Lindera, a core genus containing more than 100 species, is a member of the Litseeae tribe under the Lauraceae family. Plants of the Lindera genus are widely distributed all over the world, particularly in the tropical, subtropical and temperate regions of Asia and midwestern America. Plants from the Lindera genus are considered a rich source of essential oils and are often used in the production of aromatic cosmetic products such as soap and lubricants for their elegant fragrance. Most importantly, throughout history, many Lindera plants have been used in traditional medicine for their healing and curing capabilities for several health-related implications, such as pain, cold, urinary tract disorders, rheumatoid arthritis, gastric ulcer, abdominal pain, cholera, and beriberi.
- Lindera obtusiloba
- antioxidant
- oxidative stress
1. Introduction
Surprisingly, plants of the Lindera genus have been reported to produce almost 350 chemical constituents, which mostly belong to sesquiterpenoids, alkaloids, phenylpropanoids, butanolides, lucidones, flavonoids, etc. [1][2]. Studies have shown that Lindera genus plants possess anti-cancer, anti-inflammatory, antihypertensive, and analgesic properties. Moreover, several species of this genus have been reported as a rich source of antioxidants, including Lindera aggregata (Sims) Kosterm, Lindera erythrocarpa Makino, and Lindera pulcherrima (Nees) Hook. f. [3][4][5].
The human body is consistently fighting oxidative attacks from reactive oxygen and nitrogen species (RONS) using a complex antioxidant defence system to maintain the pro-oxidant–antioxidant balance [6]. Imbalance in the cellular antioxidant defence machinery can result from several factors, including ageing and environmental toxins [7]. However, chronic imbalance in the cellular oxidative state may lead to several age- or un-related disorders such as neurodegenerative, cardiovascular, chronic obstructive pulmonary, and chronic kidney diseases as well as cancer [8]. Reactive oxygen participates in the high-energy electron transfer mechanism and the production of adenosine-5-triphosphate (ATP) through oxidative phosphorylation [6]. Imbalance in the production of RONS and antioxidant defence in the cell leads to oxidative stress and attack of many biological molecules such as proteins, lipids, or DNA [8]. Several redox-sensitive transcriptional factors and enzymes such as nuclear factor-erythroid 2-related factor (Nrf-2) and activate antioxidant response element (ARE) have been implicated in maintaining cellular oxidative homeostasis [9][10]. Activation of ARE results in the up-regulation of several antioxidant enzymes, such as heme oxygenase-1 (HO-1), which catalyzes the oxidative degradation of heme into bilirubin and free iron and NAD(P)H quinine oxidoreductase 1 (NQO1); this latter enzyme regulates reactive oxygen species (ROS) generation by reducing quinones to hydroquinones [10]. Therefore, Nrf-2/HO-1/NQO1 are involved in maintaining the balance of oxidative stress, thus protecting cells and tissues from oxidative damage [9][10].
Antioxidants are used for the prevention and treatment of oxidative stress-induced diseases, and plants are used as natural sources of antioxidant compounds. Natural antioxidants can decrease the generation of RONS, scavenge free radicals and prevent the lipid peroxidation process [11]. Plant-derived antioxidants or plants rich with antioxidant compounds have been used for thousands of years for treating various pathological conditions associated with ageing and oxidative stress [12].
Lindera obtusiloba Blume (LO), a member of the genus Lindera, is widely distributed in northeast Asia. The use of LO as a traditional medicine for the treatment of improvement of blood circulation, inflammation fever, abdominal pain, etc., is well documented. Both extracts of the different parts of LO and its bioactive compounds have been reported to contain numerous antioxidative compounds, such as polyphenols, lignans, neolignans, flavonoids, and butenolides. The neuroprotective, cytotoxic, anti-inflammatory, anti-allergic, and antithrombotic properties of these bioactive compounds have been reported [13][14][15][16]. Therefore, LO presents a rich source of bioactive compounds, particularly antioxidants that have been investigated in many diseases associated with oxidative stress. Still, many new antioxidant compounds are being isolated from the extract of different parts of LO and need to be investigated for their antioxidant and pharmacological activity. Here, we briefly discuss the antioxidant properties of the genus Lindera and focus on their traditional and ethnopharmacological use, bioactive compounds isolated from LO extracts and their antioxidant and pharmacological properties. Newly isolated or detected compounds from LO extracts that could prove beneficial to developing new therapeutic agents to treat oxidative stress-associated disorders such as cardiovascular disorders, neurodegenerative disorders, allergy, inflammation, and cancer.
