To overcome cancer, various chemotherapeutic studies are in progress; among these, studies on nano-formulated combinatorial drugs (NFCDs) are being actively pursued. NFCDs function via a fusion technology that includes a drug delivery system using nanoparticles as a carrier and a combinatorial drug therapy using two or more drugs. It not only includes the advantages of these two technologies, such as ensuring stability of drugs, selectively transporting drugs to cancer cells, and synergistic effects of two or more drugs, but also has the additional benefit of enabling the spatiotemporal and controlled release of drugs. This spatial and temporal drug release from NFCDs depends on the application of nanotechnology and the composition of the combination drug.
- nano-formulated combinatorial drug
- ratiometric
- sequential
- spatiotemporal
- controlled release
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
1. Introduction
Many studies are being conducted to overcome cancer, a major health problem for humans in modern society. Chemotherapy has been in the spotlight as the main approach for anticancer research, but there are several limitations to this approach [1]. As chemotherapy usually uses a single anticancer drug that targets only one signaling mechanism, many problems such as drug resistance, side effects on healthy tissues, and poor pharmacokinetic profiles are encountered [2,3,4]. Therefore, combination therapy using two or more different drugs has been considered as a solution for anticancer therapy [5]. However, combination therapy also has its disadvantages. It is difficult to control the pharmacokinetics and pharmacodynamics due to a time difference in drug administration, in which individual drugs are administered in combination, and the possibility of cross-resistance induced by the administration of several drugs [6,7]. Owing to its high level of adaptability, cancer is difficult to treat owing to multiple drug resistance (MDR) that results in simultaneous resistance to multiple drugs with various chemical structures and the associated mechanisms of action [8]. To solve these problems, recent research has focused on methods using nanoparticles as nanocarriers in anticancer therapy [9,10].
Nanoparticles have been widely used as vehicles for cancer treatment because of their several advantages, and various nanocarriers such as liposomes, polymeric micelles, silica nanoparticles, chitosan nanoparticles, and protein nanoparticles are being developed [11,12,13,14,15]. The encapsulation of drugs using nanocarriers can help solubilize various poorly water-soluble drugs using nanocarriers with amphiphilic properties [16]. In addition, it is possible to protect drugs through nano-encapsulation or via nanoparticle uptake through endocytosis [17]. A key advantage of using nanoparticles in chemotherapy is the ability to differentiate cancer cells from normal cells and selectively remove cancer cells [18]. Nano-formulated drugs can avoid rapid removal from the body through an enhanced permeability and retention effect (EPR), allowing passive tumor accumulation into tumors, as well as active tumor accumulation by adding ligands for targeting cancer cells [19].
Nano-formulated combinatorial drugs (NFCDs), which can be co-delivered with these nanoparticles, have several unique advantages, such as improving synergistic treatment efficacy, drug resistance management, and the ability to temporarily control drug release [20]. NFCD treatments are considered to have high potential to solve problems such as drug toxicity and dose control, as they can simultaneously utilize the advantages of existing nanoparticles and combination therapy [8,21,22]. NFCDs in various formulations can be prepared, depending on the type of nanoparticles to be used and the drug to be combined. Due to these advantages, studies on NFCD are being actively conducted, and some studies have already reached the clinical trial stage [23,24,25,26,27].
We focused on the controlled release of NFCD and confirmed previous studies. NFCDs can be released at the same time or sequentially at intervals of time. In addition, drugs can be released into different spaces. As the anticancer efficacy of NFCDs can vary greatly depending on the drug release pattern from nanoparticles, it is necessary to carefully consider the drug release system according to time and space. Therefore, in this review paper, we classify the types of controlled release of NFCD as ratiometric drug delivery that is simultaneously released over time, sequential drug delivery that is released within cells in order, and sequential drug delivery with intercellular sequential delivery followed by intracellular sequential delivery. We also introduce the advanced technologies of controlled release for this and review the benefits of each controlled drug release pattern and the prospects of these technologies (Figure 1).

Figure 1. Schematic illustration of various spatiotemporal types of combinatorial anticancer drug release. (A) Ratiometric drug delivery and simultaneous release for synergistic drug interaction. (B) Sequential drug release to achieve both intercellular and intracellular drug action. (C) Intracellular sequential release of adjuvant and primary drug for enhanced drug efficacy.
