Iron complexes are particularly interesting as catalyst systems over the other transition metals (including noble metals) due to iron’s high natural abundance and mediation in important biological processes, therefore making them non-toxic, cost-effective, and biocompatible. Both homogeneous and heterogeneous catalysis mediated by iron as a transition metal have found applications in many industries, including oxidation, C-C bond formation, hydrocarboxylation and dehydration, hydrogenation and reduction reactions of low molecular weight molecules. These processes provided substrates for industrial-scale use, e.g., switchable materials, sustainable and scalable energy storage technologies, drugs for the treatment of cancer, and high molecular weight polymer materials with a predetermined structure through controlled radical polymerization techniques.
- iron-based catalysts
- oxidation processes
- “green”
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Environmental pollution and climate change make the introduction of “green chemistry” principles into industry one of the most urgent scientific challenges. According to this approach, it is important to reduce the use and generation of substances hazardous to the environment and human health as well as to make industrial manufacturing methods more eco-friendly and cost-effective. In the last decade, iron catalysts have received much attention due to their nontoxic, abundant, and inexpensive qualities. Tetra- and pentadentate iron complexes have found a wide application as catalysts for hydroxylation [1,2], dihydroxylation [3,4], or epoxidation [5–8] of hydrocarbon substrates providing substrates for industrial-scale use, also as switchable materials, in the effective treatment of cancer or catalysts for controlled radical polymerization. The oxidation of organic substrates with high stereo- and regioselectivity by environmentally friendly oxidants, such as dioxygen (O2) or hydrogen peroxide (H2O2) catalyzed by bioinspired iron(II) catalysts, are very important from a technological point of view. Oxidizers used in chemicals production are expensive, highly toxic, and environmentally harmful. The use of molecular oxygen or hydrogen peroxide for their oxidation provides biomimetic oxygen activation—analogous to processes produced by nature. Clean technology, and efficient and cost-effective processes are currently desired. In this context, iron catalysts stabilized by nitrogen donor ligands are a good alternative. Iron complexes bearing well-designed N donor ligands have found applications as catalysts across multiple fields of research, e.g., homogeneous catalysis, coordination chemistry, or materials science, and can prove their usability as catalysts for industrial processes in organic synthesis, pharmacy, and advanced materials technologies. The range of catalytic activity of discussed iron complexes is not confined to the oxidation processes. These complexes have found the number of applications in polymerization, C-C bond formation, hydrocarboxylation and dehydration reactions, as well as in hydrogenation and reduction reactions. Iron complexes containing N donor ligands were successfully used as photosensitizers in solar cells and in light-driven redox and catalytic processes, owing to their photochemical properties.
Catalysts based on supported metal complexes also deserve attention. The most promising group of precious group metal (PGM)-free electrocatalysts are metal-nitrogen-carbon (Me-N-C) materials, frequently heat-treated. Iron-based ones stand out, owing to good activity and low cost. Nowadays, Fe complexes containing N donor ligands are used for the synthesis of electrocatalysts investigated for oxygen reduction reaction (ORR) [9–12], oxygen evolution reaction (OER) [13,14], hydrogen evolution reaction (HER) [15,16], carbon dioxide reduction reaction (CO2RR) [17], but also in organic chemistry.
Considering the preparation of high molecular weight molecules, iron-based complexes are effective, and environmentally-friendly counterparts for copper catalysts in atom transfer radical polymerization (ATRP) techniques—a representative approach of controlled radical polymerization. Due to the disadvantages of commonly used copper catalysts, such as contamination of polymers with stable catalytic complex limiting specialist industrial manufacture, iron-based catalysts attracted attention in this context. Iron-mediated ATRP processes provided a wide range of polymer materials with different architectures and predetermined molecular weight including copolymers, branched architectures (i.e., star and bottlebrush polymers), and organic–inorganic hybrid materials for biomedical use.
2. Applications of Iron-Based Complexes Containing N Donor Ligands in Homogeneous Catalysis
Iron complexes with N donor ligands are widely used in homogeneous catalysis. Undoubtedly, one of the most important applications of these complexes is the use as catalysts for the oxidation of organic compounds. They can also be applied as switchable materials, components to produce biodegradation materials in medicine and photochemistry, and to imitate artificial photosynthesis. There are also a number of catalytic applications of the iron complexes containing N donor ligands in polymerization and C-C bond formation reactions as well as in reduction, dehydration, hydrocarboxylation, etc. In addition, these compounds may be used as novel noble metal-free photosensitizers. Therefore, besides the oxidation reactions involving this kind of iron complex, representative examples of abovementioned applications in further sections of the chapter will be presented.
