Flavonoids are metabolites widely distributed in plants and commonly present in foods, such as fruits and vegetables. Pectolinarin, which belongs to the flavone subclass, has attracted considerable attention due to its presence in many medicinal plants. It has turned out to be a good biological agent especially due to its antioxidant, anti-inflammatory, antidiabetic, and antitumor activities, evaluated both in vitro and in vivo. Its aglycone, the metabolite pectolinarigenin, is also known for a series of biological properties including anti-inflammatory and antidiabetic effects. In the first overview on the two metabolites here presented, their collection, isolation and the results of their biological evaluation are reported
- flavonoids
- flavones
- anticancer
1. Definition
Flavonoids are metabolites widely distributed in plants and commonly present in foods, such as fruits and vegetables. Based on several evidences, flavonoids have been associated with the role of preventing and managing current diseases such as cancers, diabetes, and cardiovascular disorders.
Pectolinarin, which belongs to the flavone subclass, has attracted considerable attention due to its presence in many medicinal plants. It has turned out to be a good biological agent especially due to its antioxidant, anti-inflammatory, antidiabetic, and antitumor activities, evaluated both in vitro and in vivo. Its aglycone, the metabolite pectolinarigenin, is also known for a series of biological properties including anti-inflammatory and antidiabetic effects.
2. Introduction
The glycosylated flavone pectolinarin was first isolated from Linaria vulgaris [1], a known medicinal Chinese herb used for the internal treatement of digestion problems and urinary disorders, in the external treatment of haemorrhoids, venous skin ulcer, as well as for the washing of festering wounds and skin rashes. It has also displayed anti-inflammatory effect [2] and has been used to treat coughs and asthma [3]. The structure of pectolinarin was determined to be a rutinoside conjugate of pectolinarigenin (=5,7-dihydroxy-4,6-dimethoxyflavone, C17H14O6) at the 7-O position (pectolinarigenin-7-O-rutinoside, C29H34O15) (Figure 1).
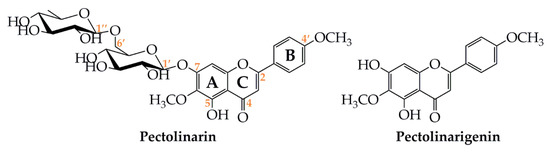
Figure 1. Molecular structures of pectolinarin and pectolinarigenin.
Later, pectolinarin and its aglycone pectolinarigenin were identified as the major constituents in many medicinal herbs from different genera around the world. Several studies reported so far prove that the presence of these two flavones has an important role in affecting the biological properties of the following herbs: i) the Korean herb Cirsium setidens (Dunn) Nakai employed for the treatment of hemostasis, hematemesis, hematuria and hypertension [4]; ii) the Chinese herb Cirsium chanroenicum used for detoxification, to treat fever and to enhance blood circulation [5]; iii) Cirsium japonicum DC. employed as an anti-hemorrhagic and uretic agent, as well as prescribed to treat liver and uterine tumours, and leukemia [6]; iv) Kickxia ramosissima (Wall.) Janch., used in Pakistan folk medicine as diuretic and against kidney stones [7], fever and rheumatism [8], and during management of snake and scorpion bites [9]; v) Lantana camara L., used for the treatment of various human ailments, such as ulcers, malaria, influenza, tumors, swellings, bilious fever, eczema eruptions, stomach ache, toothache, and as antiseptic for wounds [10]; and vi) Picnomon acama (L.) Cass., used in Greek folk medicine as hemostatic and spasmolytic agent [11].
Due to the structural similarity of pectolinarigenin to known potent flavonoids such as acacetin (5,7-dihydroxy-4′-methoxyflavone, C16H12O5), hispidulin (4′,5,7-trihydroxy-6-methoxyflavone, C16H12O6) and scutellarein (5,6,7,4′-tetrahydroxyflavone, C15H10O6) and based on the numerous data reported for both pectolinarin and pectolinarigenin, the aim of this work is to provide a comprehensive overview focusing on their isolation.
3. Isolation of Pectolinarin and Pectolinarigenin
Over the last 113 years from its first report [1], pectolinarin was isolated in most cases from the aerial parts of 87 plants belonging to 29 differents genera distributing widely around the world. Most of these plants are used in folk medicine in different parts of the world. Table 1 gathers the 87 plants from which pectolinarin was isolated.
Table 1. Isolation of pectolinarin from the indicated plants, classified according to family, genus and species, and place of collection.
| Genus | Species | Collection Place | Reference |
|---|---|---|---|
| Family: Adoxaceae | |||
| Viburnum | V. cotinifolium | Kashmir/India | [12] |
| V. mullaha | Indian Himalayan region | [13] |
|
| Family: Asteraceae | |||
| Cirsium | C. subcoriaceum | Pahuatlan/Mexico | [14] |
| C. japonicum | Daejeon/S. Korea, Oberndorf/Austria, Chengdu/China, Henan/China | [6][15][16][17][18][19] | |
| Oberndorf/Austria | |||
| Bialystok/Poland | |||
| Daejeon/S. Korea | |||
| C. setosum | Daejeon/S. Korea | [17] | |
| C. rivulare | Daejeon, Wonju, Pyongchang-gun, Gangwondo, Jeongseon-gun, Yanggu/S. Korea | [20] | |
| C. lineare | Daejeon/S. Korea | [18] | |
| C. nipponicum | Daejeon, Sancheong/S. Korea | [18][21] | |
| C. setidens | Jeongseon-gun, Jeju Island/S. Korea | [18][22][23][24][25] | |
| Laramie/USA | |||
| Laramie/USA, Vitebsk/Belarus | |||
| C. pendulum | Nemuro, Hatimandake, Memanbetsu, Onsen Kyushu, Hokkaido/Japan | [18] | |
| C. chanroenicum | [18][25][26] | ||
| C. rhinoceros | [27][28] | ||
| C. coloradense | Wyoming/USA, Japan | [29] | |
| C. arisanense | Mount Akaishi, Mount Senmai, Shizuoka, Takanomori, Nekura Valley/Japan, Vitebsk/Belarus, | ||
| C. tioganum | Mount Shirouma/Japan | ||
| C. oleraceum | la Dotze/Switzerland | [29][30][31] | |
| Hsien/Taiwan | |||
| C. microspicatum | Nemuro, Mount Shirouma /Japan, Mount Ali, Chiayi Hsien/Taiwan | [15] | |
| C. babanum | Ku Kuan, Taichung Haien/Taiwan | ||
| C. kagamontanum | Mount Shirouma, Mount Hakusan ad pedem/Japan | ||
| C. inundatum | Vancouver/Canada | ||
| C. dipsacolepis | Seongnam/Korea | ||
| C. brevicaule | |||
| C. yezoense | |||
| C. kamtschaticum | |||
| C. pectinellum | |||
| C. bitchuense | [32][33] | ||
| C. senjonse | [16][30][34][35][36] | ||
| C. spicatum | |||
| C. yezonese | |||
| C. vallis-demonii | |||
| C. gratiosum | |||
| C. indundatum | |||
| C. otayae | [34] | ||
| C. purpuratum | |||
| C. spinosissimum | [37] | ||
| C. spinosum | [33] | ||
| C. ferum | [38] | ||
| C. kawakamii | [15][39][40] | ||
| C. wallichii | [40] | ||
| C. yoshizawae | [34] | ||
| C. matsumurae | [33] | ||
| C. brevistylum | [41] | ||
| C. chlorolepis | [42] | ||
| Duranta | D. plumieri | Rajshahi/Bangladesh | [43] |
| Hemistepta | H. lyrata | Kangwon/S. Korea | [44] |
| Picnomon | P. acarna | Mount Hortiatis/Greece | [45] |
| Family: Bignoniaceae | |||
| Distictella | D. elongata | Minas Gerais State/Brazil | [46] |
| Markhamia | M. lutea | Benguluru/India | [47] |
| Family: Buddlejaceae | |||
| Buddleja | B. officinalis | Anhui/China | [48] |
| Family: Ericaceae | |||
| Rhododendron | R. arboreum | Aligarh/India | [49] |
| Family: Gesneriaceae | |||
| Corallodiscus | C. flabellate | Kunming/China | [50] |
| Aeschynanthus | A. moningeriae | Jinhua/China | [51] |
| Family: Lamiaceae | |||
| Leucosceptrum | L. canum | Tibet/China | [52] |
| Teucrium | T. hyrcanicum | Sicily/Italy | [53] |
| Family: Lythraceae | |||
| Lawsonia | L. inermis | Thanjavur/India | [54] |
| Family: Moraceae | |||
| Clerodedrum | C. phlomides | Tamil Nadu/India | [55] |
| Family: Orchidaceae | |||
| Oncidium | O. baueri | Londrina/Brazil | [56][57] |
| Family: Orobanchaceae | |||
| Melampyrum | M. roseum | Suwon/S. Korea | [58] |
| Family: Plantaginaceae | |||
| Scoparia | S. dulcis | Nanning/China | [59] |
| Family: Poaceae | |||
| Oryza | O. sativa | a) | [60] |
| Family: Ranunculaceae | |||
| Trollius | T. ledebourii | Hebei/China | [61] |
| Family: Rosaceae | |||
| Kerria | K. japonica var. | Chongqing/China | [62] |
| Crataegus | C. laevigata | Bremen/Germany | [63] |
| Family: Santalaceae | |||
| Thesium | T. chinense | Anhui/China | [64] |
| Family: Scrophulariaceae | |||
| Linaria | L. vulgaris | Sofia/Bulgaria, Tachkent/Uzbekistan | [1][65][66][67] |
| L. japonica | Heilongjiang/China | [68][69][70] | |
| L. reflexa | Tottori Prefecture/Japan | [71][72][73][74] | |
| L. vulgariformis | Constantine/Algeria, Calabria/Italy | [65] | |
| L. popovii | Tachkent/Uzbekistan | ||
| L. kurdica | |||
| L. sessili | [75] | ||
| L. kokanica | Pamir/Tajikistan | ||
| L. haelava | [76] | ||
| L. simplex | Mansoura/Egypt | [66] | |
| L. genistifolia | Sofia/Bulgaria | ||
| L. dalmatica | |||
| L. scariosa | Msila/Algeria | [77] | |
| Kickxia | K. elatine | Dustlik/Uzbekistan | [78] |
| K. heterophylla | Mansoura/Egypt | [79] | |
| K. ramosissima | Ankara/Turkey | [80][81] |
|
| K. abhaica | Baljurashi/Saudi Arabia | [82] | |
| Appennines hills/Italy | |||
| K. spuria | Saudi Arabia | [83] | |
| K. aegyptiaca | [84] | ||
| Family: Verbenaceae | |||
| Morus | M. alba L. | Hongseong/Korea | [85] |
| Lantana L. camara | Taichung/Taiwan, Palampur/India, Karachi/Pakistan, Ceará state/Brazil, Manado/Indonesia, Okinawa/Japan | [86][87][88][89][90][91] | |
| Lippia L. rubella | Minas Gerais/Brazil | [92] | |
| Family: Winteraceae | |||
| Tasmannia | T. lanceolata | Go Wild Harvest/Australia | [93] |
a Not found.