2. Ratiometric Drug Delivery
By inhibiting cancer via different mechanisms through multi anticancer agents, resistance to anticancer agents can be reduced to a higher degree than when using a single anticancer agent; moreover, a synergistic effect can occur, leading to higher efficiency. For specific combinations to achieve a synergistic anticancer effect, the drugs must be delivered to cancer cells at a fixed constant rate. However, this is difficult owing to the different pharmacokinetic properties of drugs [41]. In addition, the toxicity of drugs to normal cells can cause problems. These problems can be solved using nanoparticles as a carrier for combination drugs. Ratiometric drug release is a drug delivery system that simultaneously releases drugs that are encapsulated in a nanocarrier, and this method can integrate the pharmacokinetics of different drugs (Figure 2) [42]. In the case of ratiometric drug delivery, examples were divided according to how combinatorial drugs were simultaneously released intracellularly, and the techniques used and drug efficacy were discussed.

Figure 2. Ratiometric drug delivery of combinatorial drugs using nanoparticles is more advantageous in terms of pharmacokinetics and biodistribution of drug combinations compared to free combinatorial drugs. Reproduced with permission from [40], Journal of Controlled Release, 2016.
2.1. Release of Co-Loaded Drugs through pH Control
Penetration of drugs into tumor tissue is considerably difficult owing to the abnormal extracellular matrix and high cancer cell density [43,44]. Therefore, for better efficacy, anticancer drugs should act more selectively on cancer cells than on normal cells. Tumor tissue has a relatively low pH than normal cells; therefore, anticancer agents should promote drug release under acidic pH conditions.
To effectively penetrate tumor tissues, Xu et al. prepared cationic nanoparticles of VES-g-ε-PLL (Cur-NPs) encapsulating the well-known natural anticancer agent hydrophobic curcumin (Cur) in vitamin E succinate-grafted-ε-polylysine (VES-g-ε-PLL) [28,45]. Then, pH-sensitive core–shell nanoparticles (PDCP-NPs) were formed using doxorubicin (DOX) hydrochlorate and Cur-NPs in dopamine-modified-poly-γ-glutamic acid polymer (γ-PGA-Dopa) (Figure 3). In these nanoparticles, γ-PGA provides a drug-loading site for most primary chemotherapeutic drugs via carboxyl–metal ion coordination or electrostatic interactions. In general, these nanoparticles differ from other nanoparticles combining two drugs in a polymer because DOX is encapsulated in the outer shell. γ-PGA has high biocompatibility and biodegradability; thus, it is nontoxic to the human body and contributes to the stability of PDCP-NPs in vivo and in vitro. In addition, γ-PGA improves drug delivery efficiency by contributing to the intracellular absorption of cancer cells. The side carboxyl groups of γ-PGA coated on PDCP-NPs are protonated in acidic conditions to promote the rapid release of DOX. Free amino groups of exposed Cur-NP are also protonated to increase the release rate of Cur from PDCP. Through this process, drugs with different physical properties can be released proportionally. Cur and DOX encapsulated at a ratio of 3:1 in PDCP-NPs were released at a ratio close to 3:1 in cancer cells, and they subsequently inhibited the rapid proliferation of cancer cells and caused apoptosis. In vivo, the PDCP-NP treatment group showed stronger antitumor effects than the single-drug-loaded nanoparticle treatment group. Thus, simultaneous delivery of Cur and DOX showed better treatment efficiency than administration of single-drug-loaded nanoparticles. In addition, tumor volume increased over time in the brain of glioma rats treated with CUR/DOX complex liquid and bilayer pH-sensitive DOX nanoparticles, whereas tumor growth inhibition was observed in mice with treated with PDCP-NPs. Therefore, it was confirmed that the survival rate of mice after PDCP-NP treatment was prolonged compared that of mice after control treatment [28].
