Coffee berry borer (CBB) is the most serious insect pest of coffee worldwide, causing more than US$500M in damages annually. Reduction in the yield and quality of coffee results from the adult female CBB boring into the coffee fruit and building galleries for reproduction, followed by larval feeding on the bean itself.
- Coffea arabica
- biocontrol
- biosecurity
- cultural control
- integrated pest management
- invasion biology
- Coleoptera
1. Introduction
Coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) or CBB, is the most damaging insect pest of coffee worldwide, affecting both the yield and quality of coffee products [1][2] and causing more than US$500 million in damage annually [3]. Damage to the marketable coffee product occurs when the female beetle (Figure 1A) bores a hole into the coffee fruit (often referred to as a berry) and eventually into the seed (or “bean”) (Figure 1B), where she builds galleries for reproduction (Figure 1C), followed by larval feeding on the endosperm [4][5] (Figure 1D). In addition to being a serious pest of coffee, CBB is cosmopolitan today: over about 100 years, CBB has spread from the humid evergreen forests of Africa to nearly all coffee-producing countries in the world (the exceptions being Australia [6], China, and Nepal [5][7][8]) (Figure 2).
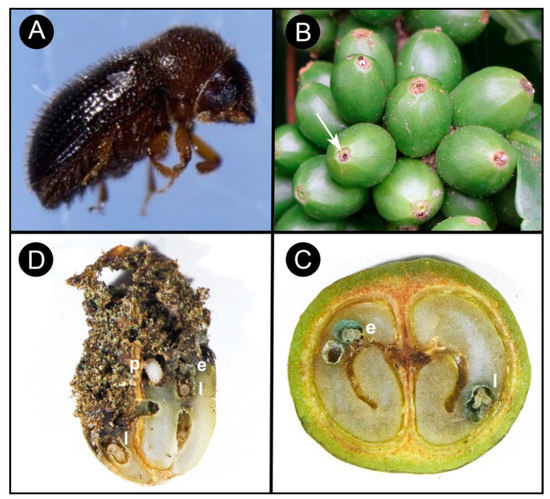
Figure 1. The coffee berry borer life cycle: (A) adult female coffee berry borer; (B) entrance hole with female coffee berry borer (CBB) (arrow) in the central disc of the developing green coffee berries; also note the presence of entomopathogenic fungus Beauveria bassiana (white mycelium protruding from female CBB); (C) reproductive galleries containing eggs (e) and larvae (l) in the coffee bean; (D) advanced stages of bean damage due to larval feeding, with pupae (p), larvae (l), and eggs (e) visible.
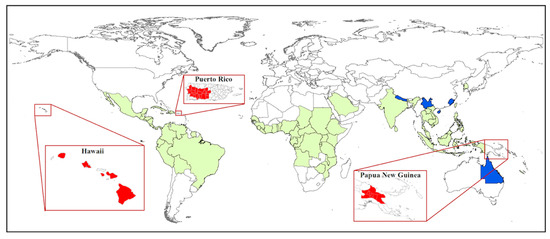
Figure 2. World distribution of the coffee berry borer (CBB). Countries with CBB prior to 2007 are highlighted in green. Island regions that were recently invaded are highlighted in red from left to right; Hawaii (2010), Puerto Rico (2007), and Papua New Guinea (2018). The only countries without CBB (Nepal, China, and Australia) are highlighted in blue.
2. Taxonomy and Identification
The genus Hypothenemus is one of the largest genera within the Scolytidae family (“bark beetles”) with 181 described species [9][10][11][12]. The majority of Hypothenemus species are poorly known, being very small (<2 mm long) wood-boring beetles that occur in tropical and subtropical areas [13]. The coffee berry borer was first described as Cryphalus hampei by Ferrari in 1867 [14] from specimens obtained in traded green coffee beans that were imported to France from an unknown location [15]. Following Eichhoff’s [16] description of the genus, the species was later moved to Stephanoderes, a genus treated as a synonym of Hypothenemus by Swaine [17]. The genus Stephanoderes was eventually moved to Hypothenemus by Browne [18], with Hypothenemus hampei being the currently accepted name for the species.