Pectolinarigenin is the aglycone part of pectolinarin, which is obtained by hydrolysis reaction [72]. It is also a natural product, isolated and identified from 136 plantes of 71 differents genera. The data are summarized in Table 2, indicating that pectolinarigenin was isolated from 20 different families, especially from Asteraceae with 33 genera and 64 species (47.1%), Lamiaceae with 9 genera and 19 species (14%) and Verbenaceae with 4 genera and 10 species (8%).
Table 2. Isolation of pectolinarigenin from the indicated plants, classified according to family, genus and species, and place of collection.
| Genus | Species | Collection Place | Reference |
|---|---|---|---|
| Family: Apiaceae | |||
| Coriandrum | C. sativum | Faisalabad/Pakistan | [94] |
| Family: Aspleniaceae | |||
| Asplenium | A. glaucophyllum | West Malysia | [95] |
| A. normale | West Malaysia | [96] | |
| Family: Asteraceae | |||
| Achillea | A. collina | wet lowlandmeadows/UK | [97] |
| A. asplenifolia | |||
| Ajania | A. potaninii | Gansu/China | [98] |
| Ambrosia | A. camphorate | Baja California/Mexico | [99] |
| Arnica | A. angustifolia | northwestCanada and Alaska | [100] |
| A. Montana | California/USA | [101] | |
| A. chamissonis | Graines Voltz/France | [102] |
|
| A. montana | Šumava Mounts/Czech | [103] | |
| Artemisia | A. mongolica | Gansu/China | [104] |
| A. judaica | St. Catherine, Sinai/Egypt | [105] | |
| A. monosperma | Cairo/Egypt | ||
| A. herba-alba | Mount Moses/Egypt | ||
| A. xerophytica | South Gobi Aimak/Mongolia | [106] |
|
| A. glabella | Karaganda/Kazakhstan | [107] | |
| A. vestita | Lhasa/Tibet | [108] | |
| Baccharis | B. trinervis | Costa Rica | [109] |
| B. decussata | Venezuela | [110] | |
| B. concave | [111] | ||
| B. uncinella | Campos do Jordão/Brazil | [112] | |
| B. conferta | Veracruz/Mexico | [113] | |
| Centaurea | C. alexandrina | Alexandria/Egypt | [114] |
| C. aspera | Ribera Baixa/Spain | [115] | |
| C. cariensis | [116] |
||
| C. collina | Valencia/Spain | [117] | |
| C. sadleriana | Jakabszállás/Hungary | [118] | |
| C. moesiaca | Malashevska planina/Bulgaria | [119] | |
| C. behen | Iran | [120] | |
| Chromolaena | C. odorata | Chonburi/Thailand | [121] |
| Chrysanthemum | C. pacificum | Tsukuba/Japan | [26] |
| C. shiwogiku | Muroto-misaki/Japan | ||
| C. kinokuniense | Tsukuba/Japan | ||
| C. rupestre | Mount Mikuni/Japan | ||
| Cirsium | C. setidens | Jeongseon-gun, Halla of jejudo, Daejeon, Kangwon, Yanggu/S. Korea; Guerrero/Mexico; | [18][23][24][25][26][27][122] |
| C. chanroenicum | Daejeon, Ulsan, Sancheong/S. Korea | [25][123] |
|
| C. japonicum | Jiang Xi/China | [124] | |
| C. arvense | Musa Khel Bannu/Pakistan | [125] | |
| C. nipponicum | Suwon/S. Korea | [126] | |
| C. rhinoceros | [127] | ||
| Dichrocephala | D. integrifolia | Shanghai/China | [128] |
| Dugaldia | D. pinetorum | Nuevo Lebn/Mexico | [129] |
| Eriocephalus | E. giessii | Aus-Koppies/Namibia | [130] |
| Eupatorium | E. cannabinum | Gronigen/Netherlands | [131] |
| E. odoratum | Kuala Pilah/Malaysia | [132][133] | |
| E. semiserratum | Arkansas/USA | [134] | |
| Fragrant | F. Eupatorium | Guangxi/China | [135] |
| Grindelia | G. glutinosa | Poconchile, Valle deLiuta, Tarapaca/Chile | [136] |
| Gutierrezia | G. mandonii | Salta/Argentina | [137] |
| Helenium | H. integrifolium | [138] | |
| Heterotheca | H. latifolia | San Luis/Argentina | [139] |
| Hemistepta | H. lyrata | Kangwon/S. Korea | [44] |
| Hymenoxys | H. jamesii | Coconino/USA | [140] |
| Iva | I. nevadensis | Tonopah/USA | [141] |
| I. frutescens | Franklin/USA | [142] | |
| Jungia | J. polita | San Martin/Argentina | [143] |
| Olearia | O. paniculata | Dunedin/New Zealand | [144] |
| Onopordon | O. corymbosum | Barracas, Castellon/Spain | [145] |
| O. nervosum | a) | [146] |
|
| Santolina | S. chamaecyparissus | Lyon/France | [147] |
| S. pinnata | Pisa/Italy | [148] | |
| Saussurea | S. elegans | Murghab/Tajikistan | [149] |
| Schkuhria | S. pinnata | Cordoba/Argentina | [150] |
| Seriphidium | S. santolium | Xinjiang Uigour/China | [151] |
| Stevia | S. laxiflora | Cuernavaca, Morelos/Mexico | [152] |
| Vernonia | V. cinerea | Pahang/Malaysia | [153] |
| Family: Betulaceae | |||
| Alnus | A. glutinosa | Darmstadt/Germany | [154] |
| A. japonica | [155] | ||
| Betula | B. ermanii | [154] | |
| B. verrucosa | a) | [156] | |
| B. pubescens | Biebrza/Poland | [157] | |
| B. pendula | |||
| Family: Bignoniaceae | |||
| Millingtonia | M. hortensis | Khon Kaen/Thailand | [158] |
| Family: Blechnaceae | |||
| Brainea | B. insignis | Yunnan/China | [159] |
| Family: Boraginaceae | |||
| Eriodictyon | E. tomentosum | Placer Co./USA | [160] |
| Family: Fabaceae | |||
| Adesmia | A. grandiflora | a) | [161] |
| A. trijuga | |||
| A. horrida | |||
| A. retrofracta | |||
| Ononis | O. fruticosa | Los Castanõs/Spain | [162] |
| O. natrix | |||
| O. rotundifolia | a) | [163] | |
| Trifolium | T. pratense | Trout Lake/USA | [164] |
| Family: Lamiaceae | |||
| Leucosceptrum | L. canum | a) | [165] |
| Mentha | M. pulegium | Petite Kabylie/Algeria | [166] |
| M. suaveolens | |||
| Ocimum | O. americanum | RoyalBotanic Gardens, Kew/England | [167] |
| Otostegia | O. fruticosa | St. Catherine/Egypt | [168] |
| Salvia | S. trilobu | Marmara island/Turkey | [169] |
| S. hypoleuca | Elbruz moun/Russia | [170] | |
| S. pedicellata | a) | [171] |
|
| S. yosgadensis | Sultanhani/Turkey | [172] | |
| S. plebeia | a) | [173] | |
| S. pilifera | Berit Mount/Turkey | [174] | |
| S. tomentosa | Sofia/Bulgaria | [175] |
|
| S. argentea | |||
| Scutellaria | S. polyodon | a) | [176] |
| S. przewalskii | Susamyr/Kyrgyzstan | [177] | |
| Sideritis | S. gomerae | Canary islands/Spain | [178] |
| Teucrium | T. chamaedrys | Eskisehir/Turkey | [179] |
| Thymus | T. longicaulis | Sar planina/Macedonia | [180] |
| T. glabrescens | Skopje/Macedonia | ||
| Family: Lythraceae | |||
