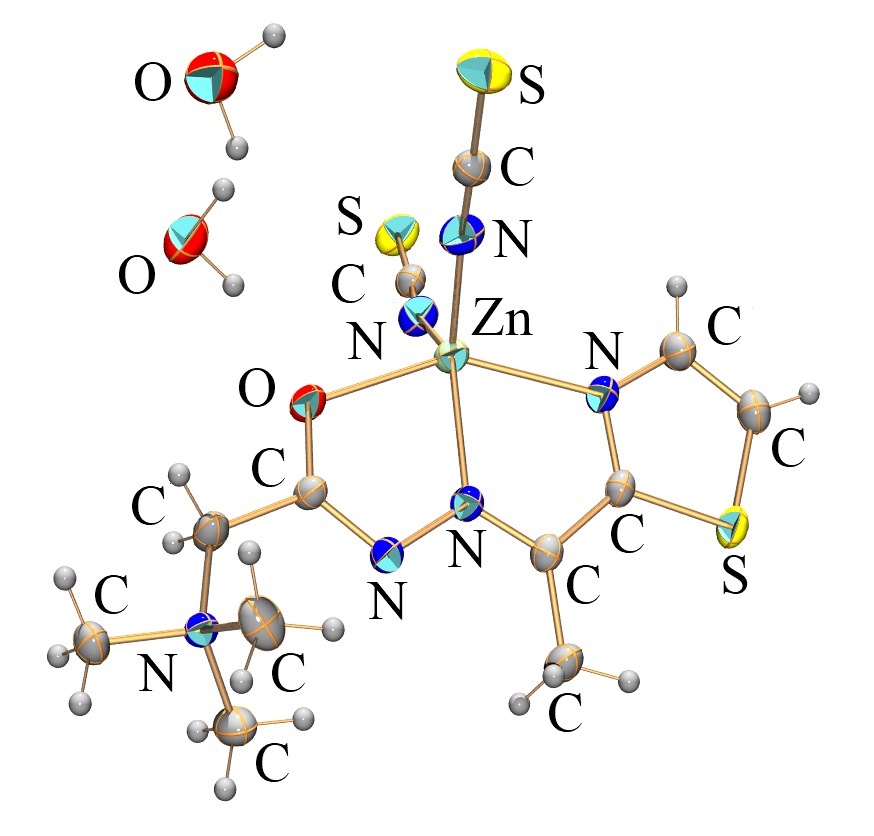
Compound Name: (E)-N,N,N-trimethyl-2-oxo-2-(2-(1-(thiazol-2-yl)ethylidene)hydrazinyl)ethan-1-aminium di(thiocyanato-κN) zink(II) dihydrate.
- Zn(II) complex,Catalytic Activity,DFT Calculations
1. The New Concept
Given that the first zinc-based homogeneous catalytic system for the ketone-amine-alkyne (KA2) coupling reaction was reported very recently (2019)[1], the use of ligands in these catalytic systems is rare. Therefore, we were interested in evaluating some well-defined zinc complexes as potential catalysts.
2. The Synthesis Process
The hydrazone ligand (HLCl), (E)-N,N,N-trimethyl-2-oxo-2-(2-(1-(thiazol-2-yl)ethylidene)hydrazinyl)ethan-1-aminium chloride, was obtained from the condensation reaction of 2-acetylthiazole and Girard’s T reagent. Zn(II) complex was synthesized by reacting Zn(OAc)2∙2H2O, Girards reagent T hydrazone ligand, and an excess of NH4SCN in a solvent mixture of water/methanol, with the composition [ZnL(NCS)2]×2H2O. The central Zn(II) ion is pentacoordinated with the 4 N and O donor atoms of the deprotonated hydrazone ligand, as well as two thiocyanate ligands coordinated through their nitrogen atoms.
3. The Structure
The complex was characterized by FTIR and NMR spectroscopies and single crystal X-ray diffraction. In this complex, Zn(II) has fivefold coordination with tridentate ligand L and two nitrogen atoms from thiocyanate ligands forming a distorted square pyramidal structure. Ligand L is coordinated to Zn(II) in the zwitterionic form through NNO-set of donor atoms forming two fused five-membered chelate rings (Zn–N–C–C–N and Zn–N–N–C–O). The dihedral angle of nearly 4.0° between two five-membered chelate rings shows the non-coplanar nature of metal-ligand system.The thiocyanate ligands are coordinated to Zn(II) ion in bent mode.
4. The Activities and Bioactivities
For evaluation of the Zn(II) complex catalytic activity in the KA2 coupling reaction we chose cyclohexanone, pyrrolidine and phenylacetylene as a model substrate triad. A promising result was obtained when Zn(II) complex was used in 10 mol% loading in toluene, affording the product in 85% isolated yield after 16 h. As expected, when ligand HLCl was used as a possible catalyst in a control experiment, the desired propargylamine was not formed. Removing the solvent while reducing the temperature and catalyst loading led to moderate yields, while using MgSO4 as a water-scavenging additive, in combination with an increase in temperature, led to the higher yield.
The DFT calculations of newly synthesized Zn(II) complex, has been carried out for its structural determination, HOMO, LUMO study and to calculate reactivity descriptors. The low kinetic stability and high reactivity of Zn(II) complex has been found from the low HOMO–LUMO energy gap value, which is in agreement with experimental data. The electrophilicity index value (6.971 eV in gas phase and 5.908 eV in toluene) indicates that Zn(II) complex is the strong electrophile. In addition, its possesses high electronegativity value (χ = 3.886 eV).
