In June 2020, the long-waited “Regulations on the Supervision and Administration of Cosmetics” (CSAR) was finally issued by the State Council of China, and this regulation will be implemented from 1 January 2021 [1]. CSAR is the first-time revision and replacement of the “Regulations on Hygiene Supervision of Cosmetics” (CHSR), which was published in 1989. During the past 30 years, substantial changes have happened both in the industry and in consumer needs, and the market has increased significantly. According to incomplete statistics, there are more than 1,800,000 valid cosmetic products in China currently in 2020. In addition, new techniques and approaches have appeared, and the concepts of supervision and administration have evolved.
- CSAR
- cosmetics
- ingredient
- categorization
- safety evaluation
- efficacy substantiation
1. CSAR and Its Regulatory System
There are 6 chapters and 80 articles in CSAR, while the previous CHSR has only 35 articles. The 6 chapters include general provisions, ingredients and products, production and distribution, supervision and administration, legal liability, and supplementary provisions. Key points concerning cosmetics are specified in CSAR, for example, the definition, product classification, ingredient management, registration and notification, requirements for production, post-market supervision and inspection, and the roles and corresponding responsibilities in cosmetics-related activities.
There is a shift of regulatory focus in CSAR when compared to CHSR, which can be reflected in the change of names. CHSR emphasizes more on the hygiene qualification of cosmetic products, while in CSAR, advanced management philosophy and measures are introduced or further promoted, such as key responsibilities of enterprises (by introducing the concepts of registration person and notification person), classified risk management, encouragement of innovation, safety evaluation, and efficacy substantiation. If a keyword in CHSR is “hygiene”, the new will be “safety” and “quality”. Similar changes once happened when the technical regulation for cosmetics, the “Hygienic Standards for Cosmetics”, was revised into “Safety and Technical Standards for Cosmetics” (STSC) in 2015.
As the fundamental regulation, CSAR provides a basis for detailed regulations and technical documents. NMPA has made a legislation plan to build up the CSAR related regulatory system , covering both the procedure rules and technical guidance. In brief, these subordinated regulations and documents can be functionally divided into four units. The first group is about registration and notification, with the procedural and technical requirements for both ingredients and final products. The second is about production and distribution, taking producers and enterprises as the main objects of administration. The third part is about toothpaste. Moreover, the last one is for standardized management of labeling. In addition, more regulations and documents are also being drafted or revised.
2. Definition, Scope and Classification of Cosmetics
2.1. Definition and Scope
The definition of cosmetics in CSAR remains unchanged when compared to CHSR, and cosmetics are divided into special cosmetics and general cosmetics in both CHSR and CSAR. Special cosmetics are regulated with registration and must get approval before production or importation, with passing technical evaluation from the National Institutes for Food and Drug Control (NIFDC), a subordinated institution of NMPA. General cosmetics can be directly put into the market with the completion of a notification.
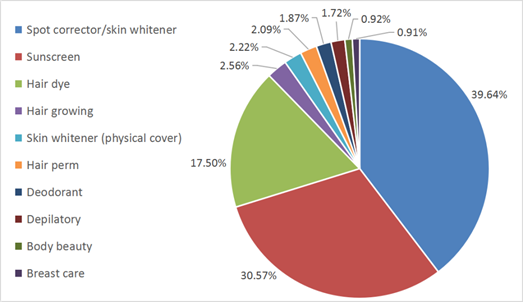
Although the definition of cosmetics remains the same, the interpretation of this definition and the scope of cosmetic products have actually been changed. In CHSR, there are nine special cosmetics, including products for/as hair dye, hair perm, sunscreen, depilatory, deodorant, spot corrector (including skin whitener), hair growth, breast care and body beauty (help to keep body shape). According to the approved products published online by NMPA, the approximate distribution of existing special cosmetics is shown in Figure 1. In CSAR, only five out of the nine still remain as special cosmetics, including products for/as hair dye, hair perm, spot corrector/skin whitener, sunscreen and preventing hair loss (instead of products for hair growth). Moreover, there is a new category in special cosmetics called “cosmetics with new efficacy claim”.
