The gluten-free diet (GFD) has gained increasing popularity in recent years, supported by marketing campaigns, media messages and social networks. Nevertheless, real knowledge of gluten and GF-related implications for health is still poor among the general population. The GFD has also been suggested for non-celiac gluten/wheat sensitivity (NCG/WS), a clinical entity characterized by intestinal and extraintestinal symptoms induced by gluten ingestion in the absence of celiac disease (CD) or wheat allergy (WA).
- gluten
- gluten-free diet
- low FODMAP diet
1. Introduction
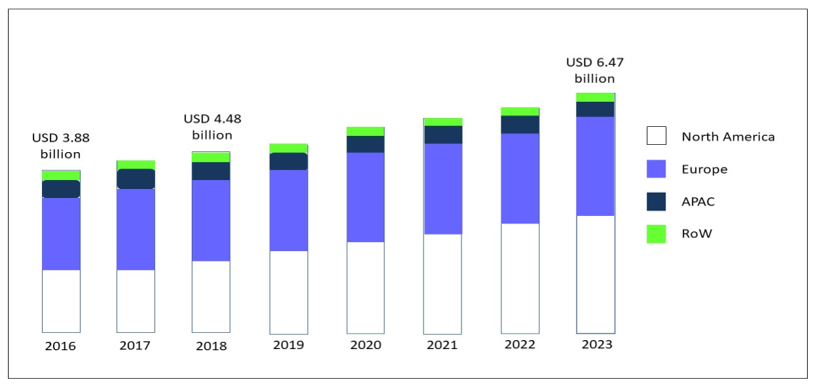
Over the last 30 years, the gluten-free diet (GFD) has gained increasing popularity associated with an exponential growth in the sales of gluten-free (GF) products [1]. The global market for GF food, driven by North America and Europe, but now spreading across the Asia-Pacific countries (APAC), was valued at USD 3.88 bn in 2016, and is foreseen to expand to USD 6.47 bn in 2023, at a compound annual growth rate (CAGR) of 7.60% [2] (Figure 1).
Figure 1. GF product market, by region, during the forecast period of 2016 to 2023, modified from Research and Markets, Report May 2019 [2]. Abbreviations: GF: gluten-free; APAC: Asia-Pacific countries; RoW: rest of the world.
In the USA, a follow-up analysis of the National Health and Nutrition Examination Survey (NHANES) revealed that self-adoption of a GF diet without a diagnosis of celiac disease (CD) tripled from 2009-2010 (prevalence 0.52%) to 2013-2014 (prevalence 1.69%) [3] and NPD’s Dieting Monitor, which tracks nutrition-related issues of consumers, in 2013 reported that nearly 30% percent of adults claimed to cut down on or avoid gluten [4]. In Italy, where bread and pasta are the foundation of food culture, is in the vanguard of the European GF sector with the range of products jumping from 280 in 2001 to the current 6500 and a market amounting to EUR 320 million, of which only 215 are dispensed on prescription for celiac patients. The launch of innovative products containing no or less gluten and dominated by the bakery product segment is on the rise and contributes to boosting this industry. Suppliers continue to invest in innovation to improve taste, texture and overall quality in GF formulations.