2. Genus Lindera and Antioxidant Properties of Its Plants
Throughout history, plant extracts have been used as remedies for several pathological conditions associated with oxidative stress [17][18]. The secondary metabolites isolated from the plants of the genus Lindera consist of several types of phytochemicals, namely sesquiterpenoids (Linderagalactone E, furanodienone, curzerenone), alkaloids (N-methyllaurotetanine, (+)-isoboldine), flavonoids (Quercetin-3-O-α-l-rhamnopyranoside, quercitrin, (−)-epicatechin, Afzelin), phenylpropanoids, butanolides ((2Z,3S,4S)-2-(11-dodecenylidene)-3-hydroxy-4-methyl butanolide and (2E,3R,4R)-2-(11-dodecenylidene)-3-hydroxy-4-methoxy-4-methyl butanolide), lucidones, etc. [1]. These secondary metabolites and bioactive compounds isolated from the plants of the Lindera genus have been reported to possess various pharmacological activities (e.g., antioxidant, anti-allergic, antimelanogenic, cytoprotective, anti-inflammatory, and antitumour) both in vitro and in vivo (Table 1) [3][19][20][21][22][23].
The Lindera genus is part of the family Lauraceae, which is widely distributed in tropical, sub-alpine and temperate regions of the Asian and American continents, with approximately 80 to 100 species [24][25]. Among them, Lindera aggregata (Sims) Kosterm, Lindera glauca (Siebold et Zucc.) Blume, Lindera neesiana (Wall. ex Nees) Kurz, Lindera pulcherrima (Nees) Hook. F., Lindera benzoin (L.) Blume, Lindera chunii Merr., Lindera obtusiloba Blume, Lindera angustifolia W.C. Cheng, and Lindera reflexa Hemsl. species are used as traditional medicines for their therapeutic effect on whitening, hepatitis C, hepatotoxicity, anti-cancer, antibacterial, antiproliferative, endothelial dysfunction, neuroprotection, antifibrotic, and effects on post-ischemic myocardial dysfunction [3][4][5][26]. However, due to the abundance of antioxidant compounds in Lindera genus plants, this genus can be considered a potential source of natural compounds that can be used for the development of therapeutic agents to treat oxidative stress-induced diseases.
L. aggregata (LA) is widely used as a tea in China and Southeast Asian countries. Both ethanolic and water extract of different parts of L. aggregate have been shown to possess antioxidant activities [19]. Water and EtOH extract of Lindera radix and the dried root of LA have been reported to decrease methane dicarboxylic aldehyde (MDA) and superoxide dismutase (SOD) levels and the expression levels of nuclear factor (NF-κB), tumour necrosis factor (TNF-α) and interleukin (IL-1β) in alcoholic liver injury. Further, the extract improved the histopathological status and decreased the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TC), and MDA and NF-κB, TNF-α, and IL-1β in liver tissues [21]. EtOH extract of LA leaves also showed free radical scavenging activity in a 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay. Eleven polyphenols were identified in this extract by HPLC. A higher amount of quercetin-3-O-α-l-rhamnoside was detected in the extract and showed strong antioxidant capacities. Two alkaloids—linderaggredin C (3), (+)-N-methyllaurotetanine and (+)-isoboldine—isolated from the extract showed significant inhibition of superoxide anion generation in human neutrophils [20][21]. Furthermore, linderanean, which is an active compound isolated from LA root, increased activation of the Nrf-2 pathway in INS-1 cells and protected it from streptozotocin-induced apoptosis [22]. Five sesquiterpene lactones—lindera, galactone E, linderane, hydroxylindestenolide, and linderalactone—were isolated from the roots of LA and showed hepatoprotective activity against H2O2-induced oxidative damage on HepG2 cells [23]. Quercetin, quercetin-3-O-α-L-rhamnopyranoside, and quercetin-3-O-α-l-rhamnoside were found in high concentrations in the LA leaves, demonstrating free radical scavenging activity and modulation of the Nrf-2 pathway [3][20][27]. Overall, most of the compounds identified in the extracts of LA were associated with a higher level of antioxidant activities in different assays; hence, LA could be a potential source of antioxidant compounds and should be further studied for its therapeutic possibilities.
