High lymphocytic infiltration (TILs) seem to reflect favorable host antitumor immune responses. In breast cancer, the variation of TILs before and after neoadjuvant chemotherapy (NAC) according to BRCA status has been poorly described.
- BRCA
- TILs
- pCR
- NAC
- immunotherapy
1. Introduction
Neoadjuvant or pre-operative chemotherapy (NAC) is classically administered to patients with inflammatory or locally advanced breast cancer (BC). Beyond increasing breast-conserving surgery rates[1][2], it also serves as an in vivo chemosensitivity test and the analysis of residual tumor burden may help understanding treatment resistance mechanisms[2]. In addition, it helps refining the prognosis of patients after NAC, as pathological complete response (pCR) after NAC is associated with a better long-term survival[1][3].
Nearly 5% of breast cancers occur in a context of genetic predisposition, mostly represented by monoallelic pathogenic variants of BRCA1, BRCA2 or PALB2 genes[4]. Patients with loss-of-function of the BRCA1 or 2 proteins have a higher cumulated breast cancer risk, with a cumulated lifetime risk at eighty years old of 72% (BRCA1) and 69% (BRCA2)[5]. The peak incidence for BRCA1 mutation carriers occurs between 41 and 50 years old (28.3 per 1000 person-years), whereas it occurs ten years later for BRCA2 mutation carriers (30.6 per 1000 person-years between 51 and 60)[5]. BRCA1 and BRCA2 are tumor-suppressor genes that code for proteins involved in homologous recombination (HR) repair. HR deficiency (HRD) occurs when the second allele is inactivated by allelic deletion (often detected by LOH), genic alteration or promoter methylation (for BRCA1 only). Biallelic BRCA1/2 inactivation results in genomic instability and theoretically increases the somatic mutational load[6].
Tumors associated with germline or somatic BRCA1/2 pathogenic mutations display different patterns when compared with sporadic BCs. Cancers occurring among BRCA1 carriers are more frequently classified as medullary [7], whereas histological subtypes among BRCA2 carriers tend to be more heterogeneous[8]. In addition, BRCA1 carriers are more frequently ER-negative, PR-negative and lack HER2 amplification (i.e., display a triple negative (TNBCs) phenotype[9]) whereas in BRCA2 carriers, a similar prevalence of ER-positive tumors has been described when compared with sporadic controls[10][11][12][13].
Most of patients with TNBCs receive chemotherapy[14][15]. Due to the alteration of BRCA1 and BRCA2 proteins in tumor cells, BRCA-mutated cells are unable to properly repair double-strand breaks, classically induced by DNA-alkylating agents[16]. Hence, BRCA deficiency has sometimes been associated with a higher sensitivity to platinum agents when compared to other types of neoadjuvant chemotherapy regimens[17][18][19]. In a recent meta-analysis of platinum-based neoadjuvant chemotherapy in TNBC, the addition of carboplatin was not associated with significantly increased pCR rate in BRCA-mutated patients (OR = 1.17, CI95% [0.51–2.67], p = 0.711)[20]. So far, the benefit of adding a platinum agent in BRCA-mutated patients receiving standard neoadjuvant chemotherapy remains a matter of debate. Nevertheless, beyond the controversy upon platinum-based agents in BRCA-deficient tumors, the effectiveness of standard NAC in all BC subtypes associated with BRCA pathogenic variants compared to controls has been poorly explored so far.
The role of tumor infiltrating lymphocytes (TILs) in BC has been extensively studied over the last decade. High levels of TILs before NAC are associated with higher pCR rates and better survival, especially for TNBC and HER2-positive BCs[21][22]. However, despite a growing interest in the field of immunity and oncology, characterization and quantification of TILs across all BC subtypes according to BRCA status has not been extensively described. Similarly, no study has evaluated so far, the evolution of immune infiltration after NAC according to BRCA status.
2. Response to Treatment and Post-NAC Immune Infiltration
2.1. Response to Treatment
At NAC completion, pCR was observed in 84 out of 266 (31%) patients and pCR rates were significantly different by BC subtype (luminal: 10% (9/89), TNBC: 45% (49/110) and HER2-positive 39% (26/67), p < 0.001). Pre-NAC str TIL levels were significantly higher in tumors for which pCR was achieved (p < 0.001) and there was a significant association between pre-NAC TIL levels and pCR status in the whole population (all: OR = 1.03, CI95% [1.02–1.05], p < 0.001) and in the TNBC subgroup (luminal: OR = 1.03, CI95% [1–1.09], p = 0.21; TNBC: OR = 1.03; CI95% [1–1.04], p = 0.007; HER2-positive: OR = 1.02, CI95% [0.99–1.06], p = 0.23).