2.1. Functionalization of C-H, C-C, C-N Bond by Iron Complexes with N Donor Ligands
From a technological point of view, high selectivity but also targeted and controlled reactivity are required from an effective catalyst. A properly activated catalyst should convert readily available hydrocarbons into valuable products that can be easily separated and purified. Moreover, they generate the least environmentally harmful waste. In this regard, metalloenzymes are still an unrivalled role model to produce new materials. Over 20 years ago a series of iron complexes, functional models of iron oxygen activating enzymes like catechol dioxygenases, methane monooxygenase, or α-keto acid-dependent enzymes were synthesized, and have been intensively explored to date [24–27]. Due to the wide range of research conducted on iron complexes with tetra- and pentadentade ligands, this paper presents the recently published results. Application of dioxygen or hydrogen peroxide as environmentally friendly oxidants is of great interest in catalytic oxidation systems.
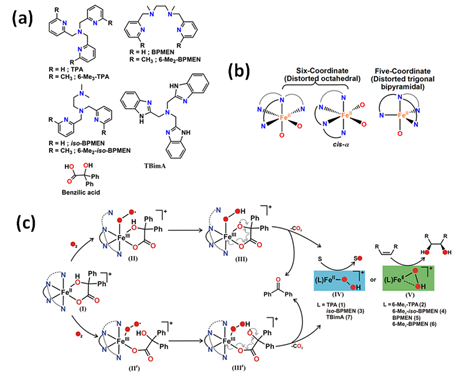
A series of iron catalysts were investigated for the hydroxylation, dihydroxylation and epoxidation of hydrocarbon substrates (Table 1) [28–31]. Paine et al. [31] discussed the influence of the use of tetradentate ligands as supporting ligands. They studied the reactivity of iron(II) benzilate complexes [(L)FeII(benzilate)]+, while L = tris(2-pyridylmethyl)amine (TPMA), N1,N1-dimethyl-N2,N2-bis(2-pyridylmethyl)ethane-1,2-diamine (iso-BPMEN), tris(6-methyl-2-pyridylmethyl)amine (6-Me3-TPMA), N1,N1-dimethyl-N2,N2-bis(6-methyl-2-pyridylmethyl)ethane-1,2-diamine (6-Me2-iso-BPMEN), N1,N2-dimethyl-N1,N2-bis(2-pyridylmethyl)ethane-1,2-diamine (BPMEN), tris(2-benzimidazolylmethyl)amine (TBimA), and N1,N2-dimethyl-N1,N2-bis(6-methyl-2-pyridylmethyl)ethane-1,2-diamine (6-Me2-BPMEN) in the reaction with dioxygen (Figure 1). All of those complexes react with dioxygen to exhibit quantitative decarboxylation of benzylic acid to benzophenone. The combination of N-boc-hydroxylamine in air atmosphere with either (TPMA)FeII or (BPMEN)FeII converts cyclohexene to the allylic hydroxylamine (tert-butyl cyclohex-2-en-1-yl(hydroxy)carbamate) [32]. Additionally, the catalytic reactivity of the iron(II) complex with TPMA [33] or iron(II)-2-aminophenolate complexes with 6-Me3-TPMA [34] towards oxygenative aromatic C-C bond cleavage in catechol [33], 2-aminophenol [33], and 2-aminophenolates [34] were presented. According to recent literature, N-pentadentate ligands-based catalysts also react with molecular oxygen oxidizing cyclohexanone derivatives to ɛ-caprolactones [35]. It was found that (N4Py)FeII is highly selective and efficiently catalyses the Baeyer-Villiger oxidation of cyclohexanone derivatives to ɛ-caprolactones with molecular oxygen in the presence of various aldehydes such as isobutyraldehyde and benzaldehyde, which may be applied in the production of polymers, pharmaceuticals, and herbicides.
Figure 1. (a) Various types of tetradentate ligand; (b) coordination geometry at the metal center of iron(II) benzilate complexes; (c) The mechanism of oxidative decarboxylation of iron(II) benzilate complexes of tridentate ligands as follows: formation of iron(III) superoxide radical intermediate (II) and analogous iron(III) superoxide radical intermediate (II′), followed by generation of iron(III) hydroperoxo oxyl radical intermediates (III and III′) due to hydrogen atom abstraction from the hydroxy group of α-hydroxy acid. Decarboxylation of III and III′ generates iron(II) hydroperoxo intermediates (IV and V) that could oxidize sulfide to sulfoxide and sulfone, and provide cis-diol product in reactions with olefins (only V) [31]. TPA is equivalent to the TPMA abbreviation. Reprinted with permission from American Chemical Society, Copyright 2016.