| Lawsonia | L. inermis | Thanjavur/India | [148] |
| Family: Nothofagaceae | |||
| Nothofagus | N. dombeyi | Altos de Lircay/Chile | [181] |
| Family: Orobanchaceae | |||
| Striga | S. passargei | a) | [182] |
| S. aspera | a) | [183] | |
| Family: Padaliacea | |||
| Sesamum | S. indicum | Gambang/Malaysia | [184] |
| Family: Plantaginaceae | |||
| Digitalis | D. trojana | Kizilcahamam, DemirkOy/Turkey | [185] |
| D. orientalis | |||
| D. lanata | a) | [186] |
|
| Hebe | H. cupressoides | Dunedin/New Zealand | [187] |
| Veronica | V. chamaedrys | Rila Mount/Bulgaria | [188] |
| Veronicastrum | V. latifolium | Yongkang/China | [189] |
| Family: Portulacaceae | |||
| Portulaca | P. oleracea | Tianjin/China | [190] |
| Family: Ranunculaceae | |||
| Trollius | T. chinensis | Hebei/China | [191] |
| Family: Rosaceae | |||
| Rosa | R. damascena | Plovdiv/Bulgaria | [192] |
| R. rugosa | Botanischer Garten der TU Darmstadt/Germany | [193] | |
| Family: Scrophulariaceae | |||
| Buddleia | B. macrostachya | Sibsagar/India | [194] |
| Kickxia | K. ramosissima | Takht-e-Nusrati/Pakistan | [195][196][197] |
| Limnophila | L. aromatica | Ho Chi Minh/Vietnam | [198] |
| Linaria | L. vulgaris | Ukrania; China | [67][199] |
| L. reflexa | Constantine/Algeria | [72] | |
| L. kurdica | Ukrania | [199] | |
| L. scariosa | Msila/Algeria | [77] | |
| Family: Verbenaceae | |||
| Clerodendrum | C. siphonenthus | Calcutta, Kalyani/India | [200][201] |
| C. phlomidis | Pondicherry, Alanthurai/India | [200][201][202][203][204][205] | |
| C. serratum | Bhilai/India | [206] | |
| C. inerme | Pondicherry/India | [207][208] | |
| C. neriifolium | a) | [209] | |
| C. indicum | a)Khao Kho/Thailand | [210][211] | |
| Duranta | D. repens | a) | [212] |
| D. plumieri | a) | [43][213] | |
| Lantana | L. camara | Taichung/Taiwan, Palampur/India, Karachi/Pakistan, Ceará state/Brazil, Manado/Indonesia, Okinawa/Japan | [86][87][88][89][90][91] |
| Lippia | L. citriodora | Athens/Greece | [214] |
| Family: Zosteraceae | |||
| Phyllospadix | P. japonica | Omaezaki/Japan | [215] |
- a) not found.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics9070417
References
- Klobb, T. Two new glucosides: Linarine and pectolinarine. Compt. Rend. 1907, 145, 331–334.
- Gruenwald, J. PDR for Herbal Medicines, 1st ed.; Medical Economics Company: Montvale, NJ, USA, 1998.
- Hua, H.; Cheng, M.; Li, X.; Pei, Y. A new pyrroloquinazoline alkaloid from Linaria vulgaris. Chem. Pharm. Bull. 2002, 50, 1393–1394.
- Lee, S.J. Korean Folk Medicine; Seoul National University Press: Seoul, Korea, 1966; pp. 145–146.
- Jeong, B.; Shin, M. Dictionary of Folk Medicine; Yeungrim Pub.: Seoul, Korea, 1989; p. 1041.
- Liu, S.; Zhang, J.; Li, D.; Liu, W.; Luo, X.; Zhang, R.; Li, L.; Zhao, J. Anticancer activity and quantitative analysis of flavone of Cirsium japonicum DC. Nat. Prod. Res. 2007, 21, 915–922.
- Pandya, P.N.; Aghera, H.B.; Ashok, B.K. Diuretic activity of Linaria ramosissima (Wall.) Janch. leaves in albino rats. Ayu 2012, 33, 576–578.
- Jain, A.; Katewa, S.S.; Galave, P.; Nag, A. Some therapeutic uses of biodiversity among the tribals of Rajasthan. Ind. J. Tradit. Med. 2008, 7, 256–262.
- Bole, P.V.; Pathak, J.M. Flora of Saurashtra; The director botanical survey of India: New Delhi, India, 1988.
- Ghisalberti, E.L. Lantana camara L. (Verbenaceae). Fitoterapia 2000, 71, 467–486.
- Dioscorides; Robert, T.G. The Greek Herbal of Dioscorides; Gunther, R.T., Ed.; Hafner Publishing Company: New York, NY, USA, 1968; p. 247.
- Muhaisen, H.M.H.; Ilyas, M.; Mushfiq, M.; Parveen, M.; Basudan, O.A. Flavonoid from Viburnum cotinifolium. J. Chem. Res. 2002, 10, 480–481.
- Singh, H.; Lily, M.K.; Dangwal, K. Viburnum mullaha D.DON fruit (Indian Cranberry): A potential source of polyphenol with rich antioxidant, anti-elastase, anti-collagenase, and anti-tyrosinase activities. Int. J. Food Prop. 2017, 20, 1729–1739.
- Martínez-Vázquez, M.T.O.; Apan, R.; Lastra, A.L.; Bye, R. A comparative study of the analgesic and anti-inflammatory activities of pectolinarin isolated from Cirsium subcoriaceum and linarin isolated from Buddleia cordata. Planta Med. 1998, 64, 134–137.
- Morita, N.; Shimizu, M.; Arisawa, M. Two new flavone glycosides from Cirsium lineare. Phytochemistry 1973, 12, 421–423.
- Park, J.C.; Lee, J.H.; Choi, J.W. Isolation and biological activity of flavone glycosides from the aerial part of Cirsium japonicum var. ussuriense in Korea. Han’guk Yongyang Siklyong Hakhoechi 1995, 24, 906–910.
- Ganzera, M.; Pöcher, A.; Stuppner, H. Differentiation of Cirsium japonicum and C. setosum by TLC and HPLC-MS. Phytochem. Anal 2005, 16, 205–209.
- Jeong, D.M.; Jung, H.A.; Choi, J.S. Comparative antioxidant activity and HPLC profiles of some selected Korean thistles. Arch. Pharm. Res. 2008, 31, 28–33.
- Ma, Q.; Jiang, J.G.; Zhang, X.M.; Zhu, W. Identification of luteolin 7-O-β-D-glucuronide from Cirsium japonicum and its anti-inflammatory mechanism. J. Funct. Foods 2018, 46, 521–528.
- Nazaruk, J.; Jakoniuk, P. Flavonoid composition and antimicrobial activity of Cirsium rivulare (Jacq.) All. flowers. J. Ethnopharmacol. 2005, 102, 208–212.
- Do, J.C.; Jung, K.Y.; Son, K.H. Isolation of pectolinarin from the aerial parts of Cirsium nipponicum. Saengyak Hakhoe Chi. 1994, 25, 73–75, CA 121:104074.
- Yoo, Y.M.; Nam, J.H.; Kim, M.Y.; Choi, J.; Park, H.J. Pectolinarin and pectolinarigenin of Cirsium setidens prevent the hepatic injury in rats caused by D-galactosamine via an antioxidant mechanism. Biol. Pharm. Bull. 2008, 31, 760–764.
- Nugroho, A.; Lim, S.C.; Karki, S.; Choi, J.S.; Park, H.J. Quantitative determination of five phenolic peroxynitrite-scavengers in nine korean native compositae herbs. Nat. Prod. Sci. 2015, 21, 155–161.
- Lee, J.H.; Jung, H.K.; Han, Y.S.; Yoon, Y.M.; Yun, C.W.; Sun, H.Y.; Cho, H.W.; Lee, S.H. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol. Med. Rep. 2016, 14, 3777–3784.