5. The Applications
Hydrazone ligands are one of the most important classes of flexible and versatile polydentate ligands which show very high efficiency in chelating various metal ions[2][3][4][5][6][7][8]. Our interest in metal complexes with hydrazone-based ligands is partly due to their potential applications as catalysts[6][7] and molecular magnets[8].
On the other hand, the propargylamines are a unique family of organic compounds, which has received ample attention by the wider scientific community[9][10]. The profound interest surrounding these compounds is partly due to the bioactive nature of certain members of their family[9][10][11][12]. The most straightforward approach towards such molecules is the ketone-amine-alkyne (KA2) multicomponent coupling reaction, for which a significant number of catalytic systems has been reported during the past decade. Since the use of ligands in these catalytic systems is rare, we were interested in testing well-defined zinc complexes as potential catalysts for the reaction operating under air.
6. Stories Behind This Compound
The hydrazone ligand (HLCl), (E)-N,N,N-trimethyl-2-oxo-2-(2-(1-(thiazol-2-yl)ethylidene)hydrazinyl)ethan-1-aminium chloride and its Zn(II) complex were synthesitzed and characterized during a Short-Term Scientific Mission (B. Čobeljić) in Slovenia at laboratory of prof. I. Turel, reference number COST-STSM-CM1305-37566. Catalytical investigation was co-funded by the European Union and Greek national funds through the Operational Program “ Human Resources Development, Education and Lifelong Learning “ (NSRF 2014-2020), under the call “Supporting Researchers with an Emphasis on Young Researchers - Cycle B” (MIS: 5047938) (C. Vougioukalakis).
This entry is adapted from the peer-reviewed paper 10.3390/molecules25184043
References
- Tzouras, N.V.; Neofotistos, S.P.; Vougioukalakis, G.C. Zn-catalyzed multicomponent KA2 coupling: One-pot assembly of propargylamines bearing tetrasubstituted carbon centers. ACS Omega 2019, 4(6), 10279–10292.
- Afkhami, F.A.; Khandar, A.A.; Mahmoudi, G.; Maniukiewicz, W.; Lipkowski, J.; White, J.M.; Waterman, R.; García-Granda, S.; Zangrando, E.; Bauzái, A.; et al. Synthesis, X-ray characterization, DFT calculations and Hirshfeld surface analysis of Zn(II) and Cd(II) complexes based on isonicotinoylhydrazone ligand. Cryst. Eng. Comm. 2016, 18, 4587–4596.
- Abedi, M.; Yeșilel, O.Z.; Mahmoudi, G.; Bauzá, A.; Lofland, S.E.; Yerli, Y.; Kaminsky, W.; Garczarek, P.; Zaręba, J.K.; Ienco, A.; et al. Tetranuclear manganese(II) complexes of hydrazone and carbohydrazone ligands: Synthesis, crystal structures, magnetic properties, Hirshfeld surface analysis and DFT calculations. Inorg. Chim. Acta. 2016, 443, 101–109.
- Romanović, M.Č.; Čobeljić, B.R.; Pevec, A.; Turel, I.; Spasojević, V.; Tsaturyan, A.A.; Shcherbakov, I.N.; Anđelković, K.K.; Milenković, M.; Radanović, D.; et al. Synthesis, crystal structure, magnetic properties and DFT study of dinuclear Ni(II) complex with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. Polyhedron 2017, 128, 30–37.
- Romanović, M.Č.; Milenković, M.R.; Pevec, A.; Turel, I.; Spasojević, V.; Grubišić, S.; Radanović, D.; Anđelković, K.; Čobeljić, B. Crystal structures, magnetic properties and DFT study of cobalt(II) azido complexes with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. Polyhedron 2018, 139, 142–147.
- Pouralimardan, O.; Chamayou, A.-C.; Janiak, C.; Hosseini-Monfared, H. Hydrazone Schiff base-manganese(II) complexes: Synthesis, crystal structure and catalytic reactivity. Inorg. Chim. Acta. 2007, 360, 1599–1608.
- Milenković, M.R.; Papastavrou, A.T.; Radanović, D.; Pevec, A.; Jagličić, Z.; Zlatar, M.; Gruden, M.; Vougioukalakis, G.C.; Turel, I.; Anđelković, K.; et al. Highly-efficient N-arylation of imidazole catalyzed by Cu(II) complexes with quaternary ammonium-functionalized 2-acetylpyridine acylhydrazone. Polyhedron 2019, 165, 22–30.
- Darmanović, D.; Shcherbakov, I.N.; Duboc, C.; Spasojević, V.; Hanžel, D.; Anđelković, K.; Radanović, D.; Turel, I.; Milenković, M.; Gruden, M.; et al. Combined experimental and theoretical investigation of the origin of magnetic anisotropy in pentagonal bipyramidal isothiocyanato Co(II), Ni(II), and Fe(III) complexes with quaternary-ammonium-functionalized 2,6-diacetylpyridine bisacylhydrazone. J. Phys. Chem. C. 2019, 123, 31142–31155.
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 2017, 117, 14091–14200.
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the A3-coupling. Chem. Soc. Rev. 2012, 41, 3790−3807
- Langston, J.W.; Irwin, I.; Langston, E.B.; Forno, L.S. Pargyline prevents MPTP-induced parkinsonism in primates. Science 1984, 225, 1480–1482.
- Chen, J.J.; Swope, D.M. Clinical pharmacology of rasagiline: A novel, second-generation propargylamine for the treatment of Parkinson disease. J. Clin. Pharmacol. 2005, 45, 878−894.