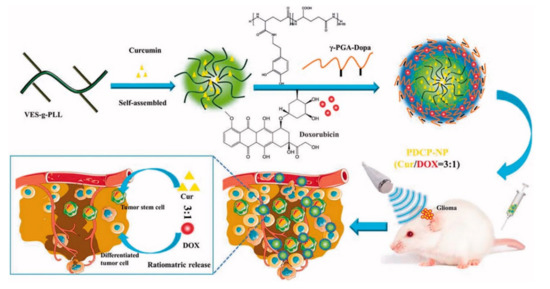
Figure 3. Schematic diagram of pH-sensitive core–shell nanoparticles for ratiometric drug release. The curcumin/doxorubicin co-loaded on the pH-sensitive core–shell nanoparticles is released at a constant ratio in cancer cells. Reproduced with permission from [28], Drug delivery, 2018.
Nanoparticles can accumulate in tumors through the EPR effect, but inefficient intracellular release results in inefficient treatment [46,47,48,49]. To solve this problem, Guo et al. studied positively charged polymer nanoparticles to improve drug bioavailability through strong adsorption of negatively charged cell membranes and cationic polymer nanoparticles [29]. The surface of cationic nanoparticles is usually decorated with amino-rich functional groups. One example is ε-poly-l-lysine (EPLYS), a naturally biodegradable homopoly(amino acid), which demonstrated no cytotoxicity with the resultant nanoparticles [29].
Thus, Guo et al. fabricated novel dual drug-loading polymeric nanoparticles using polyethylene glycol (PEG) and EPLYS that physically encapsulated lapatinib (LAP) and DOX (DMMA-P-DOX/LAP nanoparticles) [50,51,52,53,54,55,56,57]. In these polymer–drug conjugates, an acid-cleavable linker was inserted between the drug molecule and the polymer, accelerating the decomposition of the conjugate under intracellular pH conditions to accurately deliver and release drugs [58,59,60,61,62]. Therefore, DOX was conjugated to the hydrophilic PEG-EPLYS backbone through acid-labile imine bonds, and LAP was physically encapsulated into the nanoparticles; thus, after the cleavage of imine bond, the remaining hydrophobic chain was rendered insufficient, leading to rapid decomposition of nanoparticles. Through these processes, DOX and LAP were simultaneously released. The DMMA-P-DOX/LAP nanoparticle showed that, following intravenous injection, nanoparticles accumulated in the tumor tissue through the EPR effect, and the surface charge reversed from negative to positive, enhancing tumor cell internalization [29,63]. As a result, the low pH of the cells caused the cleavage of residual amino groups, thus rapidly breaking down nanoparticles. Therefore, DOX and LAP were simultaneously released accurately into the cytoplasm, effectively inhibiting cell proliferation. As a result of confirming the antitumor effect in vivo, the tumor was more suppressed in the group treated with DOX and LAP nanoparticles compared to the group treated with only free DOX, free LAP, and DOX nanoparticles. In addition, it was confirmed that tumor volume in the DMMA-P-DOX/LAP nanoparticle group decreased more rapidly than that in the DMMA-P-DOX nanoparticle group, and the tumor was completely removed after chemotherapy [29].
Nanoparticles are characterized by stimulus responsiveness for effective drug release at the target site, releasing drugs with environmental changes. Among various stimuli, the pH of the endosome/lysosome (pH 5.0) in cancer cells is relatively lower than that of the extracellular environment; therefore, pH responsiveness is most often used for ensuring drug release from nanoparticles [47,64,65].
2.2. Release of Co-Loaded Drugs through Polymeric Degradation
It is considerably difficult to formulate nanoparticles using drugs with different physicochemical properties [5]. For example, cisplatin is characterized by limited solubility in both water and oil, and gemcitabine monophosphate (GMP) is a hydrophilic drug [66,67]. Cisplatin and GMP have different physicochemical properties and have limitations for loading in nanoparticles. To solve this problem, Miao et al. formulated nanoparticles after wrapping cisplatin and GMP with different characteristics using dioleoyl phosphatidic acid (DOPA) (Figure 4) [30]. Therefore, poly(lactic-co-glycolic acid) (PLGA) nanoparticles were formed using a DOPA-coated cisplatin core (CP core), DOPA-coated GMP core (GMP core), and PLGA. To further improve the internalization of PLGA nanoparticles into cancer cells, a ligand that acts with a receptor overexpressed on the surface of cancer cells was introduced into PLGA nanoparticles. The prerequisite for control of delivery in this way is to incorporate the physicochemical properties of the dual drugs by taking advantage of the similarities of the surface and size of the core. The advantage of this method is that it avoids functional indirection between individual molecules, allowing for precise ratio loading and delivery. In addition, the optimal combination drug ratio was more effective than single nanoparticles loaded with GMP and cisplatin separately, and it showed remarkable anticancer efficacy [2,68].