Hypothenemus hampei is distinguished from other Hypothenemus species by the following morphological characters: a narrowly rounded pronotum with mixed setae and some slightly flattened (Figure 3A,B); a broadly rounded elytral declivity without a distinct transition from the elytral disc (Figure 3B); prominent interstrial bristles in uniseriate rows, and the bristles being long, narrow, and slightly flattened (Figure 3B); a broad, indistinctive frontal grove or no groove at all on the frons (Figure 3C); usually four marginal asperities [5]. Hopkins [19] noted that one of the defining characters for taxa within the genus Hypothenemus is a four-segmented antennal funicle, while five segments is characteristic of the genus Stephanoderes. However, this character is unreliable as some species can have a range of 3–5 segments [5], including H. hampei (see Figure 3C). This suggests that a detailed taxonomic review of the genus should be conducted, taking into account relevant information on morphology and phylogeny.
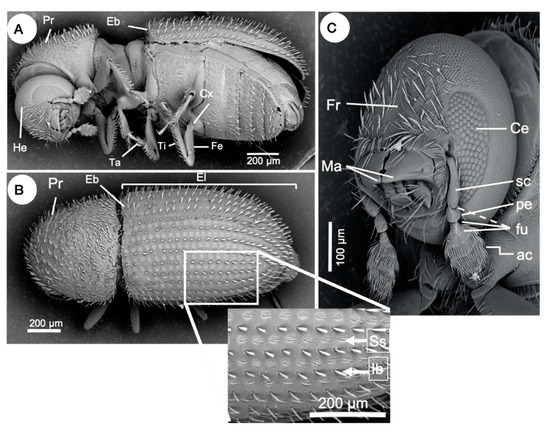
Figure 3. External anatomy of an adult of Hypothenemus hampei. (A) Lateral and inferior view showing pronotum (Pr), elytral base (Eb), head (He), legs with third coxa (Cx), femur (Fe), tibia (Ti), and tarsus (Ta). (B) Top view; detail (box) of the elytron (El) showing strial setae (Ss) and interstrial bristles (Ib). (C) Frons (Fr), mandibles (M), compound eye (Ce), antenna club (Ac), segmented funicle (Sf), pedicel (Pe), scapus or scape (Sc). Nomenclature according to Hulcr et al. [20] and Vega et al. [5].
3. Origin
CBB was first reported in coffee plantations in Liberia in 1897, followed by the Democratic Republic of Congo in 1901 [1] and Zaire in 1903 [14]. The geographic origin and initial host of CBB within Africa remains unclear. Some authors have speculated that it emerged in Ethiopia in conjunction with Coffea arabica L. (commercially known as arabica; Rubiaceae), thriving at altitudes from 1200–2000 m [15]. However, Davidson [16] concluded in 1967 that the pest was not present in Ethiopian coffee farms, and it was not until 1998 that is was reported to be a problem in the country [17]. Prior to 1984, low annual minimum temperatures in the area around Jimma (1780 m, Ethiopia) would have prevented CBB from completing a single generation per year [18], suggesting that the original host was likely the low-altitude species Coffea canephora Pierre ex Frohner (commercially known as robusta), which occurs from 250–1500 m in West and Central Africa [4][18][19]. Under this scenario, CBB could have spread from infested robusta coffee berries originating in West Africa to arabica coffee in Ethiopia or Saudi Arabia, where it was first imported before the 15th century [14].
4. Biology and Life History
CBB is distinguished from all 850 other insect species that can feed on parts of the coffee plant in that it is the only one able to feed and complete its life cycle in the coffee seed, thought to be possible by its association with Pseudomonas bacteria in its alimentary canal that acts to detoxify caffeine [21]. Adult female CBB colonize coffee berries when the dry weight content of the coffee seed is 20% and the endosperm is in the state of development known as semi-consistent [22], approximately 120–150 days after flowering [2][23]. A small entrance hole (0.6–0.8 mm wide [24]) is typically bored near or through the floral disc (Figure 1B), which provides an ideal rough surface for the beetle to grasp while boring [25][26]. Usually, there is only a single hole per berry unless the infestation is high and the availability of berries is scarce, in which case multiple females may bore entrance holes into the berry [27]. Laboratory studies have estimated that the time required to enter the berry is ≈2–8 h [27][28][29]. Four stages describe the position of the colonizing female within the berry: (A) the female has initiated penetration of the exocarp, (B) the female has penetrated the endocarp, and only part of the abdomen is visible, (C) the female is no longer visible and has bored into the endosperm, and (D) the female has constructed galleries and is reproducing within the seed [23].