The GFD is recommended as lifelong treatment for CD. However, neither government awareness campaigns and initiatives nor the improvement of diagnostic tools and increasing prevalence of CD [5] account for the overwhelming adoption of a GF lifestyle. Clinical application of GFDs continues to escalate as a therapeutic option for non-celiacs who seem to react negatively to gluten ingestion, are trying to lose weight [6] or simply want to reduce bloating after meals [7]. The reasons given for a self-imposed GFD include irritable bowel syndrome (IBS) and lactose intolerance [8]. Other persons spontaneously limit or eliminate gluten intake as a “healthy” dietary regimen without previous clinical tests due to the widespread consumer interest in free-from products and the growing adoption of specific eating patterns in pursuit of health and wellness [9]. In an Australian cross sectional population survey [10], symptomatic wheat avoidance was highly correlated with dairy avoidance, female gender and lesser and greater receptiveness to conventional and complementary medicine, respectively. While perception of the potential harm and expected benefits of gluten consumption/avoidance are high, real knowledge of gluten and GF-related implications for health is scarce. An American survey [11] found that over 30% of respondents had no specific reason for adopting a GF regimen, while less than 10% self-reported gluten sensitivity (GS). The other reasons were a healthy lifestyle, improvement of intestinal health or the presence of a gluten-sensitive family member. Different factors lie at the basis of the GF movement, mostly driven by non-scientific sources of information. While Google searches containing “low carb” and “low fat” have declined since 2004, worldwide searches for “gluten” showed a sharp upward trend, reaching the peak of food concerns from 2005 to 2014. From then on, they have remained generally steady. In Italy, an increase in the number of searches was observed until mid-2019, then there was a decline. This far exceeds lactose, genetically modified organisms (GMO) and palm oil, with ratios approaching 16:1, 6:1 and 2:1, respectively. Marketing campaigns aimed at extending the appeal of GF to every health-conscious consumer despite the high costs of products. Moreover, athletes and celebrities, together with mass media messages and social network platforms, all contribute to increasing awareness of gluten intolerance and fuel the interest in dietary treatments. Consumers commonly select GF products from aisles in major supermarkets and health food shops [12]; for many consumers, the front of package claims are more important determinants of GF product choices [13] than nutritional labeling [14]. Several studies have shown an excessive intake of fats and carbohydrates, with a lower consumption of dietary fibers, in CD patients on a GFD [15]. Moreover, clinically relevant deficiencies of iron, vitamin D, vitamin B6 and zinc have been reported in CD patients during treatment with a GFD, whereas data on deficiencies of vitamin B12, folic acid, calcium and magnesium are controversial [16]. The alarming discrepancy between media claims and scientific evidence [17] drives the motivation and reinforcement of people’s commitment to avoiding gluten, generating a great deal of confusion and misconceptions [18].
2. What About Gluten?
2.1. Gluten Structure and Genetics
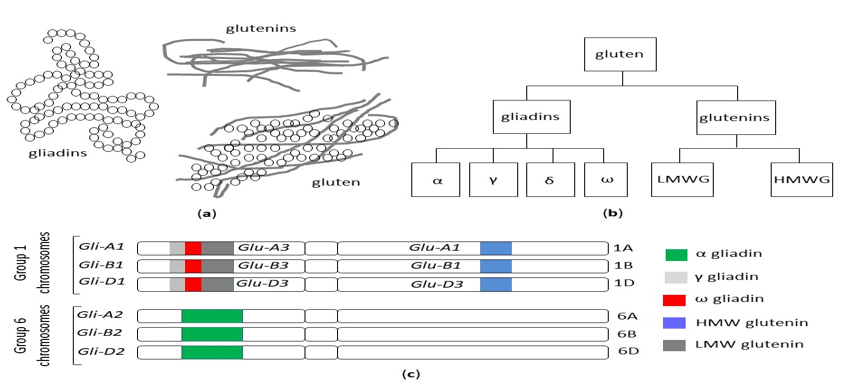
The term gluten collectively refers to a family of storage proteins, formally known as prolamins, naturally occurring in wheat, barley and rye, and their cross-bred grains [19]. Although oats also contain prolamins (avenins), they represent a small (10 to 15%) proportion of total protein content, in comparison with 80 to 85% of wheat gliadins. Moreover, avenins contain less proline than the other prolamins, are more easily digested and the peptides show less affinity for MHC II peptides encoded by HLA DQ2.5 haplotypes [20]. These properties could make oats generally safe for celiac patients, although individual hypersensitivity in some celiac patients can occur [21][22], and some varieties may display immunogenicity/toxicity [23][24]. The wheat prolamins are termed gliadins, monomeric proteins divided into four distinct subtypes, referred to as alpha, gamma, delta and omega gliadins and glutenins, and polymeric proteins composed of high (HMW-GS) and low molecular weight subunits (LMW-GS) linked by disulfide bonds [25] (Figure 2a,b).
Figure 2. (a) The structure of the gluten protein gluten network. (b) The classification of wheat gluten proteins. (c) Gliadin and glutenin loci in Triticum aestivum (AABBDD), modified from Sharma, 2020 [26]. HMW: high molecular weight, LMW: low molecular weight.
A significant proportion of prolamins are represented by repetitive sequences of glutamine and proline. The various wheat varieties differ in terms of prolamin molecular weight and microstructure (junction density, branching rate, lacunarity). These characteristics influence the strength of the network and the dough quality and, in turn, determine the tensile and cooking properties [19][25][27]. Due to its unique biochemical and functional features (water-binding and visco-elastic properties, gas retention), gluten is essential for baking but also widely used as an additive in processed food.