- Kim, M.S.; Nam, M.; Hwang, G.S. Metabolic alterations in two Cirsium Species identified at distinct phenological stages using UPLC-QTOF/MS. Phytochem. Anal. 2018, 29, 77–86.
- Uehara, A.; Nakata, M.; Kitajima, J.; Iwashina, T. Internal and external flavonoids from the leaves of Japanese Chrysanthemum species (Asteraceae). Biochem. Syst. Ecol. 2012, 41, 142–149.
- Lee, H.B.; Kwak, J.H.; Zee, O.P.; Yoo, S.J. Flavonoids from Cirsium rhinoceros. Arch. Pharmacal. Res. 1994, 17, 273–277.
- Yim, S.H.; Kim, H.J.; Lee, I.S. A polyacetylene and flavonoids from Cirsium rhinoceros. Arch. Pharm. Res. 2003, 26, 128–131.
- Gardner, R.C. Acacetin-7-O-rutinoside and pectolinarin from Cirsium coloradense. Phytochemistry 1973, 12, 223.
- Shelyuto, V.L.; Glyzin, V.I.; Ban’kovskii, A.I.; Bubon, N.T. Flavonoid glycosides of Cirsium oleraceum. Chem. Nat. Compd. 1971, 7, 372–373.
- Shelyuto, V.L.; Glyzin, V.I.; Yurchenko, G.N.; Smirnova, L.P. Flavonoids from Cirsium oleraceum flowers. Chem. Nat. Compd. 1978, 14, 336.
- Gardner, R.C. Systematics of Cirsium (Compositae) in Wyoming. Madrono 1974, 22, 239–265.
- Morita, N.; Fukuta, M.; Shimizu, M. Studies on the medicinal resources XXIII, Flavonoids of Cirsium plants (Compositae) in Japan. Components of the leaves of Cirsium microspicatum Nakai var. kiotense Kitam., C. dipsicolepis Matsum., C. brevicaule A. Gray, C. matsumurae Nakai, C. yakusimense Masamune, C. amplexifolium Kitam., C. spinosum Kitam., C. tanakae Matsum. subsp. aomorense Kitam. and C. arvense scop. var. setosum Ledeb. Yakugaku Zasshi 1964, 18, 9–11.
- Nakaoki, T.; Morita, N. Studies on the medicinal resources XIII, Flavonoids of Cirsium plants (Compositae) in Japan. Components of the leaves of Cirsium microspicatum Nakai, C. otayae Kitamura, C. yoshizawae Koidz., C. japonicum DC.; C. purpuratum Matusum. Yakugaku Zasshi 1959, 79, 1338–1340.
- Glyzinm, V.I.; Shelyuto, V.L.; Patudin, A.V.; Bubon, N.T. Flavonoids of Cirsium Mill species. Mater S’ezde. Farm. B SSR 1977, 153–156.
- Iwashina, T.; Kadota, Y.; Ueno, T.; Ootani, S. Foliar flavonoid composition in Japanese Cirsium species (Compositae), and their chemotaxonomic significance. J. Jpn. Bot. 1995, 70, 280–290.
- Christian, A.; Ivan, S.; Elisabetta, C.; Maria, D.M.; Matthias, H.; Olivier, P. Comprehensive analysis of Cirsium spinosissimum Scop., a wild alpine food plant. Food Chem. 2014, 160, 165–170.
- Morita, N.; Lin, C.N. Studies on the components of Formosan Cirsium species. Part IV. Components of Cirsium arisanense Kitamura and Cirsium ferum Kitamura. Tiawan Yao xue Zazhi 1976, 28, 40–42.
- Nakaoki, T.; Morita, N. Studies on the medicinal resources XIV, Flavonoids of Cirsium plants (Compositae) in Japan. Components of the leaves of Cirsium kagamontanum Nakai, C. inundatum Makino, and C. matsumurae Nakai var. pubescens Kitamura. Yakugaku Zasshi 1960, 80, 1296–1297.
- Lin, C.N. Components of formosan Cirsium species. III. Flavonoids of Cirsium kawakamii and Cirsium wallichii. J. Chin. Chem. Soc. (Taipei-Taiwan) 1975, 22, 275–277.
- Wallace, J.W.; Bohm, B.A. Cirsimaritin-4′-O-rutinoside, a new flavone glycoside from Cirsium brevistylum. Phytochemistry 1971, 10, 452–454.
- Cho, S.; Lee, J.; Lee, Y.K.; Chung, M.J.; Kwon, K.H.; Lee, S. Determination of pectolinarin in Cirsium spp. using HPLC/UV analysis. J. Appl. Biol. Chem. 2016, 59, 107–112.
- Makboul, A.M.; Abdel-Baki, A.M. Flavonoids from the leaves of Duranta plumieri. Fitoterapia 1981, 52, 219–220.
- Nugroho, A.; Lim, S.C.; Byeon, J.S.; Choi, J.S.; Park, H.J. Simultaneous quantification and validation of caffeoylquinic acids and flavonoids in Hemistepta lyrata and peroxynitrite-scavenging activity. J. Pharm. Biomed. Anal. 2013, 76, 139–144.
- Laskaris, G.G.; Gourneus, D.C.; Kokkalou, E. Phenolics of Picnomon acarna. J. Nat. Prod. 1995, 58, 1248–1250. [
- Simões, L.R.; Maciel, G.M.; Brandão, G.C.; Filho, J.D.; Oliveira, A.B.; Castilho, R.O. Chemical constituents of Distictella elongata (Vahl) Urb. (Bignoniaceae). An. Acad. Bras. Cienc. 2013, 85, 873–879.
- Brindha, P.; Ragamanvitha, A.; Narendran, R.; Sriram, S.; Vadivel, V. Antioxidant activity and phytochemical composition of aqueous extract of Markhamia lutea (Benth) K. Schum. leaves. Trop. J. Nat. Prod. Res. 2017, 1, 63–68.
- Chen, X.; Wang, L.; Wei, T.; Liang, M.; Huang, X. A Method for Extraction of Pectolinarin in Buddleja officinalis Flower. CN 105954405 A 20160921, 21 September 2016. [Google Scholar]
- Kamil, M.S.; Ilyas, M. Flavonoidic constituents of Rhododendron arboreum leaves. Fitoterapia 1995, 66, 371.
- Yao, Y.; Wu, C.Y.; Hao, Q.; Li, H.Z.; Li, R.T. Study on chemical constituents of Corallodiscus flabellatus. J. Kunming Univ. Sci. Tech. (Nat. Sci. Ed.) 2012, 37, 64–68.
- Liu, H.; Liao, H.; Yuan, K. Chemical constituents contained in Aeschynanthus moningeriae. Zhongguo Zhongyao Zazhi 2012, 37, 1963–1967.
- Feng, L.; Zhang, Y.; Liu, Y.C.; Liu, Y.; Luo, S.H.; Huang, C.S.; Li, S.H. Leucoflavonine, a new bioactive racemic flavoalkaloid from the leaves of Leucosceptrum canum. Bioorg. Med. Chem. 2019, 27, 27,442–446.
- Oganesyan, G.B.; Mnatsakanyan, V.A.; Gacs-Baitz, E.; Radics, L. Flavonoid glycosides of Teucrium hyrcanicum L. Armyanskii Khimicheskii Zhurnal 1989, 42, 646–653.
- Manivannan, R.; Aeganathan, R.; Prabakaran, K. Anti-microbial and anti-inflammatory flavonoid constituents from the leaves of Lawsonia inermis. J. Phytopharmacol. 2015, 4, 212–216. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants, An Illustrated Dictionary; Springer: Berlin/Heidelberg, Germany, 2007; p. 423.
- Monteiro, J.; Schuquel, I.T.A.; de Almeida, T.L.; de Oliveira Santin, S.M.; da Silva, C.C.; Chiavelli, L.U.R.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Vendramini-Costa, D.B.; Nakamura, C.V.; et al. Oncibauerins A and B, new flavanones from Oncidium baueri (Orchidaceae). Phytochem. Lett. 2014, 9, 141–148.
- Ferreira, N.P.; Chiavelli, L.U.R.; Savaris, C.R.; Silvana, M.O.; Lucca, D.L.; Milaneze-Gutierre, M.A.; Faria, R.T.; Pomini, A.M. Chemical study of the flowers of the orchid Oncidium baueri Lindley and their visiting bees Trigona spinipes Fabricius. Biochem. Syst. Ecol. 2019, 86, 103918.
- Roh, J.H.; Zee, O.P.; Moon, H.I. Phytochemical Constituents from Melampyrum roseum var. hirsutum Beauv. Korean J. Pharmacol. 2006, 31, 157–162.