Females can lay >100 eggs [30][31] over an oviposition period of 11–40 days [30][32]. The average time to complete the life cycle from egg to adult depends largely on temperature and berry moisture, with faster development times as temperatures increase and berry moisture decreases [33][34][35][36]. The sex ratio is skewed towards females, but estimates vary widely among published studies (5:1 to 494:1; reviewed in [5]). Sex determination in CBB is described as functional haplodiploidy; both males and females are diploid, but males are functionally haploid due to the paternal set of chromosomes condensing into a mass of chromatin and are therefore unincorporated or nonfunctional in somatic cells [37]. Males are smaller than females (0.99–1.3 mm long vs. 1.6–1.9 mm long) and have smaller eyes and wings [4][31]. There is a high degree of inbreeding, with almost all mating occurring between siblings. Male and female siblings mate within the berry; the males die, and the fertilized females leave their natal berry to find a new berry in which to deposit their eggs [33]. The number of CBB generations per year has been estimated to vary between 2 and 13, depending on the climatic conditions at a given location [5][30][35][36][38][39].
5. Worldwide pest status
Molecular studies have been used to track the spread of CBB from Africa to other coffee-producing countries [38, 39], and to determine which regions are likely source areas for new introductions [32][33][34][35]. Benavides et al. [32][34] used AFLP (amplified fragment length polymorphism) fingerprinting to analyze genetic variability and biogeographic patterns based on samples from 17 countries. These authors concluded that there were three separate introductions of CBB into the Americas and that West Africa was likely the origin of introductions into America and Asia. Gauthier [36] sampled CBB populations across Africa, Asia, Central America and South America, and used the mitochondrial COI gene to conduct a Bayesian clustering analysis that revealed five distinct populations: 1) Ethiopia, 2) Kenya and Uganda, 3) Brazil, 4) Central America, Columbia, and the Dominican Republic, and 5) Indonesia, New Caledonia, India, West Africa, and Jamaica. As of 2017, CBB has successfully invaded all coffee-producing countries in the world, except for Nepal and Australia (Figure 2). CBB likely entered most new regions either through infested green coffee beans which were not adequately treated before importation [36][37] or through plantation workers that unknowingly brought the pest in clothing or equipment used on infested farms [35].
This entry is adapted from the peer-reviewed paper 10.3390/insects11120882
References
- Le Pelley, R.H. Pests of Coffee; Longmans Green and Co.: London, UK, 1968; p. 590.
- Baker, P.S. The Coffee Berry Borer in Colombia; Final Report of the DFID-Cenicafé-CABI Bioscience IPM for Coffee Project; The Commodities Press: Chinchiná, Colombia, 1999; p. 154.
- Vega, F.E.; Benavides, P.; Stuart, J.A.; O’Neill, S.L. Wolbachia infection in the coffee berry borer (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 2002, 95, 374–378.
- Damon, A. A review of the biology and control of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Entomol. Res. 2000, 90, 453–465.
- Vega, F.E.; Infante, F.; Johnson, A.J. The genus Hypothenemus, with emphasis on H. hampei, the coffee berry borer. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 427–494.
- Bottrill, M.; Ludowici, V.; Turner, R. Biosecurity Plan for the Australian Coffee Industry, V. 1.0-2018; Publication No. 18/023; AgriFutures: Wagga Wagga, NSW, Australia, 2018.
- Vega, F.E.; Infante, F.; Castillo, A.; Jaramillo, J. The coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae): A short review, with recent findings and future research directions. Terr. Arthropod Rev. 2009, 2, 129–147.
- Jaramillo, J.; Muchugu, E.; Vega, F.E.; Davis, A.; Borgemeister, C.; Chabi-Olaye, A. Some like it hot: The influence and implications of climate change on coffee berry borer (Hypothenemus hampei) and coffee production in East Africa. PLoS ONE 2011, 6, e24528.
- Wood, S.L.; Bright, D.E., Jr. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic Index, Volume B. Great Basin Nat. Mem. 1992, 13, 835–1553.
- Bright, D.E.; Skidmore, R.E. A Catalog of Scolytidae and Platypodidae (Coleoptera); Supplement 1 (1990–1994); NRC Research Press: Ottawa, ON, Canada, 1997.
- Bright, D.E.; Skidmore, R.E. A Catalog of Scolytidae and Platypodidae (Coleoptera); Supplement 2 (1995–1999); NRC Research Press: Ottawa, ON, Canada, 2002.
- Bright, D.E. A catalog of Scolytidae and Platypodidae (Coleoptera), Supplement 3 (2000–2010). Insecta Mundi 2014, 0356, 1–336.