pCR rates were significantly higher in patients with BRCA-deficient breast cancers (45.7% (21/46) versus 28% (63/221) in BRCA-proficient, p < 0.035, Figure 2). After the subgroup analysis of BC subtype, this was confirmed only in the luminal BC subtype (33.3% (5/15), p = 0.006), but not in TNBC and HER2-positive BCs (48.1% (13/27), p = 0.823 and 75% (3/4), p = 0.291, respectively, Figure 2). The interaction test between BC subtype and BRCA status was nearly significant (Pinteraction = 0.056). There were no differences in pCR rates by BRCA1 or BRCA2 mutation status in patients with BRCA-deficient tumors (BRCA1, 42% (13/31) versus BRCA2, 50% (7/14), p = 0.7) but the effective of the subpopulations were limited.
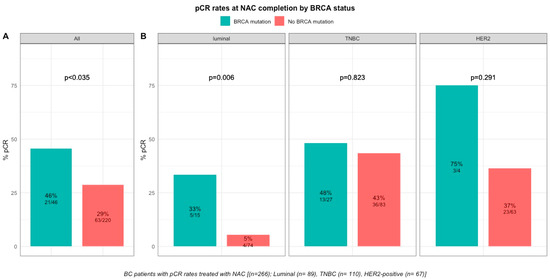
Figure 2. Barplot of associations between response to treatment and BRCA status in whole population, and by breast cancer subtype. (A), among the whole population (All (n = 266), BRCA mutation (n = 46), BRCA wild-type (n = 220)). (B), by BC subtype (Luminal (n = 89), BRCA mutation (n = 15), BRCA wild-type (n = 74); TNBC (n = 110), BRCA mutation (n = 27), BRCA wild-type (n = 83); HER2 (n = 67), BRCA mutation (n = 4), BRCA wild-type (n = 63)).
However, BRCA status was not significantly associated with pCR after multivariate analysis, and only BC subtype (TNBC, OR = 7.14, CI95% [3.39–16.57], p < 0.001; HER2-positive, OR = 5.64, CI95% [2.5–13.78], p = 0.001), tumor size (T2, OR = 0.37, CI95% [0.16–0.83], p = 0.017; T3, OR = 0.21, CI95% [0.08–0.55], p = 0.002) and pre-NAC str and IT TILs (OR = 1.03, CI95% [1.02–1.05], p = 0.001 and OR = 1.04, CI95% [1.02–1.07], p = 0.002) were independent predictors of pCR .
2.2. Post-NAC Immune Infiltration by BRCA Status
After NAC, str and IT TILs were available in 192 (72%) and 120 (45%) patients respectively. Post-NAC immune infiltration (whether intra-tumoral or stromal) was not significantly different between BRCA-deficient and BRCA-proficient carriers (Figure 3A–3E). However, both str and IT TIL levels were significantly higher in tumors with BRCA pathogenic mutations when compared with wild-type tumors in luminal BCs (median str TIL levels: 15% vs. 10%, p = 0.009 and median IT TIL levels: 10% vs. 5%, p = 0.019, respectively, Figure 3).
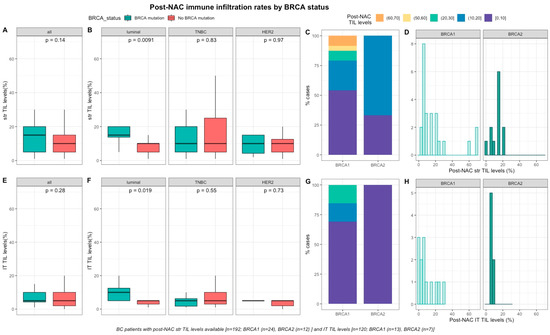
Figure 3. Associations between post-NAC TILs and BRCA status in whole population, and after stratification by breast cancer subtype. Bottom and top bars of the boxplots represent the first and third quartiles, respectively, the medium bar is the median, and whiskers extend to 1.5 times the interquartile range. (A) stromal lymphocytes among the whole population (All (n = 192), BRCA mutation (n = 36), BRCA wild-type (n = 156)). (B) stromal lymphocytes in each BC subtype (Luminal (n = 52), BRCA mutation (n = 8), BRCA wild-type (n = 44); TNBC (n = 97), BRCA mutation (n = 24), BRCA wild-type (n = 73); HER2 (n = 43), BRCA mutation (n = 4), BRCA wild-type (n = 39)). (C) Percentage of tumor according to post-NAC stromal lymphocytes levels binned by 10% increment in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)). (D) distribution of post-NAC stromal lymphocytes by gene mutations (histogram plot) in patients with BRCA-deficient (BRCA1 (n = 24), BRCA2 (n = 12)). (E) intratumoral lymphocytes among the whole population (All (n = 120), BRCA mutation (n = 20), BRCA wild type (n = 100)). (F) intratumoral lymphocytes in each BC subtype (Luminal (n = 44), BRCA mutation (n = 7), BRCA wild-type (n = 37); TNBC (n = 50), BRCA mutation (n = 12), BRCA wild-type (n = 38); HER2 (n = 26), BRCA mutation (n = 1), BRCA wild-type (n = 25)). (G) percentage of tumor according to post-NAC intratumoral lymphocytes levels binned by 10% increment in patients with BRCA-deficient (BRCA1 (n = 13), BRCA2 (n = 7)). (H) distribution of post-NAC intratumoral lymphocytes by gene mutations (histogram plot) in patients with BRCA-deficient (BRCA1 (n = 13), BRCA2 (n = 7)).