- Liu, Q.; Yang, Q.M.; Hu, H.J.; Yang, L.; Yang, Y.B.; Chou, G.X.; Wang, Z.T. Bioactive diterpenoids and flavonoids from the aerial parts of Scoparia dulcis. J. Nat. Prod. 2014, 77, 1594–1600.
- Moon, K.I.; Min, B.S.; Lee, H.K.; Zee, O.P. Antioxidant compounds of Oryza sativa L. Saengyak Hakhoechi 2002, 33, 173–176.
- Liao, M.; Cheng, X.; Zhang, X.; Diao, X.; Liang, C.; Zhang, L. Qualitative and quantitative analyses of active constituents in Trollius ledebourii. J. Chromatogr. Sci. 2018, 56, 619–635.
- Zhou, J.; Xie, G.; Yan, X. Encyclopedia of Traditional Chinese Medicines—Molecular Structures, Pharmacological Activities, Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2010; Volume 4, p. 173.
- Karar, M.G.E.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2015, 1, 102–125.
- Zou, Y.; Hong, M.; Yang, X.f. Isolation of chemical components from Thesium chinense. Zhongguo Shiyan Fangjixue Zazhi 2016, 22, 74–77.
- Kuptsova, L.P.; Ban’kovskii, A.I. New flavonoid from some species of toadflax. Chem. Nat. Compd. 1970, 6, 128–129.
- Ilieva, E.; Handjieva, N.; Bankova, V.; Popov, S.; Evstatieva, L. Iridoid and flavonoid glycosides from Linaria species. Bulg. Chem. Commun. 1992, 25, 400–406.
- Hua, H.; Sun, J.; Li, X. Flavonoids from yellow toadflax (Linaria vulgaris). Chin. Tradit. Herb. Drugs. 1999, 30, 332–334.
- Mun, G.S.; Song, H.Y.; Kim, I.G. Analysis of flavonoid-components of Linaria Japonica Miq. Punsok Hwahak 1979, 3, 28–32.
- Otsuka, H. Isolation of isolinariins A and B, new flavonoid glycosides from Linaria japonica. J. Nat. Prod. 1992, 55, 1252–1255.
- Widyowati, R.; Sugimoto, S.; Yamano, Y.; Sukardiman, Y.; Otsuka, H.; Matsunami, K. New Isolinariins C, D and E, flavonoid glycosides from Linaria japonica. Chem. Pharm. Bull. 2016, 64, 517–521.
- Tundis, R.; Deguin, B.; Loizzo, M.R.; Bonesi, M.; Statti, G.A.; Tillequin, F.; Menichini, F. Potential antitumor agents: Flavones and their derivatives from Linaria reflexa Desf. Bioorg. Med. Chem. Lett. 2005, 15, 4757–4760.
- Cheriet, T.; Aouabdia, S.; Mancini, I.; Defant, A.; Seghiri, R.; Boumaza, O.; Mekkiou, R.; Sarri, D.; León, F.; Brouard, I.; et al. Chemical constituents of Linaria reflexa Desf. (Scrophulariaceae). Der Pharm. Lett. 2014, 6, 54–57.
- Cheriet, T.; Hanfer, M.; Boudjelal, A.; Baali, N.; Mancini, I.; Seghiri, R.; Ameddah, S.; Menad, A.; Benayache, F.; Benayache, S. Glycosyl flavonoid profile, in vivo antidiabetic and in vitro antioxidant properties of Linaria reflexa Desf. Nat. Prod. Res. 2017, 31, 2042–2048.
- Cheriet, T.; Hanfer, M.; Mancini, I.; Benelhadj, S.; Laouas, N.E.; Ameddah, S.; Menad, A.; Seghiri, R. Anti-inflammatory and hemostatic effects of Linaria reflexa Desf. Nat. Prod. Res. 2019, 1–6.
- Smirnova, L.P.; Boryaev, K.I.; Ban’kovskii, A.I. Acacetin and its glycosides in plants of the genus Linaria. Chem. Nat. Compd. 1974, 10, 96–97.
- Lahloub, M.F. Flavonoid, phenylpropanoid and iridoid glycosides of Linaria haelava (Forssk.) Dil. Mansoura J. Pharm. Sci. 1992, 8, 78–95.
- Ahmed-Chaouch, M.; Cheriet, T.; Beretta, G.; Sarri, D.; Bensouici, C.; Ouelbani, R.; Mancini, I.; Sekhara, I.; Seghiri, R. Chemical composition, in vitro antioxidant, anticholinesterase and antibacterial activities of Linaria scariosa Desf. Nat. Prod. Res. 2019, 1–5.
- Yuldashev, M.P.; Batirov, E.K.H.; Malikov, V.M. Flavonoids of the epigeal part of Kickxia elatine. Chem. Nat. Compd. 1996, 32, 30–32.
- Amer, M.M.A. Glycosides of Kickxia heterophylla (Schousb.) Dandy in Andrews. Alex. J. Pharm. Sci. 1993, 7, 58–61.
- Khan, I.Z.; Aqil, M. Isolation and identification of pectolinarin and mannitol from Kickxia ramosissima (Wall). Chem. Environ. Res. 1993, 2, 287–289.
- Ahmad, V.A.; Kousar, F.; Zubair, M.; Khan, A.; Ali, M.S.; Choudhary, M.I.; Sener, B. A new iridoid glycoside from Linaria genestifolia. Fitoterapia 2006, 77, 12–14.
- Al-Rehaily, A.J.; Abdel-Kader, M.S.; Ahmad, M.S.; Mossa, J.S. Iridoid glucosides from Kickxia abhaica D.A. Sutton from Scrophulariaceae. Phytochemistry 2006, 67, 429–432.
- Venditti, A.; Frezza, C.; Serafini, I.; Ciccòla, A.; Sciubba, F.; Serafini, M.; Bianco, A. Iridoids of chemotaxonomy relevance, a new antirrhinoside ester and other constituents from Kickxia spuria subsp. integrifolia (Brot.) R. Fern. Chem. Biodiv. 2018, 15, e1700473.
- Kassem, F.F. Flavonoids of Kickxia aegyptiaca (Dum.) Nabelek. Alex. J. Pharm. Sci. 1992, 6, 62–65.
- Jeon, Y.S.; Kim, M.W. The antioxidative effects and isolation and characterization of the extracts from Morus alba L. Korean J. Food Nutr. 2011, 24, 94–100.
- Pan, W.D.; Mai, L.T.; Li, Y.J.; Xu, X.L.; Yu, D.Q. Studies on the chemical constituents of the leaves of Lantana camara. Acta Pharma. Sin. 1993, 28, 35–39.
- Mahato, S.B.; Sahu, N.P.; Roy, S.K.; Sharma, O.P. Potential antitumor agents from Lantana camara: Structures of flavonoid, and phenylpropanoid glycosides. Tetrahedron 1994, 50, 9439–9446.
- Begum, S.; Wahab, A.; Siddiqui, B.S.; Qamar, F. Nematicidal constituents of the aerial parts of Lantana camara. J. Nat. Prod. 2000, 63, 765–767.
- Juang, F.C.; Chen, Y.F.; Lin, F.M.; Huang, K.F. Constituents from the leaves of Lantana camara (IV). J. Chin. Med. 2005, 16, 149–155.
- Sousa, E.O.; Rocha, J.B.T.; Barros, L.M.; Barros, A.R.C.; Costa, J.G.M. Phytochemical characterization and in vitro antioxidant properties of Lantana camara L. and Lantana montevidensis Briq. Ind. Crops Prod. 2013, 43, 517–522.
- Abdjul, D.B.; Yamazaki, H.; Maarisit, W.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Kapojos, M.M.; Losung, F.; Ukai, K.; Namikoshi, M. Oleanane triterpenes with protein tyrosine phosphatase 1B inhibitory activity from aerial parts of Lantana camara collected in Indonesia and Japan. Phytochemistry 2017, 144, 106–112.
- Martins, G.R.; da Fonseca, T.S.; Martínez-Fructuoso, L.; Simas, R.C.; Silva, F.T.; Salimena, F.R.G.; Alviano, D.S.; Alviano, C.S.; Leitão, G.G.; Pereda-Miranda, R.; et al. Antifungal phenylpropanoid glycosides from Lippia rubella. J. Nat. Prod. 2019, 82, 566–572.
- Winnett, V.; Boyer, H.; Sirdaarta, J.; Cock, I.E. The potential of Tasmannia lanceolata as a natural preservative and medicinal agent: Antimicrobial activity and toxicity. Pharmacogn. Commnun. 2014, 4, 42–52.
- Hussain, F.; Jahan, N.; Rahman, K.; Sultana, B.; Jamil, S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ACE) inhibition potential. Oxid. Med. Cell Longev. 2018, 2018, 4643736.
- Umikalsom, Y.; Harborne, J.B. Flavonoid distribution in Asplenioid ferns. Pertanika 1991, 14, 297–300.
- Umikalsom, Y. Flavone O-glycosides and other flavonoids of Malaysian Asplenium L. Pertanika 1991, 14, 149–152.