Besides its commercial value, the detrimental effects of gluten on human health have been described, mediated by immunological or toxic reactions [28]. Due to the high number of glutamine- and proline-rich periodic sequences, gluten peptides are highly resistant to gastric and intestinal proteolytic degradation, thus giving rise to potentially immunogenic fragments. In addition, gluten alters intestinal permeability, promotes oxidative stress, exerts cytotoxic and pro-inflammatory effects and negatively affects the microbiome; cell apoptosis is increased and cell differentiation is reduced [29]. In celiac patients carrying HLA DQ2/DQ8 haplotypes, gluten triggers an innate, as well as adaptative, Th1-driven immune response, amplified by transglutaminase-mediated synthesis of negatively charged glutamate residues from glutamine [26]. Since the Neolithic Age agricultural revolution, 10,000 years ago, ancient grasses have been domesticated and spread from the Fertile Crescent of the Middle East westward through Europe [30]. Agricultural techniques increased the abundance and availability of wheat, but it is only in the past 500 years that the gluten content of foods containing wheat has significantly increased. Modern hexaploid wheat cultivars have three different genomes (A, B and D) and evolved from the original diploid wheat, called einkorn (Triticum monococcum), through thousands of years of selective breeding and the development of tetraploid varieties [31] (Figure 2c). It has been posited that the genetic evolution, introducing new sequences into the wheat genome, could have potentially led to an increase in toxic and immunogenic epitopes responsible for the increased prevalence of CD [32] and, in general, of gluten-related disorders. A high-quality genome sequence was established from the reference wheat Chinese Spring, which made a complete set of gluten protein genes available from a single hexaploid cultivar [33][34]. Nevertheless, the large number of different wheat cultivars around the world, the high allelic variation in gluten genotypes among cultivars and the large number of immunogenic gluten epitopes make it difficult to draw firm conclusions, and the real contribution of modern wheat breeding practices to the increased prevalence of CD is still a matter of debate. Data on the reduced immunogenicity of old wheat genotypes because of the absence of the D-genome [32] have not been confirmed by more recent studies [35][36][37][38] and their health-promoting properties emerging from recent studies appear to rely on other features rather than their low immunogenicity. The macro- and micronutrient contents of ancient grains seem to decrease the risk of cardiovascular disease and metabolic syndrome, ameliorate the glycolipid profile and reduce oxidative stress and the level of pro-inflammatory cytokines. Furthermore, their consumption has been reported to curtail the extent and severity of IBS-related symptoms [39].
In the absence of convincing evidence for a role of wheat breeding in the increasing prevalence of gluten-related diseases, change in per capita consumption of wheat flour and the usage of vital gluten as a processed food additive have been postulated [40].
2.2. Is There a Role for Microbiota?
In recent years, the impact of gut microbiota on the loss of gluten tolerance has received increasing attention. The intestinal microbial communities represent a complex ecosystem, which plays a central role in modulating both innate and adaptive immune responses [41][42][43][44]. They are also involved in the maintenance of mucosal barrier function, which is a crucial mediator between our body and the external environment, and prevent the entry of toxic/immunogenic molecules across the intestinal wall [43][44].
In both stools and mucosal biopsies of celiac patients, a shift toward Bacteroides, Clostridium and Escherichia coli, with reduction in protective Bifidobacteria, Firmicutes and Lactobacilli, in comparison with non-celiac controls, has been described [45][46] as being partially restored by a GFD [47][48][49]. Both genetic makeup and environmental factors contribute to shaping the composition and diversity of the intestinal microbiota. Infants with a high genetic risk of developing CD harbor a higher proportion of Firmicutes and Proteobacteria and a lower proportion of Actinobacteria [50], resulting in an increased prevalence of pathogenic bacteria compared to those with a low risk [51]. According to the hygiene hypothesis, the decreased infectious pressure observed in industrialized countries over the last several decades should prevent the development of a functional immune system during early childhood, leading to an imbalance between pro-inflammatory and anti-inflammatory responses. Additional main drivers of microbial gut colonization, such as mode of delivery, infant feeding practice and antibiotic use, were not confirmed as risk factors for CD [52][53][54][55]. Although most studies report major differences in the composition of microbiota between celiac patients and healthy controls, a specific microbial profile cannot be identified in CD [56]. Evidence on the causal relationship between dysbiosis and disease occurrence is highly heterogeneous and controversial due to inter-individual variability, small sample sizes and different methodologies, which all hamper the interpretation of results [57]. Finally, it is still unclear whether an altered microbiota in CD patients is the cause or the consequence of mucosal inflammation [58]. The exact mechanisms by which a dysbiotic status could contribute to CD development are also still unknown and include the processing of gluten peptides, activation of innate immune response and modulation of intestinal permeability [59][60].