Moreover, LA, has long been used as a traditional medicine for rheumatic, cardiac and renal diseases in Japan and other countries. The water extract of its roots has been found to scavenge free radicals in a DPPH assay, and the leaf extract showed ROS, reactive nitrogen species (RNS), and superoxide anion scavenging activity as well as inhibition of lipid peroxidation and protein oxidation [4]. In an isolated rat heart, LA root extract protected against post-ischemic left ventricular dysfunction through scavenging hydroxyl radicals and opening the mitochondrial potassium ATP (KATP) channels [4][28]. Lindenenyl acetate, a compound isolated from the MeOH extract of the roots of LA, was reported to possess strong neuroprotective activity against glutamate-induced oxidative injury in hippocampal neuronal cells, most likely via extracellular signal-regulated kinase (ERK) pathway-Nrf-2/ARE-dependent HO-1 expression. Further, lindenenyl acetate increased the expression of HO-1, accumulation of Nrf2 and increased the promoter activity of ARE in mouse hippocampal HT22 cells [29]. Overall, both extract and bioactive compounds isolated from LA showed strong neuroprotective and cardioprotective activity via modulation of the cellular oxidative imbalance.
L. erythrocarpa (LE) is a widely distributed shrub in China, Japan, Korea, and Taiwan. Its dried fruits, which are also referred to as red mountain pepper, are used in folk medicine for indigestion and pain [30]. Lucidone, a cyclopentenedione isolated from the fruits of LE, has demonstrated significant protective abilities against free-radical and inflammation stimulator 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress in human keratinocyte cells (HaCaT) through up-regulating HO-1/Nrf-2 gene expression and down-regulating the NF-κB signalling pathway [31]. In addition, lucidone suppressed hepatitis C viral replication by induction of Nrf-2-Mediated HO-1 in Ava5 cells [32]. Moreover, among eight compounds isolated from the methanol fraction of LE, (−)-epicatechin, avicularin, and quercitrin prevented apoptotic cell death of H9c2 cardiomyocytes treated with buthionine-[S,R]-sulfoximine. These compounds also reduced the propidium iodide uptake by these cells and dose-dependently decreased the release of lactose dehydrogenase (LDH) [26].Therefore, these three compounds provide a potential lead compound for the development of antioxidative, cardioprotective agents that can be used as anti-viral or cardioprotective agents.
L. pulcherrima (Nees.) Benth. (LP), also termed an evergreen shrub, is distributed in temperate Himalayan regions, and is used as a medicinal plant. The leaves and bark are used as a spice for the remedy of cold, fever, and cough. In an in vitro study, the antioxidant activity of the essential oils of LP leaf was assessed by DPPH radical scavenging and inhibition of lipid peroxidation. The essential oils of LP leaf showed potent free radical scavenging activity and inhibition of lipid peroxidation. In another study, two constituents—furanodienone and curzerenone—of the essential oils of LP leaf were investigated for free radical scavenging activity in a DPPH assay and inhibition of lipid peroxidation. These oil constituents showed the same inhibition of lipid peroxidation and free radical scavenging activity [5][33]. These findings suggest that the leaf extract of LP and its constituents have high potency for free radical scavenging and inhibition of lipid peroxidation.
L. glauca (LG), another species of the Lindera genus, has been reported to possess free radical scavenging activity and can inhibit lipid peroxidation activity. The water and EtOH extracts of LG stem increased cell viability and reduced ROS generation in tert-butyl hydroperoxide-induced oxidative stress in Chang cells. In addition, it also increased the activities of catalase, glutathione peroxidase, glutathione S-transferase, and expression of the superoxide dismutase gene of zebrafish against oxidative stress [36]. Further, ethanolic extract of LG stem and root showed free radical scavenging, nitrite scavenging, and reducing power activities. The polyphenolic content of the LG extract was 70.99 ± 1.88 μg/TAE μg. The LG extract showed high DPPH radical scavenging activity, nitrite scavenging activity and reducing power activities. In addition, stem and root extracts were found to possess high antiproliferative activities in HT-29 and HCT116 cells [37]. Moreover, eight flavonoids isolated from LG—lindeglaucol, lindeglaucone, cilicicone B, tamarixetin 3-O-α-l-rhamnoside, procyanidin A2, cinnamtannin B, cinnamtannin D1, and procyanidin A1—were tested for their inhibition of low-density lipoprotein oxidation; only four of them—procyanidin A2, cinnamtannin B1, cinnamtannin D1, and procyanidin A1—showed strong low-density lipoprotein (LDL) oxidation inhibitory activities [34].
