been effective. After failure of multiple standard treatments, Graue et al. [23] treated a small patient’s series with electron beam radiotherapy, but with curative doses (50 Gy in 2.5-Gy fractions or 60 Gy in 2.0-Gy fractions, approximately the same radiobiological equivalent dose). The local tumor control rate was 75%, with relatively few side effects; there were no second cancers.
There are selected cases in which photon beam radiotherapy can be proposed: when patients refuse or cannot have surgery, positive margins, negative surgical margins with presumed residual microscopic disease, such as adjuvant therapy [24], extra tumoral perineural infiltration, post-exenteration radiotherapy in high-risk patients, lymph node involvement and previous recurrence.
This entry is adapted from the peer-reviewed paper 10.3390/app10249071
This entry is offline, you can click here to edit this entry!