2.2. Borderline between Cosmetics and Drugs
In reality, some drugs can also be applied to the skin or other external parts of the human body and have functions similar to cosmetics. It is a common practice to set a borderline between cosmetics and drugs. For example, a guidance document and specific manual were published in the EU to clarify the applicable scope of cosmetic regulations and help distinguish cosmetics from pharmaceutical products and medical devices, as well as from toys, biocides and other articles [1][2][3]. In recent years, the concept of “cosmeceutical” is getting increasingly popular, which is often interpreted by consumers as cosmetics with medical effects. In fact, the status of “cosmeceutical” is still controversial [20]. For example, as the US Food and Drug Administration (FDA) declares, the Federal Food, Drug, and Cosmetic Act (FD&C Act) do not recognize any such category as “cosmeceutical”[4].
Compared to drugs, cosmetics are designed for some limited purposes. They differ in the use of ingredients, risk characteristics and management, tolerance of adverse reactions, regulatory requirements, supervision systems and other important aspects. In addition, cosmetics are designed for normal people, while drugs are for those with health issues, and some should only be used under doctors’ instructions. When mistaking drugs for cosmetics, one can underestimate the possible adverse effects and thus, an extra risk to users can be generated; when taking cosmetics for drugs, the precious chance to seek professional medical care can be delayed.
In January 2019, NMPA clarified it illegal to claim “medicated cosmetics” or “medical skincare products” in China [5]. This principle will be maintained in the implementation of CSAR. Foreign quasi-drugs and drugs can only be imported as cosmetics under the premise of meeting the definition and requirements in China, including the legal compliance of the product itself and a probable adaption of labeling information.
2.3. Categorization and Catalog
With a vast diversity of cosmetic products, it will be helpful to use a categorization system to reflect the characteristics of each product. For example, The US FDA employs a set of category codes to help describe a cosmetic product in the Voluntary Cosmetic Registration Program (VCRP) [6]. In Japan, there is a list of about 56 admitted efficacy claims for cosmetics, which could also be a description of product features.
On 29 July 2020, NMPA published a draft of “Rules and Catalog for Categorization of Cosmetics” online for public comments. With this regulation, a cosmetic product can be described in five dimensions and get a unique category code. The five dimensions are efficacy claim, applied area, product form, users and exposure way (rinse-off or leave-on). Detailed items and descriptions are listed in these dimensions term-by-term, with a numerical code for each one. In this catalog, industrial development and newly emerging techniques are also taken into account. For example, the efficacy of “restoring and protection” is collected considering the increasing consumption demands [7][8], and freeze-dried powder, which is becoming increasingly popular in production these years, is also collected as a product form. Once none of the numerical codes can cover a practical case, a capital letter code shall be picked up, which means “other”. Any appearance of a capital letter in efficacy claim, applied area or users can be an indication of “cosmetics with new efficacy claim”, the newly added special cosmetics in CSAR.
To give an example, according to the draft, a facial lotion with functions of sunscreen and moisture can get a code of 0409-05-02-01-02, which means “moisture and sunscreen-face-lotion-general population-leave on”; a hair shampoo made especially for infants can get a code of 01-01-03-02-01, which means “clean-hair-liquid-babies and infants (under 3 years old)-rinse off”.
The category code is a new invention in the supervision and administration of cosmetics in China. A brief sketch of the product information can be immediately delineated with the code, and this system will help in accurate statistics and analysis. Furthermore, with reading the category code and some other submitted information, for example, the formula information, a high-throughput automated judgment of regulatory compliance can be possible, which is hoped to replace some labored work in the technical evaluation of cosmetics in the future.
3. Management of Cosmetic Ingredients
3.1. Ingredient Lists in STSC
The safety of cosmetic products highly depends on the use of ingredients. In the EU, prohibited substances, restricted substances (including hair dyes), allowed colorants, allowed preservatives and allowed ultra-violet (UV) filters are, respectively, collected in the annexes of the “Regulation (EC) No 1223/2009 on Cosmetic Products” [9]. In the US, substances prohibited or restricted in cosmetic products are listed in the Code of Federal Regulations Title 21 (21CFR). FDA also pays attention to the management of colorants. Allowed colorants and related requirements are also specified in 21CFR. Sunscreen is recognized as a drug in America, and a list of sunscreen active ingredients is collected in the sunscreen OTC monograph. In some East Asian countries where consumption demands for skin whitening are strong, such as Japan and Korea, there is also a positive list of whitening agents.
In China, there are also technical lists of cosmetic ingredients. In STSC, high-risk ingredients are collected in the lists of prohibited substances, restricted ingredients, as well as allowed preservatives, UV filters, colorants and hair dyes. The use of these ingredients must strictly meet the requirements and technical standards specified in STSC. The current STSC was published in 2015 and is open to revision all the time.