- Valant-Vetschera, K.M.; Wollenweber, E. Flavonoid patterns of Achillea. Part 7. Leaf flavonoids of the Achillea millefolium group. Part II: Distribution patterns of free aglycons in leaf exudates. Biochem. Syst. Ecol. 1988, 16, 605–614.
- Liang, J.Y.; Xu, J.; Shao, Y.Z.; Yang, Y.Y.; Lu, P.Y.; Wang, J.L.; Du, S.S. Chemical constituents from the aerial sections of Ajania potaninii. Biochem. Syst. Ecol. 2019, 84, 64–66.
- Wollenweber, E.; Hradetzky, D.; Mann, K.; Roitman, J.N.; Yatskievych, G.; Proksch, M.; Proksch, P. Exudate flavonoids from aerial parts of five Ambrosia species. J. Plant Physiol. 1987, 131, 37–43.
- Schmidt, T.J.; Willuhn, G. Sesquiterpene lactone and flavonoid variability of the Arnica angustifolia aggregate (Asteraceae). Biochem. Syst. Ecol. 2000, 28, 133–142.
- Merfort, I. Methylated flavonoids from Arnica montana and Arnica chamissonis. Planta Med. 1984, 50, 107–108.
- Todorova, M.; Staneva, J.; Evstatieva, L. Phytochemical study of Arnica chamissonis less. subsp. foliosa (Nutt.) Maguire. C. R. Acad. Bulg. Sci. 2008, 61, 451–454.
- Poplawski, J.; Holub, M.; Samek, Z.; Herout, V. Terpenes. CCIX. Arnicolides - sesquiterpenic lactones from the leaves of Arnica montana. Coll. Czech Chem. Commun. 1971, 36, 2189–2199.
- Hu, J.F.; Zhu, Q.X.; Bai, S.P.; Jia, Z.J. New eudesmane sesquiterpene and other constituents from Artemisia mongolica. Planta Med. 1996, 62, 477–478.
- Saleh, N.A.M.; El-Negoumy, S.I.; Abou-Zaid, M.M. Flavonoids of Artemisia judaica, A. monosperma and A. herba-alba. Phytochemistry 1987, 26, 3059–3064.
- Belenovskaya, L.M.; Markova, L.P.; Kapranova, G.I. Phenolic compounds of Artemisia xerophytica. Chem. Nat. Compd. 1982, 18, 115.
- Kul’magambetova, E.A.; Pribytkova, L.N.; Adekenov, S.M. Flavonoids of Artemisia glabella. Chem. Nat. Compd. 2000, 36, 95–96.
- Yin, Y.; Gong, F.Y.; Wu, X.X.; Sun, Y.; Li, Y.H.; Chen, T.; Xu, Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J. Ethnopharmacol. 2008, 120, 1–6.
- Sharp, H.; Bartholomew, B.; Bright, C.; Latif, Z.; Sarker, S.D.; Nash, R.J. 6-Oxygenated flavones from Baccharis trinervis (Asteraceae). Biochem. Syst. Ecol. 2000, 29, 105–107.
- Rojas, J.; Morales, A. Study of the chemical components of Baccharis decussata (K) hieron. Ciencia (Maracaibo) 2000, 8, 251–256.
- Zamorano, R.; Aguirre, M.E.; Munoz de la Pena, A.; Cordano, G.; Medina, J.; Timmermann, B. Flavonoids from Baccharis concava Pers. Bol. Soc. Chil. Quim. 1987, 32, 101–103.
- Passero, L.F.; Bonfim-Melo, A.; Corbett, C.E.; Laurenti, M.D.; Toyama, M.H.; de Toyama, D.O.; Romoff, P.; Fávero, O.A.; dos Grecco, S.S.; Zalewsky, C.A.; et al. Anti-leishmanial effects of purified compounds from aerial parts of Baccharis uncinella C. DC. (Asteraceae). Parasitol. Res. 2011, 108, 529–536.
- Weimann, C.; Goransson, U.; Pongprayoon-Claeson, U.; Claeson, P.; Bohlin, L.; Rimpler, H.; Heinrich, M. Spasmolytic effects of Baccharis conferta and some of its constituents. J. Pharm. Pharmacol. 2002, 54, 99–104.
- Mosharrafa, S.A.M.; Mansour, R.M.A.; Abou-Zaid, M.; Saleh, N.A.M. Some biologically active flavonoids from Egyptian members of the Compositae. Bull. Chem. Soc. Ethiopia 1994, 8, 9–13.
- Cardona, M.L.; Fernandez, I.; Pedro, J.R.; Perez, B. Sesquiterpene lactones and flavonoids from Centaurea aspera. Phytochemistry 1991, 30, 2331–2333.
- Halfon, B.; Oksuz, S.; Cirpici, A. Flavonoids from Centaurea cariensis Boiss. Doga: Turk. Saglik. Bilimleri. Dergisi. 1989, 13, 138–140.
- Fernandez, I.; Garcia, B.; Grancha, F.J.; Pedro, J.R. Sesquiterpene lactones, flavonoids and coumarins from Centaurea collina. Phytochemistry 1989, 28, 2405–2407.
- Csupor, D.; Widowitz, U.; Blazso, G.; Laczko-Zold, E.; Tatsimo, J.S.N.; Balogh, A.; Boros, K.; Danko, B.; Bauer, R.; Hohmann, J. Anti-inflammatory activities of eleven Centaurea species occurring in the Carpathian Basin. Phytother. Res. 2013, 27, 540–544.
- Trendafilova, A.; Todorova, M.; Bancheva, S. Secondary metabolites from Centaurea moesiaca. Biochem. Syst. Ecol. 2007, 35, 544–548.
- Mosaddegh, M.; Tavakoli, M.; Behzad, S. Constituents of the aerial parts of Centaurea behen. Chem. Nat. Comp. 2018, 54, 1015–1017.
- Kumkarnjana, S.; Nimmannit, U.; Koobkokkruad, T.; Pattamadilok, C.; Suttisri, R.; Vardhanabhuti, N. Anti-adipogenic effect of flavonoids from Chromolaena odorata leaves in 3T3-L1 adipocytes. J. Integr. Med. 2018, 16, 427–434.
- Perez Gutierrez, R.M.; Ramirez, E.; Vargas, R. Effect of Cirsium pascuarense on blood glucose levels of normoglycaemic and alloxan-diabetic mice. Phytother. Res. 2001, 15, 552–554.
- Lim, H.; Son, K.H.; Chang, H.W.; Bae, K.H.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of pectolinarigenin and pectolinarin isolated from Cirsium chanroenicum. Biol. Pharm. Bull. 2008, 31, 2063–2067.
- Lu, M.; Xu, X.; Lu, H.; Lu, Z.; Xu, B.; Tan, C.; Shi, K.; Guo, R.; Kong, Q. Evaluation of anti-tumor and chemoresistance-lowering effects of pectolinarigenin from Cirsium japonicum Fisch ex DC in breast cancer. Trop. J. Pharm. Res. 2016, 15, 547–553.
- Khan, Z.U.H.; Ali, F.; Khan, S.U.; Ali, I. Phytochemical study on the constituents from Cirsium arvense. Mediter. J. Chem. 2011, 1, 64–69.
- Lee, J.H.; Lee, K.R. Phytochemical constituents of Cirsium nipponicum (MAX.) Makino. Saengyak Hakhoechi 2005, 36, 145–150.
- Chung, A.K.; Kwon, H.C.; Choi, S.Z.; Min, Y.D.; Lee, S.O.; Lee, W.B.; Yang, M.C.; Lee, K.H.; Nam, J.H.; Kwak, J.H. Norisoprenoids from Cirsium rhinoceros. Saengyak Hakhoechi 2002, 33, 81–84.
- Zhu, S.H.; Zhang, Q.j.; Chen, Q.; Zhou, T.; Yao, R.J. Study on chemical constituents of Dichrocephala integrifolia. Zhongguo Shiyan Fangjixue Zazhi 2010, 16, 34–36.
- Bierner, M.W. Pectolinarigenin from Dugaldia pinetorum (Standl.) Bierner. Biochem. Syst. Ecol. 1994, 22, 109–110.
- Zdero, C.; Bohlmann, F.; Mueller, M. Sesquiterpene lactones and other constituents from Eriocephalus species. Phytochemistry 1987, 26, 2763–2775.
- Stevens, J.F.; Elema, E.T.; Wollenweber, E. Exudate flavonoids of Eupatorium cannabinum. Biochem. Syst. Ecol. 1995, 23, 451–452.
- Yuan, J.; Yang, J.; Miao, J. Chemical constituents of Eupatorium odoratum. Zhongcaoyao 2005, 36, 1771–1773.
- Wu, B.; Liang, J. Pectolinarigenin promotes functional recovery and inhibits apoptosis in rats following spinal cord injuries. Exp. Ther. Med. 2019, 17, 3877–3882.