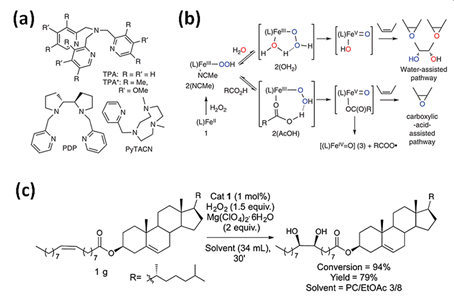
Hydrogen peroxide is another type of “green” oxidizer for organic substrates oxidation with highly stereo- and regioselective bioinspired iron(II) catalysts containing N donor ligands. Initially, it was noticed that a simple (TPMA)FeII complex reacts with H2O2 to form a powerful, metal-based oxidant for aliphatic C-H bonds oxidation with stereoretention [1]. Recently, mononuclear iron(II) complexes based on a TPMA ligand reacting with H2O2 found applications in oxidation of cyclohexane [2,36], cyclohexene [5], 1-octene [4,6], cyclooctene [6], 2-heptene [6], adamantane [2], or steroidal substrate (Figure 2c) [4] to the corresponding alcohols and ketones (Figure 2a,b) [2,6,36] or diol and epoxide [4,5]. Iron(II) complexes with tripodal ligand as TPMA and BPMEN were successfully applied to oxyfunctionalize polystyrene (PSt) [4,37]. The introduction of polar groups into the backbone of PSt increases its compatibility with polar materials which can facilitate its biodegradation [37]. The same iron(II) complexes were used to oxidize olefin (Jasmone) in the open air system. As observed, the (BPMEN)FeII catalyst mostly gave rise to mixtures of the epoxide and the trans-dihydroxylation products, while the use of the TPMA ligand led to cis-dihydroxylation products [3].
Figure 2. (a) Tetradentate ligands in bio-inspired non-heme iron-mediated olefin oxidations; (b) Activation of H2O2 by non-heme iron oxidation catalysts with the assistance of water or carboxylic acids with TPMA- (1–3) and TPMA*-based (1 *–3 *) complexes [6]. Reprinted with permission from Nature Publishing Group, a division of Macmillan Publishers Limited, Copyright 2014. (c) Gram-scale syn-dihydroxylation of cholesteryl oleate catalyzed by tips3TPMA (1) with H2O2 as oxidant [4]. Reprinted with permission from American Chemical Society, Copyright 2018.
Goh et al. [7] noticed that epoxides of plant-derived compounds are interesting substrates because of the possibility of incorporating them into various polymers as replacement compounds for traditional petroleum-based starting materials. Therefore, they used (BPMEN)FeII catalyst with H2O2 as the oxidant for the epoxidation of alkenes bearing a carboxylic acid functional group as oleic acid, undecylenic acid, 5-hexenoic acid, and 4-pentenoic acid. The presence of the carboxylic acid functional group led to the formation of epoxide product for oleic and undecylenic and the γ- and δ-lactones for 4-pentenoic and 5-hexenoic acid, respectively.
Table 1. Heterogeneous applications of iron complexes with nitrogen-containing ligands.
|
Ligand/Catalyst |
Solvent |
Type of Reaction |
Product |
Ref. |
|
TPMA 6-Me3-TPMA 6-Me2-iso-BPMEN iso-BPMEN TBimA BPMEN, 6-Me2-BPMEN |
acetonitrile |
decarboxylation of benzylic acid—O2 as oxidizer |
benzophenone |
[31] |
|
TPMA BPMEN (R,R′)-PDP |
acetonitrile |
allylic hydroxyamination of cyclohexene—O2 as oxidizer (C-N bond formation) |
tert-butyl cyclohex-2-en-1-yl(hydroxy)carbamate |
[32] |
|
TPMA |
acetonitrile-phthalate buffer |
oxygenative regioselective C-C bond cleavage of catechol and 2-aminophenol—O2 as oxidizer |
aromatic ring fission products |
[33] |
|
6-Me3-TPMA |
acetonitrile |
selectivity C-C bond cleavage of substituted 2-aminophenols—O2 as oxidizer |
2-picolinic acid, 4,6-di-tert-butyl-2H-pyran-2-imine, 4,6-di-tert-butyl-2-picolinic acid |
[34] |
|
N4Py |
acetonitrile |
oxidation of cyclohexanone derivatives—O2 as oxidizer |
ɛ-caprolactones |
[35] |
|
TPMA BPMEN |
acetonitrile |
epoxidation, cis-dihydroxylation, oxidation of aliphatic C-H bonds—H2O2 as oxidizer |
oxidized olefins |
[1] |
|
TPMA |
acetonitrile |
oxidation of cyclohexane—H2O2 and mCPBA as oxidizer |
cyclohexanol, cyclohexanone |
[36] |
|
(S,S)-PDP (S,S)-PDP* TPMA TPMA* |
acetonitrile |
hydroxylation/oxidation of cycloalkanes—H2O2, paracetic acid and mCPBA as oxidizer |
cyclohexanol, cyclohexanone |
[2] |
|
TPMA* (S,S)-PDP* |
acetonitrile |
epoxidation of cycloalkenes—H2O2 as oxidizer |
cyclohexene oxide, 2-cyclohexen-1-one, 2-cyclohexen-1-ol |
[5] |
|
TPMA* |
acetonitrile |
