This entry is associated with a review article, published in MDPI Applied Sciences on 23 April 2020, about the current knowledge on the mechanical stimulation of mesenchymal stem cells for cartilage regeneration. Loading stresses, physiologically experienced by chondrocytes, regulate the production of glycosaminoglycan and collagen, as well as promote and preserve cell viability. Therefore, there is a rising interest in the development of devices that impose mechanical stimuli, such as compression and shear stress, on mesenchymal stem cells. The mentioned review will analyze the dynamics within the joint, the physiological stimuli experienced by the chondrocytes, and how the biomechanical stimulation can be applied to a stem cell culture in order to induce chondrogenesis. In addition to that, paper lists some of the current applications in the field.
The characteristic structure of the articular cartilage is the result of the dynamic processes that occur within the joint. Chondrocytes experience biomechanical stimuli like compression, shear stress, and hydrostatic pressure [
19]. These forces are perceived as a shifting of currents, electrical fields, or changes in osmolarity and so converted into intracellular signals, influencing mechanisms like transcription, exocytosis, and activity of Na+/K+-ATPase [
21]. Growth factors such as TGF-β, IGF, and bone morphogenetic proteins (BMP)-2,-4,-7 are necessary to stimulate chondrogenic processes and require the presence of calcium ions (Ca
2+) to regulate cell functions, such as the synthesis of extracellular matrix components. Physical stimuli have been associated with the regulation of Ca
2+ entry, primarily through voltage-operated calcium channels (VOCCs), transient receptor potential (TRP) channels, and purinergic receptors [
22]. Furthermore, VOCC inhibitors have shown to reduce cartilage degradation and the progression of osteoarthritis [
23], suggesting their importance in both physiological and pathological milieux. TRP channels, such as TRP vanilloid 4 (TRPV4), which have been linked to upregulation of the SOX9 pathway, or TRPC1, able to guide chondrogenesis in stem cells and regulate the activity of other voltage-dependent ion channels, are also highly involved. Inhibitors for these two receptors, 2-aminoethoxydiphenylborane (2-APB) and Ruthenium Red, have been shown to prevent MSC chondrogenesis induced by pulsed electromagnetic fields (PEMFs) [
24].
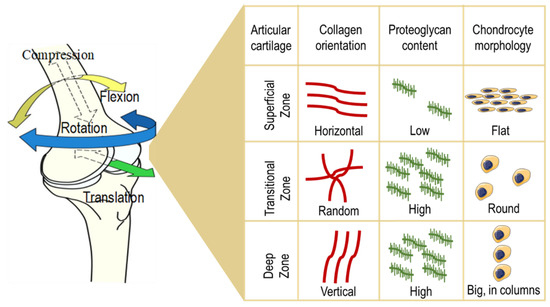
The structure of the articular cartilage comprises several layers: First, a thin superficial zone, where the collagen fibers are aligned parallel to the surface and the chondrocytes are numerous and flattened. This layer is in tight contact with the synovial fluid, and it can resist shear stresses. Under the first zone, there is a transitional zone which contains especially proteoglycans (PGs) employed for compressive resistance. Here, the collagen is arranged obliquely. Just below, the deep zone can cushion the compressions and presents collagen fibers organized perpendicular to the surface, high concentrations of proteoglycans, and columns of cells. Lastly, the tide mark separates this last zone from the calcified cartilage [
25] ().
Figure 1. Structure of the articular cartilage. The structure of the articular cartilage comprises several layers: First, a superficial zone, where the collagen fibers are aligned horizontally, the chondrocytes are numerous and flattened, and the proteoglycan content is low. Under the first zone, there is a transitional zone which contains high levels of proteoglycans, randomly arranged collagen, and round-shaped cells. Just below, the deep zone presents vertically aligned collagen fibers, a high concentration of proteoglycans, and columns of cells.
For the sake of simplicity, this tissue could be imagined like a biphasic model: one phase is represented by the interstitial water that permeates the other phase, made up of the solid components of the ECM (collagen, PGs and other proteins). These two phases are extremely interdependent in terms of functionality and biomechanics. Compression is one of the forces experienced by cartilage, which leads to an internal increase of hydrostatic pressure of the aqueous phase. As a result, the water leaks from the ECM towards the capsule, but the structure of the cartilage will remain unaltered thanks to the PG component. More precisely, the effect of uniaxial compression will be compensated by the tensile stiffness generated by the repulsive forces between the negatively charged carboxylic or sulfonic groups of the glycosaminoglycans (GAGs). When the pressure ceases, the water is again attracted inside the interstices. Tissue compressibility under load reaches even a millimeter, but, when the spring back is not able to compensate hard and long compressions, the structure can be damaged [
26]. Shear or rotational stress, which could be defined by the change in thickness with respect to the original height, is another force generated by joint movement and is caused by the tangential friction of synovial fluid on the surface. This movement allows synovial fluid to nourish the cartilage, transport waste materials, and keep the chondrocytes metabolically active with a mechanism of diffusion and fluid convection [
27]. Under this condition, the collagen network is the viscoelastic component of the tissue that exhibits cushioning ability. The collagen concentration is directly proportional to resistance to shifting [
28]. Weight-bearing articular cartilage of the hip and knee daily experience stress amplitudes from 0.5 to 7.7 MPa and average compression of 13% [
29,
30,
31]. Chondrocytes show selective responses to various mechanical stimuli. Indeed, dynamic stresses are able to improve the production of ECM components, while static compressions do not lead to great achievements in tissue engineering constructs. Mechanical stimulation triggers those pathways that culminate in maintaining functional ECM in order to provide substantial physical stability against the stresses to which the cartilage is subjected. It is a feedback cycle in which the mechanical stress influences the production of those components which sustain the stress itself. It is not surprising that, in fact, an unbalanced step in this cycle could pave the way to a pathological mechanism which could, in the end, lead to the onset of osteoarthritic features. If physiological stimulation fails, following, for example, sedentary habits, or it exceeds the ability of the tissue to sustain it, e.g., excessive mechanical loading [
32], the chondrocytes will miss most of the input to produce the new ECM, resulting in unbalanced homeostasis. It is highly recommended to patients who suffer from early osteoarthritis, who are able to conduct physical exercise, to encourage the movement of the diseased articulation in order to stimulate the restoration of the physiological cycle, which may lead to improvements in biochemical disorders. This dynamic environment should be considered in tissue engineering approaches in order to realize as realistic a construct as possible. The challenge proposed is to move from a purely biological view of the natural cell to an engineered one.
Stem cells are studied as structures able to sense and transmit physical stimuli, translating them into biological and mechanical responses, since they have greater mechanical sensitivity than adult cells [
33,
34]. As already mentioned, ion channels are paramount in triggering those signaling pathways which lead to matrix turnover and homeostasis, and an intracellular increase of Ca
2+ levels has been considered as one of the stem cell responses to mechanical load. Sequestration of calcium ions and inhibition of VOCCs and other channels have been shown to attenuate the effects of mechanical stimulation. More specifically, during physical stimulation of MSCs, Ca
2+ is known to be involved in the activation of pivotal transcription factors leading to chondrogenic differentiation [
20]. In the next section, some interesting works on the topic are presented. In some of those, no exogenous growth factors were added during the experiment. Hence, chondrogenic differentiation was achieved exclusively as a result of load applications.
This entry is adapted from the peer-reviewed paper 10.3390/app10082927