Another species of the genus Lindera, i.e., L. neesiana (Wall. ex Nees) Kurz (LN) has been reported to possess antioxidant, anti-inflammatory, and neuroprotective activities. Treatment with both water and EtOH extract of LN was found to reduce the production of NO, pro-inflammatory cytokines and iNOS and COX-2 production in lipopolysaccharide (LPS)-stimulated BV-2 cells. Furthermore, LN extract increased the phosphorylation of ERK, p38 and c-Jun N-terminal kinase (JNK) and decreased the activation of microglia cells. The water extract of LN fruit increased the secretion of Nrf-2 in N2a cells and inhibited LDH release in H2O2-stimulated BV-2 cells [35]. In another study, five kaempferol glycosides—kaempferol 3-O-β-glucopyranosyl(1→2)-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside-7-O-α-rhamnopyranoside, kaempferol 3-O-sophoroside, kaempferol 3-O-β-glucopyranosyl-(1→2)-[α-rhamnopyranosyl (1→6)]-β-glucopyranoside, kaempferol 3-O-β-glucopyranosyl(1→2)-α-rhamnopyranoside-7-O-α-rhamnopyranoside, and kaempferol 3-O-α-rhamnopyranoside—isolated from 60% EtOH extract of LN leaves and twigs showed moderate scavenging activities on DPPH radicals and potent pancreatic lipase inhibitory activity [36]. These findings suggest that LN is a rich source of potent antioxidants, which show neuroprotection and anti-inflammatory activity.
Several other species of the Lindera genus, such as L. reflexa, L. fruiticosa, L. angustifolia, L. oxyphila, and L. umbrellata have been reported to possess antioxidant activity in separate studies [37][38][39][40][41][42]. Hence, this genus represents a natural source of highly active antioxidant compounds that can scavenge free radicals and inhibit lipid peroxidation. Both extracts and bioactive compounds of this genus can modulate several oxidative pathways, including Nrf-2/HO-1, ERK, JNK, mitogen-activated protein kinase (MAPK), and ARE that are involved in oxidative stress-mediated cell death, cell proliferation, inflammation, etc.
3. Ethnomedicinal Use of Lindera obtusiloba
Lindera obtusiloba Blume is ubiquitously distributed in the north and southeast parts of Asia and has been used in traditional Chinese, Korean, and Japanese medicine over centuries [43][44]. In Korea and China, it is traditionally used for restoring blood stasis and inflammatory disorders [45]. The leaves of LO are traditionally consumed as both tea and food [46]. The consumable aqueous extract of LO demonstrated significant physiological beneficial effects, such as the inhibition of adipogenesis [47]. Further, in Korean traditional medicine, its leaf or branch extracts are widely used to treat liver diseases and for improving blood circulation, insomnia, and anxiety [48]. The young leaves of LO are fried and traditionally used as a Buddhist ceremonial dish. Furthermore, the oil extracted from LO is used as hair oil in some cultures [49]. The barks of LO are used to treat rheumatism in Chinese medicinal practice by heating the bark under the patient’s knee [50].