epoxidation of 1-octene, cyclooctene or 2-heptene—H2O2 as oxidizer |
alcohols and ketones corresponding to the substrate |
[6] |
|
tips3TPMA LN4Me2 Me,MePyTACN |
acetonitrile, acetone, γ-butyrolactone, ethyl hexanoate, ethyl acetate, propylene carbonate |
dihydroxylation/oxidation of olefins, cholesteryl oleate—H2O2 as oxidizer |
diols and epoxides corresponding to the substrate |
[4] |
|
TPMA BPMEN |
dichloromethane |
oxyfunctionalization of polystyrene—H2O2 as oxidizer |
polymer product with keto and hydroxyl groups |
[37] |
|
TPMA BPMEN |
acetonitrile |
cis-dihydroxylation of alkenes—H2O2 as oxidizer |
epoxide and the trans-dihydroxylation, cis-dihydroxylation products, cis-diols |
[3] |
|
BPMEN |
acetonitrile |
epoxidation of alkenes bearing a carboxylic acid functional groups—H2O2 as oxidizer |
epoxides, lactones |
[7] |
|
TPMA BPyA |
water |
water oxidation—sodium periodate as oxidizer |
O2, H2 |
[38] |
|
TPMA Me3TACN LN4Me2 |
water |
water oxidation—cerium(IV) ammonium nitrate as oxidizer |
O2, H2 |
[39] |
|
PyTACN |
water |
water oxidation—cerium(IV) ammonium nitrate as oxidizer |
O2, H2 |
[40] |
|
N4Py |
water/acetonitrile |
photodriven water oxidation—plastoquinone as oxidazer |
O2, H2 |
[41] |
|
TPMA |
methanol, ethanol, acetonitrile, dichloromethane |
- |
spin-crossover compounds |
[42] |
|
TPMA |
methanol, water |
- |
spin-crossover compounds |
[43] |
|
acetylacetonato ligand a) |
acetonitrile |
redox processes |
p-type dye-sensitized solar cells |
[44] |
|
PyTACN H2pmen |
acetonitrile/ionic liquid |
redox processes |
redox flow batteries |
[45] |
|
TPMA |
DMSO/water DMSO/Dulbecco’s modified eagle’s medium |
redox processes |
drug with anti-proliferative and anti-inflammatory activities |
[46] |
|
N4Py |
DMSO/water |
oxidative DNA cleavage |
drug antitumor activity |
[47] |
|
BPBP 6-Me2-BPBP BPMCN Me2PyTACN MePy2TACN |
water |
oxidative DNA cleavage |
drug antitumor activity |
[48] |
|
(S,S′)-BPBP Me2PyTACN |
water |
oxidative DNA cleavage |
peptide conjugates with antitumor activity |
[49] |
|
NCCN |
acetonitrile |
epoxidation of olefins - H2O2, tert-butyl hydroperoxide and urea hydrogen peroxide adduct as oxidizer |
epoxides |
[8] |
|
NCCN |
acetonitrile |
hydroxylation of benzene and toluene-H2O2 as oxidizer |
phenol, cresols (o-, m- and p-) |
[50] |
|
NCCN analogous-bis(N-heterocyclic carbene)- bis(pyridine), tetra(N-heterocyclic carbene) |
acetonitrile |
olefination of aldehyde |
E-ethyl cinnamate |
[51] |
|
3-methyl-1-(pyridin-2-ylmethyl)-benzimidazol-2-ylidene 3-benzyl-1-(pyridin-2-ylmethyl)-benzimidazol-2-ylidene 3-(4-tert-butyl-benzyl)-1-(pyridin-2-ylmethyl)-benzimidazol-2-ylidene 1,3-di-(2-ethylenepyridyl methyl)-benzimidazol-2-ylidene |
isopropanol |
transfer hydrogenation of aldehydes, ketones |
alcohols |
[52] |
|
enolate chelating N-heterocyclic carbenes |
toluene |
ring-opening polymerization of ε-caprolactone |
polycaprolactone |
[53,54] |
|
bis(amidinato)-N-heterocyclic carbene ancillary ligand |
1,2-dimethoxyethane |
polymerization of (rac)-lactide |
poly(lactic acid) |
[55] |
|
CNC |
acetonitrile/water |
water reduction |
H2 |
[56] |
|
N-heterocyclic carbene with strong electron-donating substituents |
acetonitrile |
redox processes |
iron-based dye sensitized solar cells |
[57] |
|
N-heterocyclic carbene ligands |
water/DMSO |
- |
iron complexes with antibacterial activities |
[58] |
|
C-functionalized neutral and anionic tris(pyrazolyl)methanes |
acetonitrile/water |
hydrocarboxylation of cyclohexane |
cyclohexanecarboxylic acid |
[59] |
|
tris(pyrazolyl)methanes |
- |
oxidation of cyclohexane with ozone |
adipic acid |
[60] |
|
tris(pyrazolyl)methanes |
- |
CO2 hydrogenation with H2 |
methanol |
[61] |
|
tris(pyrazolyl)methanes |
acetonitrile |
oxidation of o-, m- or p-xylene—H2O2 as oxidizer |
corresponding methylbenzyl alcohols, tolualdehydes and toluic acids |
[62] |
|
tris(pyrazolyl)methanes |
acetonitrile, IL, sCO2 IL/sCO2 |
oxidation of cyclohexane-tert-butyl hydroperoxide as oxidizer |
cyclohexanol, cyclohexanone |
[63] |
- a) perylene–thiophene–triphenylamine (PMI-6T-TPMA) sensitizer.