- Wood, S.L. A reclassification of the genera of Scolytidae (Coleoptera). Great Basin Nat. Mem. 1986, 10, 1–126.
- Ferrari, J.A.G. Die Forst- und Baumzuchtschädlichen Borkenkäfer; Druck und Verlag von Carl Gerold’s Sohn: Wien, Austria, 1867; pp. 12–13.
- Waterhouse, D.F.; Norris, K.R. Biological Control: Pacific Prospects; Supplement 1; ACIAR Monograph No. 12; Australian Centre for International Agricultural Research: Canberra, Australia, 1989; p. 125.
- Eichhoff, W.J. Neue exotische Tomiciden-Arten. Berl. Entomol. Z. 1871, 15, 131–137.
- Swaine, J.M. Catalogue of the described Scolytidae of America, north of Mexico. 24th Report of the State Entomologist on Injurious and other Insects of the State of New York 1908, Appendix B., Education Department Bulletin no. 455, New York State Museum. Mus. Bull. 1909, 134, 76–159.
- Browne, F.G. Taxonomic notes on Scolytidae (Coleoptera). Entomol. Ber. 1963, 23, 53–59.
- Hopkins, A.D. Classification of the Cryphalinæ, with Descriptions of New Genera and Species; Contributions from the Bureau of Entomology, Report No. 99; United States Department of Agriculture: Washington, DC, USA, 1915; p. 75.
- Hulcr, J.; Atkinson, T.H.; Cognato, A.I.; Jordal, B.H.; McKenna, D.D. Morphology, taxonomy, and phylogenetics of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, Eds.; Academic Press: San Diego, CA, USA, 2015; Chapter 2; pp. 41–84.
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618.
- Barrera, J.F. Dynamique des Populations du Scolyte des Fruits du Caféier, Hypothenemus Hampei (Coleoptera: Scolytidae), et Lutte Biologique Avec le Parasitoide Cephalonomia Stephanoderis (Hymenoptera: Bethylidae), au Chiapas, Mexique. Ph.D. Thesis, Université Paul Sabatier Toulouse, Toulouse, France, 1994; p. 301.
- Bustillo, A.E.; Cárdenas, R.; Villalba, D.A.; Benavides, P.; Orozco, J.; Posada, F.J. Manejo Integrado de la Broca del Café Hypothenemus Hampei (Ferrari) en Colombia; Centro Nacional de Investigaciones de Café (Cenicafé): Chinchiná, Colombia, 1998; p. 134.
- Varón, E.H.; Hanson, P.; Borbón, O.; Carballo, M.; Hilje, L. Potencial de hormigas como depredadoras de la broca del café (Hypothenemus hampei) in Costa Rica. Manejo Integr. Plagas Agroecol. 2004, 73, 42–50.
- Costa, J.N.M.; da Silva, R.B.; Ribeiro, P.A.D.; Teixeira, C.A.D. Flutuação Populacional da Broca-Do-Café (Hypothenemus Hampei, Ferrari) no Estado de Rondônia. In Proceedings of the 2nd Simpósio de Pesquisa dos Cafés do Brasil, Vitória, Brazil, 24–27 September 2001; pp. 1944–1950.
- Cárdenas, M.R.; Posada Flórez, F.J. Los Insectos y Otros Habitantes de Platanales y Cafetales; Centro Nacional de Investigaciones de Café: Chinchiná, Colombia, 2001; p. 250.
- Wrigley, G. Coffee; Longman Scientific & Technical: Essex, UK, 1988; p. 369.
- Penagos-Dardón, H.; Flores, J.C. Hábito y tiempo de penetración de la broca del café, Hypothenemus hampei (Ferrari). Rev. Cafe. 1974, 137, 5–15.
- Sponagel, K.W. La Broca del Café Hypothenemus Hampei en Plantaciones de Café Robusta en la Amazonía Ecuatoriana: Presencia, Posibilidades de Control y Consideraciones Socio-Económicas del Cultivo en Relación a Sistemas Alternativos de Producción Agropecuaria en la Region; Wissenschaftlicher Fachverlag: Berlin, Germany, 1994; p. 185.
- Jaramillo, J.; Chabi-Olaye, A.; Kamonjo, C.; Jaramillo, A.; Vega, F.E.; Poehling, H.; Borgemeister, C. Thermal tolerance of the coffee berry borer Hypothenemus hampei: Predictions of climate change impact on a tropical insect pest. PLoS ONE 2009, 4, e6487.