4. Bioactive Compounds of Lindera obtusiloba
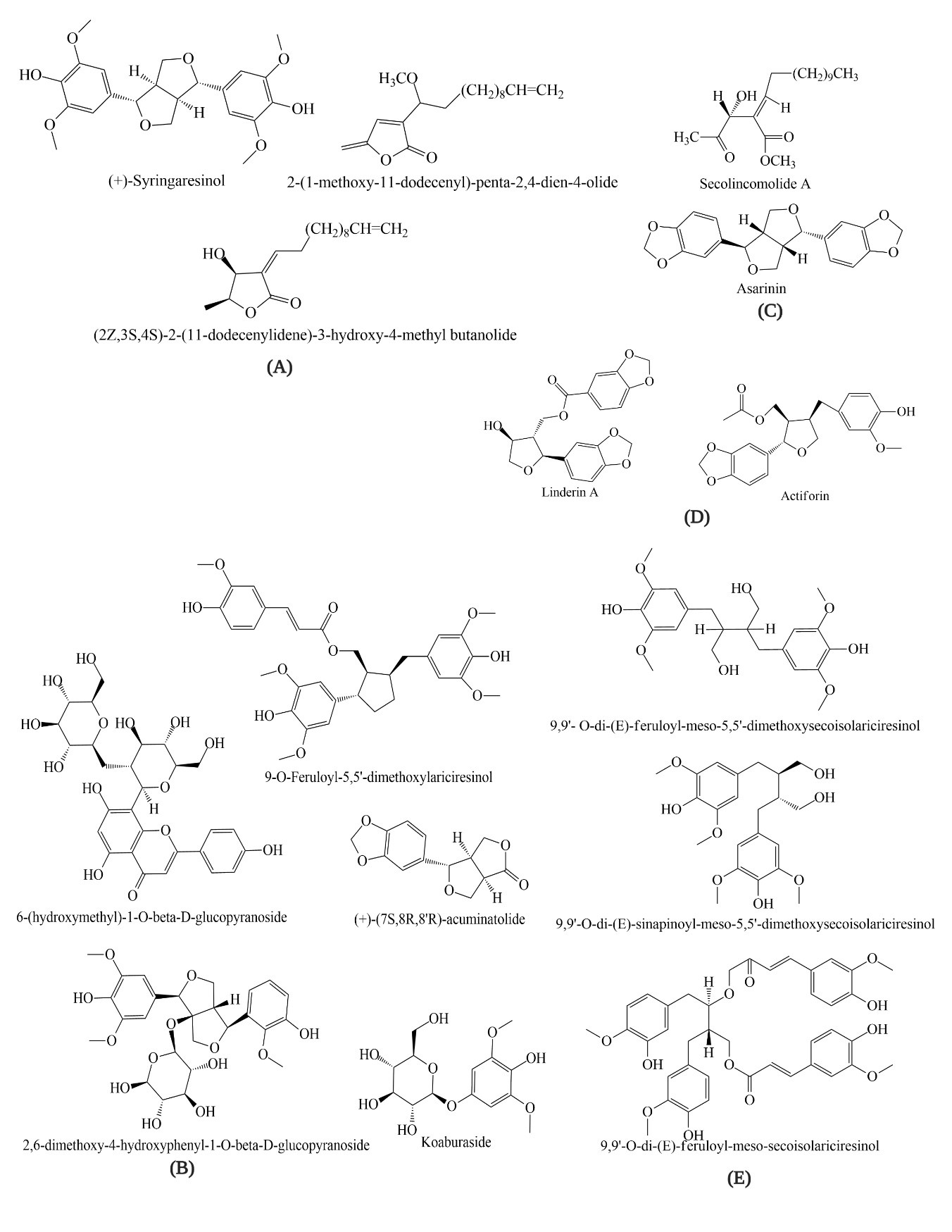
There is an array of evidence suggesting that LO is a potential source of antioxidant compounds. Hong et al. (2013) investigated quercitrin (62.9%) and afzelin (22.0%) in a 70% ethanolic extract of LO. Choi et al. (2013) isolated eight phenolic glycosides—tachioside, isotachioside, koaburaside, 2,6-dimethoxy-4-hydroxyphenyl-1-O-beta-d-glucopyranoside, 4,6-dihydroxy-2-methoxyphenyl-1-O-beta-d-glucopyranoside, a mixture of erigeside C and salidroside, and 6-hydroxyphenyl)-1-O-beta-d-glucopyranoside—from the stems of LO and investigated their anti-allergic inflammatory activities [51][52]. Seven neolignans—linderin A, (+)-xanthoxyol, pluviatilol, actiforin, (+)-syringaresinol, (+)-(7S,8R,8′R)-acuminatolide and (+)-9′-O-trans-feruloyl-5,5′-dimethoxylariciresinol—isolated from the stem extract of LO were investigated for their anti-allergic inflammatory effects [53]. Moreover, three new butanolides—2-(1-methoxy-11-dodecenyl)-penta-2,4-dien-4-olide, (2Z,3S,4S)-2-(11-dodecenylidene)-3-hydroxy-4-methyl butanolide and (2E,3R,4R)-2-(11-dodecenylidene)-3-hydroxy-4-methoxy-4-methyl butanolide—from the stems of LO have been reported by Kwon et al. (2000) [54]. The constituents of essential oils from LO leaves, mesocarps, seeds, and barks ware detected by different chromatographic methods. GC-MS analysis of the extract of LO bark detected α-cadinol (11.8%), hedycaryol (9.8%), α-eudesmol (9.7%), caryophyllene (6.4%), T-cadinol (6.2%), terpinolene (5.7%), eudesmol (5.1%), α-elemene (4.8%), cadinene (4.3%), elemene (4.0%), etc [50]. Nil et al. (1983) reported the major oil components of LO; myrcene (20.60%), α-humulene (21.45%), humulol (6.03%) and bornyl acetate (5.06%) in mesocarp; 5-dodecanolide (15.29%), lauric acid (8.74%), bornyl acetate (5.01%) and cis-4-dode-cenoic acid (4.07%) in seed; caryophyllene (7.37%), elemol (5.06%), and unidentified sesquiterpene oxidated compound (7.96%) in leaf [55]. However, many compounds have not been further studied regarding their antioxidant activities. Therefore, these compounds can be considered as potential candidates for further pharmacological studies of different disease models (Figure 1).
Figure 1. Structures of chemical constituents of Lindera obtusiloba; Groups (A). Cytotoxic; (B). Anti-histamine; (C). Antiplatelet; (D). Anti-inflammatory; (E). Neuroprotective.