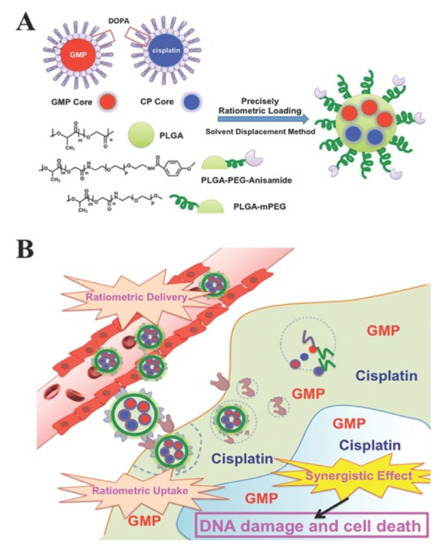
Figure 4. (A) Schematic diagram of PLGA-PEG-Anisamide NP (PLGA NP) including a dioleoyl phosphatidic acid (DOPA)-coated cisplatin core (CP core) and DOPA-coated gemcitabine monophosphate core (GMP core) through solvent substitution method. (B) Ratiometric drug delivery of CP core and GMP core co-loaded PLGA nanoparticles (combination NP) into cancer cells. Reproduced with permission from [30], Advanced Functional Materials, 2014.
It was confirmed that the IC50 value of PLGA NP loaded into CP cores and GMP cores (combo NP) was smaller than the GMP nanoparticle and cisplatin nanoparticle. In addition to in vivo antitumor efficacy for tumor transplant models, combo free administration showed that the weight of the tumor was lower than that of the tumor when free cisplatin and free GMP were injected. On the other hand, the IC50 value of combo NP was larger than that of combo free, but there was no significant difference. The tumor weight was lower when a separate NP was injected than that when cisplatin NP and GMP NP were administered, and the tumor weight when combo NP was administered was the lowest. These results show that combination NPs containing cisplatin and GMP exhibited improved anticancer effects compared with a single drug.
In another study, rapamycin (RAPA), an mTOR inhibitor, was combined with cisplatin. It was shown that co-delivery of RAPA with cisplatin could significantly promote the efficacy of RAPA through microenvironment regulation [69,70]. However, encapsulating these drugs in PLGA NPs was inefficient owing to the incompatibility between the two drugs and the polymer matrix. Here, as in the previous example, the nano precipitate (cores) of the drug was coated with DOPA to make cisplatin hydrophobic [66,71,72]. Guo et al. attempted to co-encapsulate DOPA-coated cisplatin and RAPA in PLGA NPs using a solvent displacement method to improve the encapsulation and loading efficiency of the hydrophobic drugs [31]. The combination NPs showed sustained release of both cisplatin and RAPA, with similar release rates. This release rate suggests that the explosive release of cisplatin from PLGA NPs was prevented by the hydrophobic DOPA coating. In addition, the IC50 of combined drug was lower than that of the single drugs, and the IC50 of the PLGA-NP-encapsulated drug was lower than that of the free drug, showing better anticancer effect. In addition, in the in vivo experiment, it was confirmed that the tumor size decreased when (RAPA + cisplatin) NP was administered compared to when cisplatin NPs, RAPA NPs, and RAPA NPs + cisplatin NPs were administered [31].
Therefore, encapsulation of a hydrophilic phosphorylated drug generated via lipid coating of the surface layer of the calcium phosphate core using DOPA is an efficient method for achieving ratiometric drug delivery.
2.3. The Release of Co-Loaded Drugs through Enzymatic Degradation
Drug delivery systems that use polymer–drug conjugates not only have advantages such as reduction of drug toxicity, tumor accumulation through the EPR effect, and improvement of bioavailability, but they can also control the molar ratio of different drugs more elaborately than the drug encapsulation method using nanoparticles [73,74,75,76,77]. Drug–polymer conjugates have succeeded in maintaining the ratio between several different drugs, but the ratiometric release of different drugs from a carrier is still a difficult task due to the variability of drug and polymer interaction and the steric hindrance of drugs [20,78,79,80,81].