2.3. Gluten Consumption
The phenomenon of globalization is driving a revolution in food systems (supply, marketing and distribution) as well as in dietary patterns. Major changes in food culture are closely associated with urbanization, increasing incomes, capital flow and market liberalization, and are characterized by dietary convergence, a phenomenon occurring as a result of increased reliance on a narrow base of staple foods, among which the dominant staple grain is wheat [61]. Palatability, ease of large-scale cultivation, industrial food processing and low prices have all contributed to the global spread of wheat gluten consumption. Wheat production has increased sharply since 1955, showing an impressive tenfold increase in the annual rate of yield improvement, particularly in the 1960s, and gradually afterwards. This was thanks to a technology shift commonly labeled the “green revolution” [62][63]. The green revolution resulted in the development of rust-resistant semi-dwarf, high-yield wheat. Between 1980 and 2013, the world’s annual wheat yield increased by 1.41% [64]. Currently, North America maintains the leading position in the wheat gluten market, followed by Europe. The abundance of applications in the food industry and high demand for high-fiber and meat-free foods among an increasingly health-conscious and vegan/vegetarian population are considered key factors boosting the growth of the wheat gluten industry in Western countries [65]. The global wheat protein market was estimated to be valued at USD 2.04 billion in 2017 and it was foreseen to grow at a compound annual growth rate (CAGR) of 4.8% from 2017, to reach USD 2.58 billion by 2022 [66].
In highly populated, developing countries, particularly those in the Asian region, the growing middle class, adopting Western-style diets with a higher content of wheat products, have contributed to increasing its consumption [64]. Recently, global change in consumption patterns and consumer attitudes during coronavirus lockdowns, and in particular the boom of home baking, boosted a sharp increase in wheat consumption: the Spanish Minister of Agriculture, Luis Planas, revealed that sales of flour quadrupled during the third week of lockdown; Nielsen data showed that in March 2020, the retail flour sales in France, the US and Italy increased by 140, 154 and 185 percent, respectively, compared with the same period in 2019.
Vital wheat gluten (VWG) is obtained from wheat flour by removing soluble fibers and starch fractions and recovering gliadins and glutenins [67]. VWG is widely used as an additive in bakery products and pasta dough to increase yields and improve rheological, microstructure and quality characteristics [68][69]. Due to its visco-elasticity and the range of functional properties at a lower price than competitors, such as milk and soy proteins, have contributed to spreading its use in the food industry, leading to a tripled consumption since 1977, consistent with the epidemiology of CD [40].
2.4. Gluten Exorphins
Exogenous peptides with opioid-like activities, which include gluten exorphins (wheat), casomorphins (milk), rubiscolins (spinach) and soymorphins (soybean) [70], display regulatory functions both for the gastrointestinal and central nervous systems. In rodent behavioral models, food-derived, opioid-like peptides affect nociception, spontaneous behavior and memory. After oral, intracerebroventricular or intraperitoneal administration, some food-derived opioids also affect intestinal motility, hormone release, appetite, mucus production and local immunity [71][72]. In rats, the opioid antagonist naloxone drastically reduces the intake of preferred foods [73][74].
Enzymatic breakdown of gliadin from wheat by intestinal pepsin, leucine aminopeptidase and elastase generates morphine-like peptides, also known as gluten exorphins [75]. In healthy volunteers, early research showed that gluten exorphins induced a significant increase in gastrointestinal transit time, reversible after administration of the opioid antagonist naloxone [75][76].
In rodents, orally administered gluten exorphin A5 suppressed the endogenous pain-inhibitory system induced by socio-psychological stress and modified spontaneous behavior and learning/memory processes during several laboratory stressors, indicating that the peptides may cross the blood–brain barrier [77]. It has been suggested that the effects of food exorphins could be amplified if they are absorbed in excess through a disrupted mucosal barrier [78].
This entry is adapted from the peer-reviewed paper 10.3390/nu12123785
References
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M.W. Gluten-Free Diet Indications, Safety, Quality, Labels, and Challenges. Nutrients 2017, 9, 846, doi:10.3390/nu9080846.
- Research and Markets. Global Gluten-Free Food Market (2018–2023) Report, ID: 4856374; Research and Markets: Dublin, Ireland, 2019.