- Herz, W.; Govindan, S.V.; Kumar, N. Sesquiterpene lactones and other constituents of Eupatorium lancifolium and E. semiserratum. Phytochemistry 1981, 20, 1343–1347.
- Xiao, Y.; Li, K.; Wang, Z.; Fu, F.; Shao, S.; Song, F.; Zhao, J.; Chen, W.; Liu, Q.; Xu, J. Pectolinarigenin prevents bone loss in ovariectomized mice and inhibits RANKL-induced osteoclastogenesis via blocking activation of MAPK and NFATc1 signaling. J. Cell Physiol. 2019, 234, 13959–13968.
- Timmermann, B.; Wollenweber, E.; Doerr, M.; Valant-Vetschera, K.M.; Fuentes, E.R. External flavonoids in two Grindelia species. Z. Naturforsch. 1994, 49, 395–397.
- Alarcon, R.; Ocampos, S.; Pacciaroni, A.; Colloca, C.; Sosa, V. Constituents of Gutierrezia mandonii (Asteraceae). Biochem. Syst. Ecol. 2009, 37, 683–685.
- Ozawa, A.T.; Rivera, P.A.; Romo de Vivar, A. Active principles of the toxic plant Helenium integrifolium. Rev. Latinoam. Quím. 1983, 14, 40–43.
- Rojo, A.L.; Palacios, P.S.; Acevedo, C.; Spegazzini, E.D.; Debenedetti, S.L. 6-Methoxyflavonoids from Heterotheca latifolia (Asteraceae). Biochem. Syst. Ecol. 2004, 32, 351–353.
- Ahmed, A.A.; Mohamed, A.Y.; Spring, O.; Bierner, M.W.; Mabry, T.J. Sesquiterpene lactones and flavonoids from Hymenoxys jamesii (Asteraceae) and their systematic significance. Biochem. Syst. Ecol. 2002, 30, 487–491.
- Farkas, L.; Nogradi, M.; Sudarsanam, V.; Herz, W. Constituents of Iva species. V. Isolation, structure, and synthesis of nevadensin, a new flavone from Iva nevadensis and Iva acerosa. J. Org. Chem. 1966, 31, 3228–3232.
- Herz, W.; Bhat, S.V.; Sudarsanam, V. Constituents of Iva species. XII. Sesquiterpene lactones and flavones of Iva frutescens. Phytochemistry 1972, 11, 1829–1831.
- Ybarra, M.I.; Catalan, C.A.N.; Diaz, J.G.; Herz, W. A cyperane and trixanes from Jungia polita. Phytochemistry 1992, 31, 3627–3629.
- Chivers, H.; Corbett, R.E.; Mitchell, R.E.M. Extractives from the leaves of Olearia paniculata. J. Chem. Soc. C: Org. 1966, 20, 1814–1816.
- Cardona, M.L.; Garcia, B.; Pedro, J.R.; Sinisterra, J.F. Flavonoids, flavonolignans and a phenylpropanoid from Onopordon corymbosum. Phytochemistry 1990, 29, 629–631.
- Gonzalez Collado, I, Macias, FA, Massanet, GM, Oliva, J, Maria Rodriguez Luis, F, Vergara, C Chemical components of Onopordum nervosum Boiss. Anales de Quimica, Serie C Quimica Organica y Bioquimica 1984, 80, 100–101.
- Becchi, M.; Carrier, M. 6-Methoxyflavones of Santolina chamaecyparissus. Planta Med. 1980, 38, 267–268.
- Flamini, G.; Ghelli, G.C.; Pistelli, L.; Morelli, I. Phenolic compounds from Santolina pinnata. Planta Med. 1994, 60, 97.
- Sham’yanov, I.D.; Batirov, E.K.; Yuldashev, M.P.; Mallabaev, A. Components of Saussurea elegans. Chem. Nat. Compd. 1983, 19, 763–764.
- Pacciaroni, A.D.V.; Sosa, V.E.; Espinar, L.A.; Oberti, J.C. Sesquiterpene lactones from Schkuhria pinnata. Phytochemistry 1995, 39, 127–131.
- Deng, Y.R.; Song, A.X.; Wang, H.Q. Chemical components of Seriphidium santolium Poljak. J. Chin. Chem. Soc. 2004, 51, 629–636.
- Ortega, A.; Mondragon, P.; Maldonado, E. Guaianolides of Stevia laxiflora. Rev. Soc. Quim. Mex. 1999, 43, 100–102.
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H.; Kabbashi, N.A. Metabolic profiling of flavonoids, saponins, alkaloids, and terpenoids in the extract from Vernonia cinerea. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 722–731.
- Wollenweber, E.; Bouillant, M.L.; Lebreton, P.; Egger, K. Rare flavonoid-aglycons in the lipophile excretion of Alnus glutinosa. Z. Naturforsch. B 1971, 26, 1188–1190.
- Wollenweber, E. Flavonoids from Alnus crispa, A. japonica, A. koehnei and A. sinuate. Phytochemistry 1974, 13, 2318–2319.
- Popravko, S.A.; Kononenko, G.P.; Tikhomirova, V.I.; Vul’fson, N.S. Secondary metabolites of the birch. IV. Identification of the group of flavonoid aglycons in birch buds (Betula verrucosa). Bioorg. Khim. 1979, 5, 1662–1667.
- Isidorov, V.; Szoka, L.; Nazaruk, J. Cytotoxicity of white birch bud extracts: Perspectives for therapy of tumours. PLoS ONE 2018, 13, e0201949.
- Hase, T.; Ohtani, K.; Kasai, R.; Yamasaki, K.; Picheansoonthon, C. Revised structure for hortensin, a flavonoid from Millingtonia hortensis. Phytochemistry 1995, 40, 287–290.
- Wang, K.; Li, M.M.; Chen, X.Q.; Peng, L.Y.; Cheng, X.; Li, Y.; Zhao, Q.S. Phenolic constituents from Brainea insignis. Chem. Pharm. Bull. 2010, 58, 868–871.
- Bacon, J.D.; Hannan, G.L.; Fang, N.; Mabry, T.J. Chemosystematics of the Hydrophyllaceae: Flavonoids of three species of Eriodictyon. Biochem. Syst. Ecol. 1986, 14, 591–595.
- Agnese, A.M.; Juliani, H.R.; Cabrera, J.L. Phytochemical study of species of genus Adesmia (Fabaceae). Anales de la Asociacion Quimica Argentina 1989, 77, 287–291.
- Wollenweber, E.; Doerr, M.; Rivera, D.; Roitman, J.N. Externally accumulated flavonoids in three Mediterranean Ononis species. Z. Nat. C J. Biosci. 2003, 58, 771–775.
- Wollenweber, E. On the distribution of exudate flavonoids among angiosperms. Rev. Lat. Quim. 1990, 21, 115–121.
- He, X.G.; Lin, L.Z.; Lian, L.Z. Analysis of flavonoids from red clover by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. A 1996, 755, 127–132.
- Pu, X.; Zhou, J. Studies on the chemical components from Leucosceptrum canum. Yunnan Zhiwu Yanjiu 1989, 11, 263–266.
- Zaidi, F.; Voirin, B.; Jay, M.; Viricelt, M.R. Free flavonoid aglycons from leaves of Mentha pulegium and Mentha suaveolens (Labiatae). Phytochemistry 1998, 48, 991–994.
- Vieira, R.F.; Grayer, R.J.; Paton, A.J. Chemical profiling of Ocimum americanum using external flavonoids. Phytochemistry 2003, 63, 555–567.
- Abdelshafeek, K.A.; Elgendy, H.A.; El Missiry, M.M.; Seif El Nasr, M.M. Structure elucidation of phenolic acids, flavonoids and hypocholesterolemic activity of Nepeta septemcrenata and Otostegia fruticosa. Der Pharm. Chem. 2016, 8, 357–362.
- Ulubelen, A.; Öztürk, S.; Iśildatici, S. A new flavone from Salvia triloba L.f (Labiatae). J. Pharm. Sci. 1968, 57, 1037–1038.
- Wollenweber, E.; Dörr, M.; Rustaiyan, A.; Roitman, J.N.; Graven, E.H. Exudate flavonoids of some Salvia and a Trichostema species. Z. Naturforsch. 1992, 47, 782–784.
- Ulubelen, A.; Tuzlaci, E. Flavonoids and triterpenoids from Salvia euphratica and S. longipedicellata. Fitoterapia 1990, 61, 185.
- Topcu, G.; Ulubelen, A.; Tam, T.C.M.; Tao-Che, C. Ses-terterpenes and other constituents of Salvia yosgadensis. Phytochemistry 1996, 42, 1089–1092.
- Han, G.H.; Li, Z.L.; Sun, L.; Hua, H.M. Chemical constituents of the whole herbs of Salvia plebeia R. Br. Shenyang Yaoke Daxue Xuebao 2009, 26, 896–899.