This entry is adapted from the peer-reviewed paper 10.3390/plants9121765
References
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: A source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2016, 15, 869–906. [Google Scholar] [CrossRef]
- Han, Z.; Zheng, Y.; Chen, N.; Luan, L.; Zhou, C.; Gan, L.; Wu, Y. Simultaneous determination of four alkaloids in Lindera aggregata by ultra-high-pressure liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2008, 1212, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xu, B.; Amin, A.; Li, H.; Yu, X.; Gong, M.; Zhang, L. Quercetin‑3‑O‑α‑L‑rhamnopyranoside derived from the leaves of Lindera aggregata (Sims) Kosterm. evokes the autophagy‑induced nuclear factor erythroid 2‑related factor 2 antioxidant pathway in human umbilical vein endothelial cells. Int. J. Mol. Med. 2019, 43, 461–474. [Google Scholar] [CrossRef]
- Noda, Y.; Mori, A. Antioxidant activities of Uyaku (Lindera strychnifolia) leaf extract: A natural extract used in traditional medicine. J. Clin. Biochem. Nutr. 2007, 41, 139–145. [Google Scholar] [CrossRef]
- Joshi, S.C.; Verma, A.R.; Mathela, C.S. Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem. Toxicol. 2010, 48, 37–40. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. Transl. Integr. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1985. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Element, A.R. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Reddy, A.; Majeti, N.V.; Singhal, S.S. Therapeutic potential of natural antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-W.; Ha, J.-H.; Shin, H.-G.; Jeong, S.-H.; Kim, J.-H.; Lee, J.; Park, J.-Y.; Kwon, H.-J.; Jung, K.; Lee, W.-S.; et al. Lindera obtusiloba Attenuates Oxidative Stress and Airway Inflammation in a Murine Model. of Ovalbumin-Challenged Asthma. Antioxidants 2020, 9, 563. [Google Scholar] [CrossRef]
- Freise, C.; Querfeld, U. The lignan (+)-episesamin interferes with TNF-α-induced activation of VSMC via diminished activation of NF-ĸB, ERK1/2 and AKT and decreased activity of gelatinases. Acta Physiol. 2015, 213, 642–652. [Google Scholar] [CrossRef]
- Hong, C.O.; Lee, H.A.; Rhee, C.H.; Choung, S.Y.; Lee, K.W. Separation of the antioxidant compound quercitrin from Lindera obtusiloba Blume and its antimelanogenic effect on B16F10 melanoma cells. Biosci. Biotechnol. Biochem. 2013, 77, 58–64. [Google Scholar] [CrossRef]
- Jung, S.-H.; Han, J.-H.; Park, H.-S.; Lee, J.-J.; Yang, S.-Y.; Kim, Y.H.; Heo, K.-S.; Myung, C.-S. Inhibition of collagen-induced platelet aggregation by the secobutanolide secolincomolide A from Lindera obtusiloba Blume. Front. Pharmacol. 2017, 8, 560–571. [Google Scholar] [CrossRef]
- Nanni, V.; Canuti, L.; Gismondi, A.; Canini, A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Nardi, G.M.; Januario, A.G.F.; Freire, C.G.; Megiolaro, F.; Schneider, K.; Perazzoli, M.R.A.; Raap Do Nascimento, S.; Gon, A.C.; Bolda Marino, L.N.; Wgner, G.; et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacogn. Res. 2016, 8, S42–S49. [Google Scholar]
- Wang, J.W.; Chen, X.Y.; Hu, P.Y.; Tan, M.M.; Tang, X.G.; Huang, M.C.; Lou, Z.H. Effects of Linderae radix extracts on a rat model of alcoholic liver injury. Exp. Ther. Med. 2016, 11, 2185–2192. [Google Scholar] [CrossRef]
- Xu, C.; Yang, B.; Zhu, W.; Li, X.; Tian, J.; Zhang, L. Characterisation of polyphenol constituents of Linderae aggregate leaves using HPLC fingerprint analysis and their antioxidant activities. Food Chem. 2015, 186, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Wu, Y.-H.; Hung, H.-Y.; Lam, S.-H.; Ma, G.-H.; Kuo, L.-M.; Hwang, T.-L.; Kuo, D.-H.; Wu, T.-S. Anti-inflammatory principles from Lindera aggregata. Bioorganic Med. Chem. Lett. 2020, 30, 127224. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. 7, 8-Dihydroxycoumarin (daphnetin) protects INS-1 pancreatic β-cells against streptozotocin-induced apoptosis. Phytomedicine 2017, 24, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.S.; Zheng, Y.L.; Mo, J.X.; Liu, X.; Li, X.H.; Zhou, C.X. Sesquiterpene lactones from the root tubers of Lindera aggregata. J. Nat. Prod. 2009, 72, 1497–1501. [Google Scholar] [CrossRef]