- Kim, H.S.; Patel, K.G.; Orosz, E.; Kothari, N.; Demyen, M.F.; Pyrsopoulos, N.; Ahlawat, S.K. Time Trends in the Prevalence of Celiac Disease and Gluten-Free Diet in the US Population. Results From the National Health and Nutrition Examination Surveys 2009–2014. JAMA Intern. Med. 2016, 176, 1716–1717, doi:10.1001/jamainternmed.2016.5254.
- NPD Group. Percentage of U.S. Adults Trying to Cut Down or Avoid Gluten in Their Diets Reaches New High in 2013. Available online: http://www.npd.com/wps/portal/npd/us/news/press-releases/percentage-of-us-adults-trying-to-cut-down-or-avoid-gluten-in-their-diets-reaches-new-high-in-2013-reports-npd (accessed on 27 June 2020).
- Gatti, S.; Lionetti, E.; Balanzoni, L.; Verma, A.K.; Galeazzi, T.; Gesuita, R.; Scattolo, N.; Cinquetti, M.; Fasano, A.; Catassi, C. Celiac Screening Team. Increased Prevalence of Celiac Disease in School-age Children in Italy. Gastroenterol. Hepatol. 2020, 18, 596–603, doi:10.1016/j.cgh.2019.06.013.
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204, doi:10.1053/j.gastro.2014.12.049.
- Zannini, E.; Arendt, E.K. Low FODMAPs and gluten-free foods for irritable bowel syndrome treatment: Lights and shadows. Food Res. Int. 2018, 110, 33–41, doi:10.1016/j.foodres.2017.04.001.
- Tanpowpong, P.; Broder-Fingert, S.; Katz, A.J.; Camargo, C.A., Jr. Predictors of dietary gluten avoidance in adults without a prior diagnosis of celiac disease. Nutrition 2015, 31, 236–238, doi:10.1016/j.nut.2014.07.001.
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97, doi:10.1007/s40279-018-01034-0.
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499, doi:10.1017/S1368980014000652.
- The Hartman Group, Inc. The Hartman Group’s Health & Wellness 2015 and Organic & Natural 2014 Reports. Available online: http://www.hartman-group.com/acumenPdfs/gluten-free-2015-09-03.pdf (accessed on 22 December 2015).
- Gorgitano, M.T.; Sodano, V. Gluten-Free Products: From Dietary Necessity to Premium Price Extraction Tool. Nutrients 2019, 11, 1997, doi:10.3390/nu11091997.
- Zysk, W.; Głąbska, D.; Guzek, D. Role of Front-of-Package Gluten-Free Product Labeling in a Pair-Matched Study in Women with and without Celiac Disease on a Gluten-Free Diet. Nutrients 2019, 11, 398, doi:10.3390/nu11020398.
- Hartmann, C.; Hieke, S.; Taper, C.; Siegrist, M. European consumer healthiness evaluation of ‘Free-from’ labelled food products. Food Qual. Prefer. 2018, 68, 377–388, doi:10.1016/j.foodqual.2017.12.009.
- Di Liberto, D.; Carlisi, D.; D’Anneo, A.; Emanuele, S.; Giuliano, M.; De Blasio, A.; Calvaruso, G.; Lauricella, M. Gluten Free Diet for the Management of Non Celiac Diseases: The Two Sides of the Coin. Healthcare 2020, 8, 400, doi:10.3390/healthcare8040400.
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500, doi:10.3390/nu12020500.
- Di Sabatino, A.; Corazza, G.R. Nonceliac gluten sensitivity: Sense or sensibility? Intern. Med. 2012, 156, 309–311, doi:10.7326/0003-4819-156-4-201202210-00010.
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Hepatol. (N. Y.) 2018, 14, 82–91.
- Biesiekierski, J.R. What is gluten? Gastroenterol. Hepatol. 2017, 32, 78–81, doi:10.1111/jgh.13703.
- Hoffmanová, I.; Sánchez, D.; Szczepanková, A.; Tlaskalová-Hogenová, H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients 2019, 11, 2345, doi:10.3390/nu11102345.
- Lundin, K.E.; Nilsen, E.M.; Scott, H.G.; Løberg, E.M.; Gjøen, A.; Bratlie, J.; Skar, V.; Mendez, E.; Løvik, A.; Kett, K. Oats induced villous atrophy in coeliac disease. Gut 2003, 52, 1649–1652, doi:10.1136/gut.52.11.1649.
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, e1, doi:10.1371/journal.pmed.0010001.
- Silano, M.; Pozo, E.P.; Uberti, F.; Manferdelli, S.; Del Pinto, T.; Felli, C.; Budelli, A.; Vincentini, O.; Restani, P. Diversity of oat varieties in eliciting the early inflammatory events in celiac disease. J. Nutr. 2014, 53, 1177–1186, doi:10.1007/s00394-013-0617-4.