- Kolak, U. New diterpenoids from the aerial parts of Salvia pilifera. Turk. J. Chem. 2007, 31, 363–369.
- Nikolova, M.T.; Grayer, R.J.; Genova, E.; Porter, E.A. Exudate flavonoids from Bulgarian species of Salvia. Biochem. Syst. Ecol. 2006, 34, 360–364.
- Davydov, V.S.; Nikitina, G.K.; Bandyukova, V.A. Flavonoids in aerial parts of Scutellaria polyodon Juz. Rastitel’nye Resursy 1991, 27, 50–54.
- Denikeeva, M.F.; Litvinenko, V.I.; Borodin, L.I. Flavonoid compounds of Scutellaria przewalskii. Khimiya Prirodnykh Soedinenii 1970, 6, 534–539.
- Gonzalez, A.G.; Fraga, B.M.; Hernandez, M.G.; Larruga, F.; Luis, J.G.; Ravelo, A.G. Flavones from some canary species of Sideritis. Lloydia 1978, 41, 279–280.
- Ulubelen, A.; Topcu, G.; Kaya, U. Steroidal compounds from Teucrium chamaedrys subsp. chamaedrys. Phytochemistry 1994, 36, 171–173.
- Marin, P.D.; Grayer, R.J.; Kite, G.C.; Matevski, V. External leaf flavonoids of Thymus species from Macedonia. Biochem. Syst. Ecol. 2003, 31, 1291–1307.
- Thoison, O.; Sévenet, T.; Niemeyer, H.M.; Russell, G.B. Insect antifeedant compounds from Nothofagus dombeyi and N. pumilio. Phytochemistry 2004, 65, 2173–2176.
- Aqil, M. Flavonoids from Striga passargei. Ultra Sci. Phys. Sci. 1995, 7, 105–107.
- Aqil, M.; Khan, I.Z. Cirsimiaritin 5-galactoside from Striga aspera. Sci. Phys. Sci. 1993, 5, 95–97.
- Olalere, O.A.; Abdurahman, H.N.; Gan, C.Y. Microwave-enhanced extraction and mass spectrometry fingerprints of polyphenolic constituents in Sesamum indicum leaves. Ind. Corps Prod. 2019, 131, 151–159.
- Imre, S.; Islimyeli, S.; Oztunc, A.; Buyuktimkin, N. Flavonoid aglycons in some Digitalis species. Planta Med. 1984, 50, 360.
- Hiermann, A.; Kartnig, T. Flavonoids in the leaves of Digitalis lanata (Ehrhart). Part 2. Planta Med. 1978, 34, 225–226.
- Perry, N.B.; Foster, L.M. Antiviral and antifungal flavonoids, plus a triterpene, from Hebe cupressoides. Planta Med. 1994, 60, 491–492.
- Nikolova, M.; Gevrenova, R. A HPLC analysis on interpopulational variations in the flavonoid composition of Veronica chamaedrys. Int. J. Bot. 2007, 3, 7–10.
- Yin, L.; Han, H.; Zheng, X.; Wang, G.; Li, Y.; Wang, W. Flavonoids analysis and antioxidant, antimicrobial, and anti-inflammatory activities of crude and purified extracts from Veronicastrum latifolium. Ind. Crops Prod. 2019, 137, 652–661.
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of purslane (Portulaca oleracea L.) extract on anti-browning of freshcut potato slices during storage. Food Chem. 2019, 283, 445–453.
- Wei, J.; Li, D.; Hua, H.; Li, Z. Isolation and identification of chemical constituents from flowers of Trollius chinensis (II). J. Shenyang Pharm. Univ. 2012, 29, 12–15. [
- Papanov, G.; Malakov, P.; Tomova, K. Aromatic compounds, flavones, and glycosides from extracted flowers of Rosa damascena. Nauchni Trudove-Plovdivski Universitet Paisii Khilendarski 1984, 22, 221–226.
- Wollenweber, E.; Doerr, M. Flavonoid aglycones from the lipophilic exudates of some species of Rosaceae. Biochem. Syst. Ecol. 2008, 36, 481–483.
- Singhal, A.K.; Sharma, R.P.; Thyagarajan, G.; Herz, W.; Govindan, S.V. New prenylated isoflavones and a prenylated dihydroflavonol from Millettia pachycarpa. Phytochemistry 1980, 19, 929–934.
- Singh, M.; Prakash, L. A new flavone glycoside and other chemical constituents from Kickxia ramosissima Wall. (Scrophulariaceae). Pharmazie 1987, 42, 490–491.
- Khan, I.Z.; Aqil, M.; Kolo, B.G. A new flavone glycoside Kickxia ramosissima (Wall). Ultra Sci. Phys. Sci. 2001, 13, 112–115.
- Amin, A.; Tuenter, E.; Foubert, K.; Iqbal, J.; Cos, P.; Maes, L.; Exarchou, V.; Apers, S.; Pieters, L. In vitro and in silico antidiabetic and antimicrobial evaluation of constituents from Kickxia ramosissima (Nanorrhinum ramosissimum). Front. Pharmacol. 2017, 8, 232.
- Bui, M.L.; Grayer, R.J.; Veitch, N.C.; Kite, G.C.; Tran, H.; Nguyen, Q.C.K. Uncommon 8-oxygenated flavonoids from Limnophila aromatica (Scrophulariaceae). Biochem. Syst. Ecol. 2004, 32, 943–947.
- Stetskov, V.V.; Krivut, B.A. Chromatospectrophotometric method for the quantitative determination of pectolinarigenin in Linaria vulgaris and L. kurdica. Chem. Nat. Compd. 1982, 5, 553–555.
- Pal, S.; Chowdhury, A.; Adityachaudhury, N. Isolation of rice weevil feeding inhibitors uncinatone and pectolinarigenin from Clerodendron siphonenthus. J. Agric. Food Chem. 1989, 37, 234–236.
- Barua, A.S.; Pal, S.; Chowdhury, A.; Adityachaudhury, N. Occurrence of pectolinarigenin and cirsimaritin in Clerodendron siphonanthus. Ind. J. Chem. Sect. B Org. Chem. Includ. Med. Chem. 1989, 28, 198.
- Subramanian, S.S.; Nair, A.G.R. Scutellarein and pectolinarigenin from Clerodendron phlomides and Duranta rupens. Phytochemistry 1972, 11, 3095–3096.
- Seth, K.K.; Pandey, V.B.; Dasgupta, B. Flavonoids of Clerodendron phlomidis flowers. Pharmazie 1982, 37, 74–75.
- Roy, R.; Pandey, V.B. Flavonoids of Clerodendron phlomidis. Indian J. Nat. Prod. 1995, 11, 13–14.
- Muthu, C.; Baskar, K.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Bioefficacy of pectolinaringenin from Clerodendrum phlomidis Linn. F. against Anopheles stephensi and bhendi fruit borer, Earias vittella fab. Braz. Arch. Biol. Technol. 2015, 58, 358–366.
- Singh, M.K.; Khare, G.; Iyer, S.K.; Sharwan, G.; Tripathi, D.K. Clerodendrum serratum: A clinical approach. J. Appl. Pharm. Sci. 2012, 2, 11–15.
- Vendantham, T.N.; Subramanian, S.S.; Harborne, J.B. 4′-methyl-scutellarein and pectolinarigenin from Clerodendron inerme. Phytochemistry 1977, 16, 294–295.
- Chethana, G.S.; Savitha, H.; Jyothi, N.; Hari Venkatesh, K.R.; Gopinath, S.M. Pharmacognostic investigations on different parts of Clerodendrum inerme. Glob. J. Res. Med. Plants Indigen. Med. 2013, 2, 485–491.
- Ganapaty, S.; Rao, D.V. Constituents of Clerodendrum neriifolium. Fitoterapia 1989, 60, 381.
- Rahman, A.A.; Azam, A.T.M.Z.; Gafur, M.A. Brine shrimp lethality bioassay with extracts and two flavonoids from Clerodendrum indicum Linn. Pak. J. Pharmacol. 2000, 17, 1–6.
- Somwong, P.; Suttisri, R. Cytotoxic activity of the chemical constituents of Clerodendrum indicum and Clerodendrum villosum roots. J. Integr. Med. 2018, 16, 57–61.
- Ganapaty, S.; Babu, G.J.; Naidu, K.C. Phytochemical studies of roots of Duranta repens. Ind. J. Nat. Prod. 1997, 13, 11–14.
- Babu, G.J.; Naidu, K.C.; Ganapaty, S. Phytoconstituents from the stem of Duranta plumieri Jacq. Indian Drugs 1998, 35, 514–516.
- Skaltsa, H.; Shammas, G. Flavonoids from Lippia citriodora. Planta Med. 1988, 54, 465.
- Takagi, M.; Funahashi, S.; Ohta, K.; Nakabayashi, T. Flavonoids in the sea-grass, Phyllospadix japonica. Agric. Biol. Chem. 1979, 43, 2417–2418.