- Hyland, B.P.M. A revision of Lauraceae in Australia (excluding Cassytha). Aust. Syst. Bot. 1989, 2, 135–367. [Google Scholar] [CrossRef]
- Wofford, B.E. A new Lindera (Lauraceae) from North. America. J. Arnold Arbor. 1983, 64, 325–331. [Google Scholar] [CrossRef]
- Kim, J.A.; Jung, Y.S.; Kim, M.Y.; Yang, S.Y.; Lee, S.; Kim, Y.H. Protective effect of components isolated from Lindera erythrocarpa against oxidative stress-induced Apoptosis of H9c2 Cardiomyocytes. Phytother. Res. 2011, 25, 1612–1617. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Yuan, L.; Wu, Y.; Peng, X.; Kai, G.; Liu, Y. Aqueous extracts of Lindera aggregate (Sims) Kosterm leaves regulate serum/hepatic lipid and liver function in normal and hypercholesterolemic mice. J. Pharmacol. Sci. 2020, 143, 45–51. [Google Scholar] [CrossRef]
- Wang, N.; Minatoguchi, S.; Arai, M.; Uno, Y.; Hashimoto, K.; Chen, X.H.; Fujiwara, H. Lindera strychnifolia is Protective Against Post-ischemic Myocardial Dysfunction Through Scavenging Hydroxyl Radicals and Opening the Mitochondrial K ATP Channels in Isolated Rat Hearts. Am. J. Chin. Med. 2004, 32, 587–598. [Google Scholar] [CrossRef]
- Li, B.; Jeong, G.S.; Kang, D.G.; Lee, H.S.; Kim, Y.C. Cytoprotective effects of lindenenyl acetate isolated from Lindera strychnifolia on mouse hippocampal HT22 cells. Eur. J. Pharmacol. 2009, 614, 58–65. [Google Scholar] [CrossRef]
- Ichino, K.; Tanaka, H.; Ito, K.; Tanaka, T.; Mizuno, M. Two new dihydrochalcones from Lindera erythrocarpa. J. Nat. Prod. 1988, 51, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Yang, H.L.; Tsai, Y.C.; Hung, P.C.; Chang, S.H.; Lo, H.W.; Chou, C.W. Lucidone protects human skin keratinocytes against free radical-induced oxidative damage and inflammation through the up-regulation of HO-1/Nrf2 antioxidant genes and down-regulation of NF-κB signaling pathway. Food Chem. Toxicol. 2013, 59, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Wang, S.Y.; Chiu, C.C.; Tseng, C.K.; Lin, C.K.; Wang, H.C.; Lee, J.C. Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob. Agents Chemother. 2013, 57, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C.; Mathela, C.S. Antioxidant and antibacterial activities of the leaf essential oil and its constituents furanodienone and curzerenone from Lindera pulcherrima (Nees.) Benth. ex hook. f. Phcog. Res. 2012, 4, 80–84. [Google Scholar]
- Huh, G.W.; Park, J.H.; Kang, J.H.; Jeong, T.S.; Kang, H.C.; Baek, N.I. Flavonoids from Lindera glauca Blume as low-density lipoprotein oxidation inhibitors. Nat. Prod. Res. 2014, 28, 831–834. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P.; Do, M.H.; Lee, T.H.; Kim, S.Y. Anti-neuroinflammatory and neuroprotective effects of the Lindera neesiana fruit in vitro. Phytomedicine 2016, 23, 872–881. [Google Scholar] [CrossRef]
- Adhikari-Devkota, A.; Dirar, A.I.; Kurizaki, A.; Tsushiro, K.; Devkota, H.P. Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana. Separations 2019, 6, 10. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, Y.; Wang, K. Antinociceptive and free radical scavenging activities of alkaloids isolated from Lindera angustifolia Chen. J. Ethnopharmacol. 2006, 106, 408–413. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Wang, L.; Chen, S. Pharmacokinetics and Tissue Distribution Study of Pinosylvin in Rats by Ultra-High.-Performance Liquid Chromatography Coupled with Linear Trap Quadrupole Orbitrap Mass Spectrometry. Evid. Based Complementary Altern. Med. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, J.; Chen, S.; Sun, X.; Zhao, P.; Xie, Z. Screening, and identification of the binding position, of xanthine oxidase inhibitors in the roots of Lindera reflexa Hemsl using ultrafiltration LC–MS combined with enzyme blocking. Biomed. Chromatogr. 2019, 33, e4577. [Google Scholar] [CrossRef] [PubMed]