Oxidation of two water molecules to form dioxygen is an attractive reaction enabling the implementation of processes imitating artificial photosynthesis. This reaction can be used to produce solar fuels. Carrying out this process in laboratory conditions requires catalysts that can mediate O-O bond formation. Recently, iron(II) with TPMA [38,39] and 1-(2′-pyridylmethyl)-4,7-dimethyl-1,4,7-triazacyclononane (PyTACN) [40] complexes were considered as water oxidation catalysts. It was proved that the introduction of an internal base in the coordination sphere of the iron complex reduces the energy of the O-O bond formation barrier. It has a clear impact on the future design of iron(II) N-dentate catalyst for water oxidation [40]. Also, N-pentadentate catalysts such as (N4Py)FeII have been successfully applied in photodriven water oxidation reaction, using p-benzoquinone derivatives as plastoquinone analogues [41].
2.2. Application Complexes Containing N Donor Ligands as Switchable Material, Sensitizer
Iron(II) TPMA complexes are an example of switchable material, also named spin-crossover complexes, that could potentially be used in the fields of information storage and display technology. A spin-switching phenomenon occurs for 3d4–3d7 transition metals. Spin states of Fe(TPMA) complexes can be finely tuned between the high- and the low-spin state of the metal centres. Tao et al. [42] found that the encapsulation of different solvents in a crystal lattice can effectively tune the structural packing modes. External stimuli (light, temperature, pressure) regulate the colour, magnetic properties and electrical conductivity of these compounds, which makes them promising materials for storing high-density information, sensors, switches or spintronics. Reactions of TPMA ligand and Fe(ClO4)2·6H2O in the presence tricyanometallate precursors (Tp4-Me-tri(4-methyl-pyrazol-1-yl)borate), (Ph3PMe)[(Tp4-Me)Fe(CN)3·0.5CH3CN] and MeTp-methyltris(pyrazolyl)borate, (NBu4)[(MeTp)Fe(CN)3]) provided two new cyanido-bridged mixed-valence (FeIII2FeII2) molecular squares with high thermal stability, thermally-induced single spin transition (FeIILS2/FeIIILS2 ↔ FeIIHS2/FeIIILS2) behaviour and reversible single-crystal to single-crystal (SC-SC) transformations induced by guest desorption and re-sorption or solvent exchange, useful in the development of new multistable materials like magnetic sensors and molecular switches [43].
Suitably modified TPMA ligands are also used as a sensitizer, achieving high efficiency of energy conversion and low production cost in dye-sensitized solar cells. Iron complexes, as a redox mediator, were applied in p-type dye-sensitized solar cells in conjunction with the PMI-6T-TPMA (perylene–thiophene–triphenylamine) sensitizer [44]. More recently, iron(II) complexes with a PyTACN ligand, capable of stabilizing both high and low oxidation states of metal centres for redox flow batteries, were successfully studied. Redox flow batteries are a promising technology for scalable energy storage. They proposed a design principle for charge carriers that could be used in redox flow batteries based on combining PyTACN ligands that could promote multiple redox events and high open circuit voltage values [45].