- Poley, J.R. The Gluten-Free Diet: Can Oats and Wheat Starch Be Part of It? Am. Coll. Nutr. 2017, 36, 1–8, doi:10.1080/07315724.2015.1085815.
- Shewry, P. What Is Gluten-Why Is It Special? Nutr. 2019, 6, 101, doi:10.3389/fnut.2019.00101.
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Nutr. 2020, 7, 6, doi:10.3389/fnut.2020.00006.
- Li, S.; Liu, Y.; Tong, J.; Yu, L.; Ding, M.; Zhang, Z.; Rehman, A.U.; Majzoobi, M.; Wang, Z.; Gao, X. The overexpression of high-molecular-weight glutenin subunit Bx7 improves the dough rheological properties by altering secondary and micro-structures of wheat gluten. Food Res. Int. 2020, 130, 108914, doi:10.1016/j.foodres.2019.108914.
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644, doi:10.3390/nu8100644.
- Lerner, A.; Shoenfeld, Y.; Matthias, T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Rev. 2017, 75, 1046–1058, doi:10.1093/nutrit/nux054.
- Balfourier, F.; Bouchet, S.; Robert, S.; De Oliveira, R.; Rimbert, H.; Kitt, J.; Choulet, F.; Paux, E. International Wheat Genome Sequencing Consortium; BreedWheat Consortium. Worldwide phylogeography and history of wheat genetic diversity. Adv. 2019, 5, eaav0536, doi:10.1126/sciadv.aav0536.
- Venske, E.; Dos Santos, R.S.; Busanello, C.; Gustafson, P.; Costa de Oliveira, A. Bread wheat: A role model for plant domestication and breeding. Hereditas 2019, 156, 16, doi:10.1186/s41065-019-0093-9.
- Van den Broeck, H.C.; de Jong, H.C.; Salentijn, E.M.; Dekking, L.; Bosch, D.; Hamer, R.J.; Gilissen, L.J.; van der Meer, I.M.; Smulders, M.J. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: Wheat breeding may have contributed to increased prevalence of celiac disease. Appl. Genet. 2010, 12, 1527–1539, doi:10.1007/s00122-010-1408-4.
- Huo, N.; Zhang, S.; Zhu, T.; Dong, L.; Wang, Y.; Mohr, T.; Hu, T.; Liu, Z.; Dvorak, J.; Luo, M.C.; et al. Gene Duplication and Evolution Dynamics in the Homeologous Regions Harboring Multiple Prolamin and Resistance Gene Families in Hexaploid Wheat. Plant Sci. 2018, 9, 673, doi:10.3389/fpls.2018.00673.
- Huo, N.; Zhu, T.; Altenbach, S.; Dong, L.; Wang, Y.; Mohr, T.; Liu, Z.; Dvorak, J.; Luo, M.C.; Gu, Y.Q. Dynamic Evolution of α-Gliadin Prolamin Gene Family in Homeologous Genomes of Hexaploid Wheat. Rep. 2018, 8, 5181, doi:10.1038/s41598-018-23570-5.
- Malalgoda, M.; Meinhardt, S.W.; Simsek, S. Detection and quantitation of immunogenic epitopes related to celiac disease in historical and modern hard red spring wheat cultivars. Food Chem. 2018, 264, 101–107, doi:10.1016/j.foodendritichem.2018.04.131.
- Prandi, B.; Tedeschi, T.; Folloni, S.; Galaverna, G.; Sforza, S. Peptides from gluten digestion: A comparison between old and modern wheat varieties. Food Res. Int. 2017, 91, 92–102, doi:10.1016/j.foodres.2016.11.034.
- Ficco, D.B.; Prandi, B.; Amaretti, A.; Anfelli, I.; Leonardi, A.; Raimondi, S.; Pecchioni, N.; De Vita, P.; Faccini, A.; Sforza, S.; et al. Comparison of gluten peptides and potential prebiotic carbohydrates in old and modern Triticum turgidum ssp. genotypes. Food Res. Int. 2019, 120, 568–576, doi:10.1016/j.foodres.2018.11.007.
- Ribeiro, M.; Nunes, F.M. We might have got it wrong: Modern wheat is not more toxic for celiac patients. Food Chem. 2019, 278, 820–822, doi:10.1016/j.foodchem.2018.12.003.