- Bergamin, J. Contribuição para o conhecimento da biologia da broca do café “Hypothenemus hampei (Ferrari, 1867)” (Col. Ipidae). Arq. Inst. Biológico São Paulo 1943, 14, 31–72.
- Friederichs, K. De Bestrijding van de Koffiebessenboeboek op de Onderneming Karang Redjo. Meded. Koffiebessenboeboek Fonds 1922, 1, 7–21.
- Baker, P.S.; Barrera, J.F.; Rivas, A. Life-history studies of the coffee berry borer (Hypothenemus hampei, Scolytidae) on coffee trees in southern Mexico. J. App. Ecol. 1992, 29, 656–662.
- Jaramillo, J.; Chabi-Olaye, A.; Borgemeister, C. Temperature dependent development and emergence pattern of Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae) from coffee berries. J. Econ. Entomol. 2010, 103, 1159–1165.
- Giraldo-Jaramillo, M.; Garcia, A.G.; Parra, J.R. Biology, thermal requirements, and estimation of the number of generations of Hypothenemus hampei (Ferrari, 1867) (Coleoptera: Curculionidae) in the state of São Paulo. Brazil J. Econ. Entomol. 2018, 111, 2192–2200.
- Hamilton, L.J.; Hollingsworth, R.G.; Sabado-Halpern, M.; Manoukis, N.C.; Follett, P.A.; Johnson, M.A. Coffee berry borer (Hypothenemus hampei) (Coleoptera: Curculionidae) development across an elevational gradient on Hawai’i Island: Applying laboratory degree-day predictions to natural field populations. PLoS ONE 2019, 14, e0218321.
- Brun, L.O.; Stuart, J.; Gaudichon, V.; Aronstein, K.; French-Constant, R.H. Functional haplodiploidy: A mechanism for the spread of insecticide resistance in an important international insect pest. Proc. Natl. Acad. Sci. USA 1995, 92, 9861–9865.
- Ticheler, J.H.G. Étude Analytique de l’Épidémiologie du Scolyte des Graines de Café, Stephanoderes Hampei Ferr., en Côte d’Ivoire; Diss. Veenman: Utrecht, The Netherlands, 1961.
- Ticheler, J. Estudio analítico de la epidemiología del escolítido de los granos de café, Stephanoderis hampei Ferr. en Costa de Marfil. Rev. Cenicafé 1963, 14, 223–294.
- Friederichs, K. De Bestrijding van de Koffiebessenboeboek op de Onderneming Karang Redjo. Meded. Koffiebessenboeboek Fonds 1922, 1, 7–21.
- Baker, P.S.; Barrera, J.F.; Rivas, A. Life-history studies of the coffee berry borer (Hypothenemus hampei, Scolytidae) on coffee trees in southern Mexico. J. App. Ecol. 1992, 29, 656–662.
- Jaramillo, J.; Chabi-Olaye, A.; Borgemeister, C. Temperature dependent development and emergence pattern of Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae) from coffee berries. J. Econ. Entomol. 2010, 103, 1159–1165.
- Giraldo-Jaramillo, M.; Garcia, A.G.; Parra, J.R. Biology, thermal requirements, and estimation of the number of generations of Hypothenemus hampei (Ferrari, 1867) (Coleoptera: Curculionidae) in the state of São Paulo. Brazil J. Econ. Entomol. 2018, 111, 2192–2200.
- Hamilton, L.J.; Hollingsworth, R.G.; Sabado-Halpern, M.; Manoukis, N.C.; Follett, P.A.; Johnson, M.A. Coffee berry borer (Hypothenemus hampei) (Coleoptera: Curculionidae) development across an elevational gradient on Hawai’i Island: Applying laboratory degree-day predictions to natural field populations. PLoS ONE 2019, 14, e0218321.
- Brun, L.O.; Stuart, J.; Gaudichon, V.; Aronstein, K.; French-Constant, R.H. Functional haplodiploidy: A mechanism for the spread of insecticide resistance in an important international insect pest. Proc. Natl. Acad. Sci. USA 1995, 92, 9861–9865.
- Ticheler, J.H.G. Étude Analytique de l’Épidémiologie du Scolyte des Graines de Café, Stephanoderes Hampei Ferr., en Côte d’Ivoire; Diss. Veenman: Utrecht, The Netherlands, 1961.
- Ticheler, J. Estudio analítico de la epidemiología del escolítido de los granos de café, Stephanoderis hampei Ferr. en Costa de Marfil. Rev. Cenicafé 1963, 14, 223–294.