To compensate for this problem, Luo et al. studied a novel method of loading double drugs into a macromolecular carrier at different molar ratios of DOX and mitomycin C (MMC), which are widely known anticancer agents; however, they have serious side effects when administered as free drugs [32,76,82]. As a drug carrier, xyloglucan (XG), a natural and nontoxic polysaccharide was used, and tripeptide Gly-Leu-Gly which is degraded by lysosomal enzymes was used as a linker capable of attaching DOX and MMC to the XG [83,84,85]. XG-MMC/DOX was formulated using the anticancer drugs DOX and MMC, the carrier XG, and the linker tripeptide Gly-Leu-Gly that attaches the drug and the carrier. XG-MMC/DOX accumulates in the tumor via the EPR effect and reaches the lysosomal compartment of cancer cells, whereas the linker is degraded by the lysosomal enzymes, leading to drug release from the XG, thereby enabling efficient ratiometric drug release in cancer cells [86]. XG-MMC/DOX showed a superior anticancer effect in in vitro and in vivo cytotoxicity studies compared to cocktail mixtures of anticancer drugs such as XG-MMC and XG-DOX. Therefore, a polymer–drug conjugate complex, which uses a linker such as tripeptide Gly-Leu-Gly, is considered to have sufficient advantages for ratiometric drug delivery for anticancer therapy.
Ratiometric drug release is an important release technology that increases the efficacy of drugs by controlling the pharmacokinetics of two different drugs, delivering drugs better to the target than cocktail therapy. However, more advanced strategies are needed that further consider the MDR.
3. Sequential Drug Release
Although the co-administration of multiple drugs is a major strategy to overcome drug resistance, it might limit the synergistic effects of drugs in a heterogeneous tumor environment [81,87,88]. As the research on NFCDs continued, it was confirmed through advanced studies that not only were NFCDs released in a ratiometric manner in cancer cells but also sequentially (time or space differences). As drugs show differences in solubility or cancer-inhibitory mechanisms, there are cases where sequential release of drugs from NFCD complexes is advantageous. In addition, sequential release can be spatiotemporal, in which drugs are released only in the predetermined order of release within cancer cells. Therefore, we divided sequential drug release into intracellular sequential drug release, in which drugs are released only inside cancer cells in a certain release sequence, and spatiotemporal drug release, in which drugs are released site-specifically outside and inside cancer cells.
3.1. Intracellular Sequential Drug Release
The sequential release of NFCDs in cells is a temporal concept of drug release. The characteristic of this technology is that, unlike in conventional ratiometric release, the order in which drugs are released affects their anticancer efficacy. For example, in the delivery of a P-glycoprotein (P-gp) inhibitor and an anticancer drug, rather than releasing the two drugs ratiometrically into the cell at the same time, the P-gp inhibitor is released first to inhibit P-gp, followed by the release of the anticancer agent to more efficiently overcome MDR [89]. In addition, sequential release of drugs can lead to improved safety and reduced toxicity. Therefore, there is a need for nanoparticle formulations that can sequentially release several drugs at the target site. We divided the cases into the mechanism via which two drugs directly affect cancer cells through sequential release and the mechanism via which one drug amplifies the effect of other drug.
3.2. Sequential Drug Release to Achieve both Intercellular and Intracellular (Spatiotemporal) Drug Release
The aforementioned studies suggested that, even if the drug release profile of NFCDs is ratiometric or sequential, it is difficult to completely treat cancer using these release profiles owing to the characteristics of solid tumor microenvironment (TME). The TME is both heterogeneous and complex as it contains various tumor tissues, including subgroups of genetically diverse cancer cells and nonmalignant stromal cells, which promote tumor cell survival, growth, and resistance to drugs during treatment [155,156,157,158,159]. Therefore, an effective cancer strategy that not only targets cancer cells but also the TME is needed. Thus, taking TME into consideration, NFCDs capable of spatiotemporal controlled release, which is a new paradigm where drugs related to TME treatment are first released in the TME and then internalized into cancer cells to release anticancer agents, are being developed in recent anticancer studies [160].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics12121156