2.3. Complexes with N Donor Ligands as Materials in the Effective Treatment of Cancer
Antiproliferative activity of TPMA was successfully combined with anti-inflammatory characteristics of diclofenac, forming [(TPMA)FeII(diclofenac)]+ complex. The iron(II) complex exhibited an anti-proliferative effect on a human breast cancer cells line (MDA-MB-231), which provides useful insight into the development of non-platinum anti-cancer drugs with anti-inflammatory effects [46]. Also, N-pentadentate iron(II) complexes of N4Py are excellent synthetic mimics of bleomycin—natural antibiotics used clinically to induce antitumor activity. In the presence of cellular metal ions and oxygen, those catalysts are capable of mediating oxidative DNA cleavage, which is believed to be the major contributor to their antitumor activity. Treatment of SKOV-3 and MDA-MB-231 cancer cells with N4Py-based mimics iron(II) complexes gave rise to double-strand DNA breaks as efficient as with the use of bleomycin. The results indicate that the designed synthetic bioinorganic model complexes were as active as the parent natural products [47]. Peptide-mediated drug delivery constitutes another versatile tool for releasing therapeutic agents into cancer cells. One of the most common approaches to facilitate the cellular uptake of anticancer drugs is based on their conjugation to a cell-penetrating peptide. Various iron-based ligands were demonstrated to chelate intracellular iron in cancer cells. The resulting redox-active moieties promote apoptosis via iron-dependent pro-oxidant mechanisms. The anti-cancer activity of amine-pyridine-based iron complexes relies on different inter-dependent processes, involving intracellular Fe(II) chelation, generation of reactive oxygen species, DNA fragmentation through oxidative mechanisms, induction of cell cycle arrest, and apoptosis [48]. Moreover, iron complexes with a nitrogen-rich tetradentate ligand (Me2PyTACN) form powerful oxidation species upon reaction with peroxides. The conjugation of a 1,4-dimethyl-7-(2-pyridylmethyl)-1,4,7-triazacyclononane (Me2PyTACN) ligand to a cell-penetrating peptide CPP, such as BP16, was envisaged. The results initiate synthesis of novel peptide conjugates for pro-oxidant anticancer therapies. Proposed peptide conjugates incorporate the N-based ligands at the N-terminus of the cell-penetrating peptide BP16. Analysis of the cytotoxicity of the peptide conjugates showed that the position of the ligand influenced the IC50 values and the incorporation of the βAla-Lys dipeptide rendered non-active sequences [49].
Besides the catalysts containing N donor ligands discussed above, undoubtedly crucial for homogeneous iron catalysis are iron N-heterocyclic carbene complexes (iron NHC complexes) and C-scorpionate iron(II) complexes, that will be presented below. The most important uses of the above-described iron compounds in catalysis focused on various oxidation processes, whereas the major applications of the iron NHC and C-scorpionate complexes, alongside oxidation reactions, e.g., polymerization, C-C bond formation, hydrogenation and hydrocarboxylation reactions, are included as well.
2.4. Iron N-Heterocyclic Carbene Complexes
Grubbs and Louie reported the first example of iron NHC complexes successfully used as catalysts [64]. In their work iron(II) halides bearing imidazolylidene ligands were applied as catalysts for the atom transfer radical polymerization (ATRP) of styrene and methyl methacrylate. Products of the homogeneous polymerizations—PSt and poly(methyl methacrylate) (PMMA) are among the most important and widely used polymers with numerous applications.
Organometallic compounds as N-heterocyclic carbene-ligated iron complexes are highly resistant toward decomposition due to strong σ-electron donating properties of NHC ligands that usually form tough bonds with most metal centres. Among accessibility and steric and electronic tunability, chemical stability is one of the most important features of iron NHCs. Owing to their catalytic properties, these complexes found widespread use in many important reactions, e.g., C-C bond formation (including Kumada and Suzuki cross-coupling reactions [65,66], and allylic alkylation [65]), C-X, C-B [65,66] and C-N [65–68] bond formation, oxidation [65–68] and epoxidation [68] reactions, reduction [65–68], dehydration [67], cyclization [65] and polymerization reactions [65,66]. Applicability of iron NHC complexes in homogeneous catalysis has been frequently successfully demonstrated and this class of compounds might find applications in industrial chemistry and pharmaceutical syntheses [65–68].
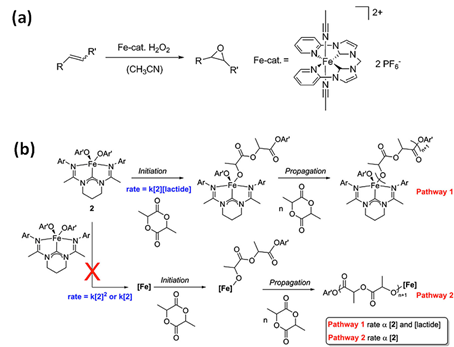
Kühn et al. [8] applied the iron(II) NHC complex bearing a tetradentate di(o-imidazol-2-ylidenepyridine)methane (NCCN) ligand, stable in air and water, as catalysts for the epoxidation of olefins with H2O2, resulting in high yields and selectivity (Figure 3a). Epoxides are a significant group of industrial chemicals commonly used as intermediates in various technological processes. Hydroxylation of benzene catalyzed by iron(II) complex with NCCN ligand in mild temperature with the use of aqueous H2O2 [50] afforded high selectivity for phenol. Hydroxylation of toluene in analogous reaction [50] brought cresols (o-, m- and p-) as the main products with high selectivity for ring hydroxylation. The selective direct oxidation of aromatics compounds is currently one of the most challenging process in organic chemistry, therefore abovementioned reactions might be particularly relevant in this regard.