Median pre-NAC str TIL were higher than after NAC (20% vs. 10%, 11.95%), also according to BRCA status and type (Figure 4). There was no correlation between pre and post NAC str TILs (correlation coefficient of 0.13 and p < 0.06) and there was a weak, positive, linear relationship between pre and post NAC IT TIL levels (correlation coefficient of 0.31 and p < 0.001).
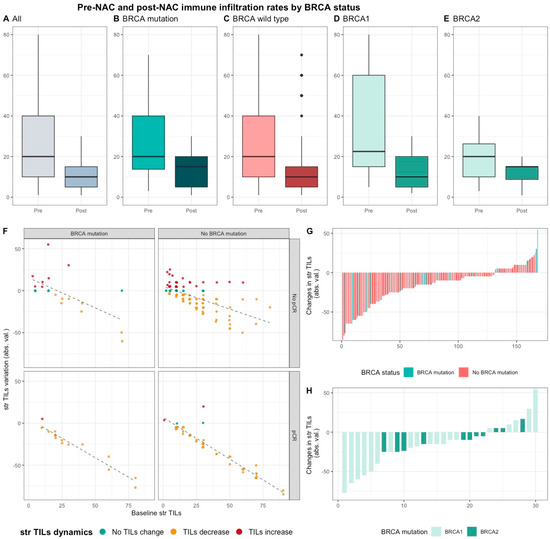
Figure 4. Pre-NAC and post-NAC stromal immune infiltration rates in the whole population and by BRCA status. (A–E) bar plots of str TIL levels before and after NAC in the whole population and in BRCA pathogenic variant. Bottom and top bars of the boxplots represent the first and third quartiles, respectively, the medium bar is the median, and whiskers extend to 1.5 times the interquartile range. (All (n = 192); BRCA mutation (n = 36), BRCA wild-type (n = 156); BRCA1 (n = 24), BRCA2 (12)). (F) variation of str TIL levels according to the pre-NAC str TIL levels binned by BRCA status and response to chemotherapy. Points represent the difference between pre- and post-NAC paired TIL levels values of a given patient and are colored according to TIL variation category (TIL level decrease: yellow/no change: green/increase: red) (All (n = 191), BRCA mutation (n = 36), BRCA wild-type (n = 155)). (G–H) waterfall plot representing the variation of TIL levels according to BRCA-deficient (BRCA1-deficient, BRCA2-deficient); each bar represents one sample, and samples are ranked by increasing order of TIL level change. Paired samples for which no change was observed have been removed from the graph. (All (n = 191), BRCA mutation [(n = 36), BRCA1, n = 24; BRCA2 = 12)], BRCA wild-type (n = 155)).
2.3. Survival Analysis
After a median of follow-up of 90.4 months (range from 0.2 to 187 months), 73 patients experienced relapse, and 38 died. RFS and OS were not significantly different between carriers of a BRCA pathogenic variant and BRCA-proficient patients, neither were they in screened population nor after the subgroup analysis of BC subtype.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12123681
References
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785.
- Reyal, F.; Hamy, A.S.; Piccart, M.J. Neoadjuvant treatment: The future of patients with breast cancer. ESMO Open 2018, 3, e000371.
- Luangdilok, S.; Samarnthai, N.; Korphaisarn, K. Association between Pathological Complete Response and Outcome Following Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer Patients. J. Breast Cancer 2014, 17, 376–385.