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Abbate, R.; Gensini, G.F.; Benedettelli, S.; Casini, A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: A double-blinded randomised dietary intervention trial. J. Nutr. 2014, 111, 1992–1999, doi:10.1017/S000711451400018X.
- Kasarda, D.D. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? Agric. Food Chem. 2013, 61, 1155–1159, doi:10.1021/jf305122s.
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506, doi:10.1038/s41422-020-0332-7.
- Spencer, S.P.; Fragiadakis, G.K.; Sonnenburg, J.L. Pursuing Human-Relevant Gut Microbiota-Immune Interactions. Immunity 2019, 51, 225–239, doi:10.1016/j.immuni.2019.08.002.
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, F1000 Faculty Rev-69, doi:10.12688/f1000research.20510.1.
- Alam, A.; Neish, A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 2018, 6, 1539595, doi:10.1080/21688370.2018.1539595.
- Chibbar, R.; Dieleman, L.A. The Gut Microbiota in Celiac Disease and probiotics. Nutrients 2019, 11, 2375, doi:10.3390/nu11102375.
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. Clin. Pathol. 2009, 62, 264–269, doi:10.1136/jcp.2008.061366.
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832, doi:10.3390/nu12061832.
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45, doi:10.1186/s13073-016-0295-y.
- Reddel, S.; Putignani, L.; Del Chierico, F. The Impact of Low-FODMAPs, Gluten-Free, and Ketogenic Diets on Gut Microbiota Modulation in Pathological Conditions. Nutrients 2019, 11, 373, doi:10.3390/nu11020373.
- Olivares, M.; Neef, A.; Castillejo, G.; Palma, G.D.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 2015, 64, 406–417, doi:10.1136/gutjnl-2014-306931.
- Olivares, M.; Benítez-Páez, A.; de Palma, G.; Capilla, A.; Nova, E.; Castillejo, G.; Varea, V.; Marcos, A.; Garrote, J.A.; Polanco, I.; et al. Increased prevalence of pathogenic bacteria in the gut microbiota of infants at risk of developing celiac disease: The PROFICEL study. Gut Microbes 2018, 9, 551–558, doi:10.1080/19490976.2018.1451276.
- Lebwohl, B.; Murray, J.A.; Verdú, E.F.; Crowe, S.E.; Dennis, M.; Fasano, A.; Green, P.H.; Guandalini, S.; Khosla, C. Gluten Introduction, Breastfeeding, and Celiac Disease: Back to the Drawing Board. J. Gastroenterol. 2016, 111, 12–14, doi:10.1038/ajg.2015.219.
- Koletzko, S.; Lee, H.S.; Beyerlein, A.; Aronsson, C.A.; Hummel, M.; Liu, E.; Simell, V.; Kurppa, K.; Lernmark, Å.; Hagopian, W.; et al. Cesarean Section on the Risk of Celiac Disease in the Offspring: The Teddy Study. Pediatr. Gastroenterol. Nutr. 2018, 66, 417–424, doi:10.1097/MPG.0000000000001682.
- Kołodziej, M.; Patro-Gołąb, B.; Gieruszczak-Białek, D.; Skórka, A.; Pieścik-Lech, M.; Baron, R.; Szajewska, H. Association between early life (prenatal and postnatal) antibiotic administration and coeliac disease: A systematic review. Dis. Child. 2019, 104, 1083–1089, doi:10.1136/archdischild-2019-317174.
- Kemppainen, K.M.; Vehik, K.; Lynch, K.F.; Larsson, H.E.; Canepa, R.J.; Simell, V.; Koletzko, S.; Liu, E.; Simell, O.G.; Toppari, J.; et al. Association between Early-Life Antibiotic Use and the Risk of Islet or Celiac Disease Autoimmunity. JAMA Pediatr. 2017, 171, 1217–1225, doi:10.1001/jamapediatrics.2017.2905.
- Verdu, E.F.; Galipeau, H.J.; Jabri, B. Novel players in coeliac disease pathogenesis: Role of the gut microbiota. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506, doi:10.1038/nrgastro.2015.90.
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac Disease and the Microbiome. Nutrients 2019, 11, 2403, doi:10.3390/nu11102403.
- Cenit, M.C.; Olivares, M.; Codoñer-Franch, P.; Sanz, Y. Intestinal Microbiota and Celiac Disease: Cause, Consequence or Co-Evolution? Nutrients 2015, 7, 6900–6923, doi:10.3390/nu7085314.