The iron(II) NCCN complexes are able to catalyze olefinic C-C bond generation in a Wittig reaction, as has been shown in recent work [51]. A one-pot reaction protocol for aldehyde olefination with high olefin yields with very good E-selectivity with the use of iron(II) complex with NCCN analogous was presented. The Wittig reaction is one of the important C-C bond formation processes and is widely used in organic synthesis for the development of alkenes.
Figure 3. (a) Catalytic epoxidation of olefins using N-heterocyclic carbene complex (NCCN) and H2O2 as the oxidant [8]. Reprinted with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Copyright 2014. (b) N-heterocyclic carbene complex-(carbenodiamidine)Fe(4-methoxyphenoxide)2 (2) in polymerization of lactide [55]. Reprinted with permission from Elsevier Ltd., Copyright 2014.
Piano-stool iron(II) NHC complexes, besides the N-heterocyclic carbene motif, bear cyclopentadienyl (Cp) ligands [65,67]. An efficient catalyst for transfer hydrogenation of carbonyl compounds, namely piano-stool iron(II) complex [Fe(L)Cp(CO)]X, L = substituted derivatives of 1-(pyridin-2-ylmethyl)-benzimidazol-2-ylidene group, was synthesized [52]. Hydrogenation of carbonyl substrates bringing hydroxyl compounds including aldehydes conversion into corresponding alcohols is a notable transformation in chemical technology.
Ring-opening polymerization of e-caprolactone catalyzed by aryloxo-functionalized NHC complexes of iron(II) was effectively conducted [53,54]. The complexes were subsequently modified to achieve better stability and higher catalytic activity. The product, polycaprolactone, is a biodegradable polyester often used as an additive for resins improving their impact resistance and biodegradability or as a plasticizer to polyvinyl chloride. Polycaprolactone also showed biomedical and other applications, e.g., in part fabrication or rapid prototyping.
Novel iron bis(alkoxide) complex bearing bis(amidinato)-N-heterocyclic carbene ancillary ligand, (carbenodiamidine)Fe(4-methoxy-phenoxide)2 was successfully applied as a catalyst for controlled polymerization of (rac)-lactide [55]. The substrate of the reaction, (rac)-lactide is derived from renewable resources (e.g., sugar cane, corn starch). The product, poly(lactic acid) (PLA), is a completely biodegradable polymer used for the production of packaging materials, textiles and is applied in the biomedical industry, making this area of research highly attractive.
Iron compounds as naturally abundant and low cost materials are increasingly considered as photocatalysts and photosensitizers in photochemistry as alternatives of photofunctional complexes based on rare or heavy metals. Light-driven applications of iron NHC complexes have been recently a subject of much research [69]. Bauer et al. [56] developed a new synthesis protocol for heteroleptic terpy-NHC iron(II) complexes and presented these novel Fe(II)-NHC compounds as noble metal-free photosensitizers (PS) for light-driven water reduction. Photocatalytic water reduction as a clean and sustainable source of hydrogen provides green energy and, in addition, employing for hydrogen production another green energy source of unlimited abundancy—the sunlight. The observed activity of complexes [(CNC)Fe-η3-terpy]X2, CNC = 2,6-bis-[3-(2,6-diisopropylphenyl)-imidazol-2-ylidene]pyridine, X = PF6¯ or BPh4¯, compared to the activity of established iridium PS [Ir(ppy)2bpy][PF6], proved the potential for new Fe(II)-NHC complexes for photocatalytic water reduction. Novel iron(II) NHC dye sensitizers were synthesized and tested in working solar cells [57]. The development of efficient iron-based dye sensitized solar cells (DSSCs) is a great challenge for photovoltaics. This work presents characterization of iron(II) NHC sensitized photoelectrodes in working DSSCs, and the results may be the basis for developing efficient iron-based dyes for photoelectrochemical applications. Exploiting solar energy for effective electricity production and storage constitutes one of the most important technological tasks.
Iron NHCs widely used in catalysis and photochemical applications have recently found potential medicinal implementations. A group of iron(II) NHC complexes has been developed using a microwave-assisted route and evaluated over antibacterial activity [58]. The results, obtained for tests with standard bacterial strains (Staphylococcus aureus and Escherichia coli), gave some perspectives for the development of new compounds having antibacterial properties and opening the way for devising a new class of antibiotics.