Moreover, as shown in Figure 1, spot corrector/skin whitener product is the biggest share in all the special cosmetics in China. As a result, it is important to consider the necessity to build a list of whitening agents in STSC in the future, where Japan and Korea have already provided some experience.
3.2. New Cosmetic Ingredients
Another key point in the management of cosmetic ingredients in China is to distinguish between “existing ingredient” and “new ingredient”. New cosmetic ingredients refer to the natural or artificial ingredients used in cosmetics for the first time within China. To better help identify new ingredients, an Inventory of Existing Cosmetic Ingredients in China (IECIC) was published in 2014 and revised in 2015, generating a collection of 8783 items of ingredients (some are collected as a group of ingredients, and besides the 8783 items, ingredients collected in the restricted or positive lists in STSC are also part of IECIC). IECIC is only an objective collection of cosmetic ingredients already used in China, different from a “positive list of cosmetic ingredients”. One, who intends to use the ingredients in it, should take a safety evaluation before using.
Ingredients excluded by IECIC are regarded as new ingredients. According to CHSR, new ingredients can only be used after approval. In CASR, the policy is optimized by dividing new ingredients into different risk levels: ingredients that function as a preservative, UV filter, colorant, hair dye or spot corrector/skin whitener are considered to be relatively high-risk and will be regulated with a registration-based system by NMPA continuously; others can be immediately used after notification to NMPA. On the basis of scientific development, NMPA can submit an application to adjust the range of high-risk ingredients.
Innovative management for the use of new ingredients in CSAR is the three-year period of monitoring after registration or notification. Within the three years, the registration person or notification person shall submit a feedback report to NMPA about the use and safety situations every year, and any emergency shall be reported immediately. When a certain safety issue occurs, if any, this registration or notification can be withdrawn by NMPA. In order to protect the interest of enterprises and encourage the development of new ingredients, within the monitoring period, the ingredient will still be regarded and managed as a new ingredient: any other person who intends to use the ingredient shall complete the registration or notification of all independently, or obtain use permission from any previous registration person or notification person. Ingredients successfully passing the three-year monitoring period will have the chance to be incorporated into IECIC.
This entry is adapted from the peer-reviewed paper 10.3390/cosmetics7040098
References
- Sonia, S. Overlapping Definitions of Drugs, Topical Medical Devices, Cosmetics. The cosmetic efficacy: Myth or reality? J. Appl. Cosmetol. 2011, 29, 129–133.
- Su, Z.; Xing, S.; Wang, G. The Worldwide Regulation of Cosmeceutical and Its Enlightenment to China. Chin. Pharm. Aff. 2019, 33, 1383–1390.
- Food and Drug Administration. Is It a Cosmetic, a Drug, or Both? (Or Is It Soap?). Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap#Cosmeceutical (accessed on 15 October 2020).
- Food and Drug Administration. Code of Federal Regulations, Title 21, Part 352: Sunscreen Drug Products for Over-the-counter Human Use. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=58025e96360c4105ae8364a55fedde8f&mc=true&node=pt21.5.352&rgn=div5 (accessed on 3 December 2020).
- Food and Drug Administration. Code of Federal Regulations, Title 21, Part 350: Antiperspirant Drug Products Over-the-counter Human Use. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=58025e96360c4105ae8364a55fedde8f&mc=true&node=pt21.5.350&rgn=div5 (accessed on 3 December 2020).
- Food and Drug Administration. Cosmetic Product Category Codes. Available online: https://www.fda.gov/cosmetics/paper-registration-voluntary-cosmetic-registration-program-vcrp/cosmetic-product-category-codes (accessed on 20 October 2020).
- Cork, M. Cork mjthe importance of skin barrier function. J dermatolog treat 8:S7-s13 (abstr). J. Dermatol. Treat. 2009, 8, S7–S13.
- Draelos, Z. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatol. 2012, 30, 345–348.
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council, of 30 November 2009, on Cosmetic Products; Official Journal of the European Union, 2009. Available online: https://ec.europa.eu/health/sites/health/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf (accessed on 1 December 2020).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council, of 30 November 2009, on Cosmetic Products; Official Journal of the European Union, 2009. Available online: https://ec.europa.eu/health/sites/health/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf (accessed on 1 December 2020).