- Caminero, A.; Meisel, M.; Jabri, B.; Verdu, E.F. Mechanisms by which gut microorganisms influence food sensitivities. Rev. Gastroenterol. Hepatol. 2019, 16, 7–18, doi:10.1038/s41575-018-0064-z.
- Caminero, A.; Verdu, E.F. Celiac disease: Should we care about microbes? J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G161–G170, doi:10.1152/ajpgi.00099.2019.
- Food and Agriculture Organization of the United Nations. Globalization of Food Systems in Developing Countries: Impact on Food Security and Nutrition; FAO Food and Nutrition Paper 2004, n. 83; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 1–14, ISSN 0254-4725.
- Food and Agriculture Organization of the United Nations. Crop Breeding: The Green Revolution and the Preceding Millennia. Available online: http://www.fao.org/english/newsroom/focus/2003/gmo2.htm (accessed on 28 June 2020).
- Karim, M.B. The Green Revolution: An International Bibliography; Greenwood: Westport, CT, USA, 1986; pp. 1–28.
- Enghiad, A.; Ufer, D.; Countryman, A.M.; Thilmany, D.D. An Overview of Global Wheat Market Fundamentals in an Era of Climate Concerns. J. Agron. 2017, 2017, 3931897, doi:10.1155/2017/3931897.
- Tiwari, H. Wheat Gluten Market: Global Industry Analysis 2014–2018 and Opportunity Assessment 2019–2029; Future Market Insights: London, UK, 2020.
- MarketsandMarkets Wheat Protein Market; Report code: FB 5949; MarketsandMarkets: Pune, India, 2018.
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90, doi:10.1016/j.tifs.2005.10.003.
- Dhiraj, B.; Prabhasankar, P. Influence of Wheat-Milled Products and Their Additive Blends on Pasta Dough Rheological, Microstructure, and Product Quality Characteristics. J. Food Sci. 2013, 2013, 538070, doi:10.1155/2013/538070.
- Giannou, V.; Tzia, C. Addition of Vital Wheat Gluten to Enhance the Quality Characteristics of Frozen Dough Products. Foods 2016, 5, 6, doi:10.3390/foods5010006.
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Pharm. Des. 2003, 9, 1331–1344, doi:10.2174/1381612033454856.
- Liu, Z.; Udenigwe, C.C. Role of food-derived opioid peptides in the central nervous and gastrointestinal systems. Food Biochem. 2019, 43, e12629, doi:10.1111/jfbc.12629.
- Lister, J.; Fletcher, P.J.; Nobrega, J.N.; Remington, G. Behavioral effects of food-derived opioid-like peptides in rodents: Implications for schizophrenia. Biochem. Behav. 2015, 134, 70–78, doi:10.1016/j.pbb.2015.01.020.
- Glass, M.J.; Grace, M.; Cleary, J.P.; Billington, C.J.; Levine, A.S. Potency of naloxone’s anorectic effect in rats is dependent on diet preference. J. Physiol. 1996, 271, R217–R221, doi:10.1152/ajpregu.1996.271.1.R217.
- Boggiano, M.M.; Chandler, P.C.; Viana, J.B.; Oswald, K.D.; Maldonado, C.R.; Wauford, P.K. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Neurosci. 2005, 119, 1207–1214, doi:10.1037/0735-7044.119.5.1207.
- Morley, J.E.; Levine, A.S.; Yamada, T.; Gebhard, R.L.; Prigge, W.F.; Shafer, R.B.; Goetz, F.C.; Silvis, S.E. Effect of exorphins on gastrointestinal function, hormonal release, and appetite. Gastroenterology 1983, 84, 1517–1523.
- Corazza, G.R.; Frazzoni, M.; Strocchi, A.; Prati, C.; Sarchielli, P.; Capelli, M. Alimentary exorphin actions on motility and hormonal secretion of gastrointestinal tract. In Opioid Peptides in the Periphery; Fraioli, F., Isidori, A., Mazzetti, M., Eds.; Elsevier Sciences Publisher: Amsterdam, NL, USA, 1984; pp. 243–247.
- Takahashi, M.; Fukunaga, H.; Kaneto, H.; Fukudome, S.; Yoshikawa, M. Behavioral and pharmacological studies on gluten exorphin A5, a newly isolated bioactive food protein fragment, in mice. J. Pharmacol. 2000, 84, 259–265, doi:10.1254/jjp.84.259.
- Bressan, P.; Kramer, P. Bread and Other Edible Agents of Mental Disease. Hum. Neurosci. 2016, 10, 130, doi:10.3389/fnhum.2016.00130.