2.5. C-Scorpionate Iron(II) Complexes
Scorpionate ligands, i.e., poly(azolyl)borates and poly(azolyl)alkanes are a suitable and versatile class of ligands due to the type and number of azolyl groups, moreover, the substituents on these groups or on the B or C centres are flexible and can be readily modified to receive ligands with various steric and electronic profiles [70]. In this consideration, these compounds found applications in several areas of research including homogeneous catalysis, organic synthesis, prospective advanced materials, and modelling of active sites of metalloenzymes [71].
The use of metallic complexes with scorpionate ligands as catalysts expanded a range of developments. For instance, homoscorpionate tris(pyrazol-1-yl)methane metal complexes were used as catalysts in the oxidative functionalization of alkanes, alkenes, and ketones under mild conditions in homogeneous catalytic systems [72]. Most recent works show that transition metal complexes of C-homoscorpionate tris(pyrazol-1-yl)methanes were employed as catalysts for an increasing number of challenging and industrially significant reactions, e.g., selective oxidation of alkanes and alcohols, hydrocarboxylation of Cn alkanes into Cn+1 carboxylic acids, and carbon dioxide hydrogenation to methanol. Heck, Sonogashira Henry C-C coupling reactions and azide-alkyne Huisgen cycloaddition have been also reported [73].
C-Scorpionate iron(II) complexes [FeCl2{κ3-HC(pz)3}] (pz = pyrazol-1-yl), Li[FeCl2{κ3-SO3C(pz)3}] and [FeCl2{κ3-HOCH2C(pz)3}] received straightway from iron(II) chloride and appropriate scorpionate ligands in ethanol or water were applied as catalysts for hydrocarboxylation of cyclohexane to cyclohexanecarboxylic acid in aqueous/acetonitrile medium at a mild temperature, receiving very good yields (up to 69%) and high selectivity (up to 98%) [59]. Cyclohexane was a model substrate of the reaction due to the particular significance of cyclohexanecarboxylic acid, used in industry as a flavouring agent; a stabilizer for rubber; and as an additive for paints, varnishes, soaps, and lubricating oils.
A highly catalytic active bio-inspired C-scorpionate iron(II) complex was developed, focusing on developing more sustainable, alternative catalytic systems for crucial industrial processes [60–63]. The presented iron(II) complex catalyzed synthesis of adipic acid directly from cyclohexane via single-pot oxidation with ozone as an oxidizing agent and pyrazine carboxylic acid as a promoter of the reaction [60]. Adipic acid, owing to its versatility as a building block, is a highly valuable chemical, produced on a large scale worldwide and used as a monomer for nylon-6,6 synthesis and also to produce polyurethane foams and resins, polyesters, lubricants, and adhesives. Environmentally benign, solvent- or amine-free synthesis of methanol (one of the most important building block in the chemical industry) was achieved via single-pot hydrogenation of carbon dioxide catalyzed by [FeCl2{κ3-HC(pz)3}] complex at a mild temperature [61]. This highly efficient reaction brought turnover numbers up to 2300 with 44% yield. Fast conversion of xylenes (o-, m- and p-) to the corresponding methylbenzyl alcohols, tolualdehydes, and toluic acids was attained in the reaction catalyzed by [FeCl2{κ3-HC(pz)3}] complex under mild conditions (35 °C, low catalysts loading) and with green oxidant (30% aqueous H2O2) in the presence of the co-catalyst (nitric acid) [62]. The iron(II) complex [FeCl2{κ3-HC(pz)3}] and its alcohol derivative [FeCl2{κ3-HOCH2C(pz)3}] complex were successfully applied as catalysts for cyclohexane oxidation to cyclohexanol and cyclohexanone with tert-butyl hydroperoxide in ionic liquids (IL), supercritical carbon dioxide (scCO2), and mixtures of these solvents (scCO2-IL) [63]. The reactions provided remarkable yields of the products, the finest in mixed solvents (scCO2-IL) media.
As presented, iron as a non-noble metal catalyst in the context of homogeneous catalysis can be considered as a versatile tool in chemical technologies and global production. It was successfully applied in a wide range of type of the reaction, including oxidation processes of low molecular weight molecules giving substrates for industrial-scale production; redox flow batteries or solar cells application; and also biological macromolecules as DNA providing anti-proliferative, anti-inflammatory, and anti-tumour drugs. It is worth noticing that iron complexes with N donor ligands also can be used as homogeneous catalysts of H2 evolution and CO2 reduction catalysts that use H2O as a proton source, co-solvent, or reaction medium. Those processes are important transformations in the synthesis of renewable fuels from abundant water or the greenhouse gas CO2 is a major step toward creating sustainable and scalable energy storage technologies [74]. Iron-based catalysts were also applied in heterogeneous catalysis, expanding the field of their potential applications.
This entry is adapted from the peer-reviewed paper 10.3390/pr8121683