- Huang, K.-L.; Mashl, R.J.; Wu, Y.; Ritter, D.I.; Wang, J.; Oh, C.; Paczkowska, M.; Reynolds, S.; Wyczalkowski, M.A.; Oak, N.; et al. Pathogenic Germline Variants in 10,389 Adult Cancers. Cell 2018, 173, 355–370.e14.
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416.
- Ferreira, E.N.; Brianese, R.C.; de Almeida, R.V.B.; Drummond, R.D.; de Souza, J.E.; da Silva, I.T.; de Souza, S.J.; Carraro, D.M. Influence of BRCA1 Germline Mutations in the Somatic Mutational Burden of Triple-Negative Breast Cancer. Transl. Oncol. 2019, 12, 1453–1460.
- Eisinger, F.; Jacquemier, J.; Charpin, C.; Stoppa-Lyonnet, D.; Bressac-de Paillerets, B.; Peyrat, J.P.; Longy, M.; Guinebretière, J.M.; Sauvan, R.; Noguchi, T.; et al. Mutations at BRCA1: The medullary breast carcinoma revisited. Cancer Res. 1998, 58, 1588–1592.
- Phillips, K.A. Immunophenotypic and pathologic differences between BRCA1 and BRCA2 hereditary breast cancers. J. Clin. Oncol. 2000, 18, 107S–112S.
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomark. Prev. 2012, 21, 134–147.
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J.; Anderson, T.J.; Osin, P.P.; McGuffog, L.; Easton, D.F. The pathology of familial breast cancer: Predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002, 20, 2310–2318.
- Armes, J.E.; Trute, L.; White, D.; Southey, M.C.; Hammet, F.; Tesoriero, A.; Hutchins, A.M.; Dite, G.S.; McCredie, M.R.; Giles, G.G.; et al. Distinct molecular pathogeneses of early-onset breast cancers in BRCA1 and BRCA2 mutation carriers: A population-based study. Cancer Res. 1999, 59, 2011–2017.
- Palacios, J.; Honrado, E.; Osorio, A.; Cazorla, A.; Sarrió, D.; Barroso, A.; Rodríguez, S.; Cigudosa, J.C.; Diez, O.; Alonso, C.; et al. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res. Treat. 2005, 90, 5–14.
- Lakhani, S.R.; Reis-Filho, J.S.; Fulford, L.; Penault-Llorca, F.; van der Vijver, M.; Parry, S.; Bishop, T.; Benitez, J.; Rivas, C.; Bignon, Y.-J.; et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin. Cancer Res. 2005, 11, 5175–5180.
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet Lond. Engl. 2017, 389, 2430–2442.
- Mancini, P.; Angeloni, A.; Risi, E.; Orsi, E.; Mezi, S. Standard of Care and Promising New Agents for Triple Negative Metastatic Breast Cancer. Cancers 2014, 6, 2187–2223.
- Godet, I.; Gilkes, D.M. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr. Cancer Sci. Ther. 2017, 4.
- Byrski, T.; Gronwald, J.; Huzarski, T.; Grzybowska, E.; Budryk, M.; Stawicka, M.; Mierzwa, T.; Szwiec, M.; Wiśniowski, R.; Siolek, M.; et al. Pathologic Complete Response Rates in Young Women With BRCA1-Positive Breast Cancers After Neoadjuvant Chemotherapy. J. Clin. Oncol. 2009, 28, 375–379.
- Wunderle, M.; Gass, P.; Häberle, L.; Flesch, V.M.; Rauh, C.; Bani, M.R.; Hack, C.C.; Schrauder, M.G.; Jud, S.M.; Emons, J.; et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res. Treat. 2018, 171, 85–94.
- Sella, T.; Gal Yam, E.N.; Levanon, K.; Rotenberg, T.S.; Gadot, M.; Kuchuk, I.; Molho, R.B.; Itai, A.; Modiano, T.M.; Gold, R.; et al. Evaluation of tolerability and efficacy of incorporating carboplatin in neoadjuvant anthracycline and taxane based therapy in a BRCA1 enriched triple-negative breast cancer cohort. Breast Edinb. Scotl. 2018, 40, 141–146.
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; La Valle, G.; Del Mastro, L.; De Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508.
- Solinas, C.; Ceppi, M.; Lambertini, M.; Scartozzi, M.; Buisseret, L.; Garaud, S.; Fumagalli, D.; de Azambuja, E.; Salgado, R.; Sotiriou, C.; et al. Tumor-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: A meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2017, 57, 8–15.
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast Edinb. Scotl. 2017, 35, 142–150.