- Song, M.C.; Nigussie, F.; Jeong, T.S.; Lee, C.Y.; Regassa, F.; Markos, T.; Baek, N.I. Phenolic Compounds from the Roots of Lindera f ruticosa. J. Nat. Prod. 2006, 69, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.; Hadi, H.A.; Mohamad, J.; Khalilzadeh, M.A.; Cheahd, S.-C.; Fadaeinasab, M. Flavonoids and Linderone from Lindera oxyphylla and their Bioactivities. Comb. Chem. High Throughput Screen. 2013, 16, 160–166. [Google Scholar] [PubMed]
- Kuroda, M.; Sakurai, K.; Mimaki, Y. Chemical constituents of the stems and twigs of Lindera umbellata. J. Nat. Med. 2011, 65, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Lee, M.S.; Her, S.; Cho, S.; Lee, C.H.; Kim, I.H.; Han, D. Antidepressant-like effects of Lindera obtusiloba extracts on the immobility behavior of rats in the forced swim test. Molecules 2016, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Yook, C.S. Medicinal Plants of Korea; Jinmyeong Publishing Co: Seoul, Korea, 1981; p. 392. [Google Scholar]
- Kim, J.H.; Lee, J.; Kang, S.; Moon, H.; Chung, K.H.; Kim, K.R. Antiplatelet and antithrombotic effects of the extract of lindera obtusiloba leaves. Biomol. Ther. 2016, 24, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Lee, J.O.; Kang, S.H.; Sohn, J.D.; Kim, J.H.; Lim, J.W.; Lee, S.W. Method for Preventing and Treating Thrombotic Disorders Using a Pharmaceutical Composition Comprising an Extract of Lindera Obtusiloba. US Patent 2014/0255529 A1, 11 September 2014. [Google Scholar]
- Freise, C.; Erben, U.; Neuman, U.; Kim, K.; Zeitz, M.; Somasundaram, R.; Ruehl, M. An active extract of Lindera obtusiloba inhibits adipogenesis via sustained Wnt signaling and exerts anti-inflammatory effects in the 3T3-L1 preadipocytes. J. Nutr. Biochem. 2010, 21, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Ruehl, M.; Erben, U.; Neumann, U.; Seehofer, D.; Kim, K.Y.; Somasundaram, R. A hepatoprotective Lindera obtusiloba extract suppresses growth and attenuates insulin like growth factor-1 receptor signaling and NF-kappaB activity in human liver cancer cell lines. Bmc Complementary Altern. Med. 2011, 11, 39. [Google Scholar] [CrossRef]
- Facciola, S. Cornucopia: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1990. [Google Scholar]
- Zekun, L.; Haixia, C. GC-MS analysis of essential oil from the bark of Lindera obtusiloba. Chem. Nat. Compd. 2012, 48, 696–697. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, H.D.; Kim, S.H.; Na, M.K.; Kim, J.A.; Lee, S.H. Phenolic glycosides from Lindera obtusiloba and their anti-allergic inflammatory activities. Nat. Prod. Commun. 2013, 8, 1934578X1300800212. [Google Scholar] [CrossRef]
- Hong, C.O.; Rhee, C.H.; Won, N.H.; Choi, H.D.; Lee, K.W. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem. Toxicol. 2013, 53, 214–220. [Google Scholar] [CrossRef]
- Choi, H.G.; Choi, Y.H.; Kim, J.H.; Kim, H.H.; Kim, S.H.; Kim, J.A.; Lee, S.H. A new neolignan and lignans from the stems of Lindera obtusiloba Blume and their anti-allergic inflammatory effects. Arch. Pharmacal Res. 2014, 37, 467–472. [Google Scholar] [CrossRef]
- Kwon, H.C.; Baek, N.I.; Choi, S.U.; Lee, K.R. New cytotoxic butanolides from Lindera obtusiloba BLUME. Chem. Pharm. Bull. 2000, 48, 614–616. [Google Scholar] [CrossRef]
- Nii, H.; Furukawa, K.; Iwakiri, M.; Kubota, T. Constituents of essential oils of Lindera obtusiloba Blume and Parabenzoin trilobum (Sieb, et Zucc.) Nakai fruit. J. Agric. Chem. Soc. Jpn. 1983, 57, 663–666. [Google Scholar]