Most of the fungi from the Fusarium genus are pathogenic to cereals, vegetables, and fruits, and the products of their secondary metabolism mycotoxins may accumulate in foods and feeds. Non-ribosomal cyclodepsipeptides are one of the main mycotoxin groups and include beauvericins (BEAs), enniatins (ENNs), and beauvenniatins (BEAEs). When ingested, even small amounts of these metabolites significantly affect human and animal health. On the other hand, in view of their antimicrobial activities and cytotoxicity, they may be used as components in drug discovery and processing and are considered as suitable candidates for anti-cancer drugs. Therefore, it is crucial to expand the existing knowledge about cyclodepsipeptides and to search for new analogues of these compounds. The present manuscript aimed to highlight the extensive variability of cyclodepsipeptides by describing chemistry, biosynthesis, and occurrence of BEAs, ENNs, and BEAEs in foods and feeds. Moreover, the co-occurrence of Fusarium species was compared to the amounts of toxins in crops,
vegetables, and fruits from different regions of the world.
- phytopathogens
- Fusarium
- mycotoxin contamination
1. Introduction
Fungi belonging to the Fusarium genus produce a wide range of secondary metabolites, including the non-ribosomal depsipeptide mycotoxins, such as beauvericins (BEAs), beauvenniatins (BEAEs), enniatins (ENNs), and their analogues [1,2,3,4]. BEAs, BEAEs, and ENNs were included in the cyclodepsipeptide group of compounds, often found in high concentrations in grains, crops, vegetables, fruits, and even eggs, as a result of fungal infection [5,6,7,8,9]. They are involved in plant-pathogen interaction and may lead to many plants′ diseases, which can be very dangerous for animals′ health, including humans [10,11,12,13,14]. For example, ENNs produced by Fusarium species may act synergistically as a phytotoxin complex, which causes wilt and necrosis of plant tissue [15]. Moreover, ENN B affects mouse embryo development by inducing the dosage-related apoptosis or necrosis in mouse blastocytes [16]. On the other hand, BEA demonstrated neurotoxic properties in mice. In higher concentrations (7.5 and 10 µM), it affected the skeletal muscle fibers [17].
Additionally, BEA has a harmful influence on the reproductive system. The progesterone synthesis in cumulus cells was decreased when exposed to BEA [18]. Moreover, BEA inhibited estradiol and progesterone synthesis in bovine granulosa cells [19]. Also, ENN B reduced progesterone, testosterone, and cortisol secretion in human adrenocortical carcinoma cells and modulated the expression of genes involved in steroidogenesis [20]. The cytotoxicity of cyclodepsipeptides (BEAs, BEAEs, ENNs) is related to their ionophoric properties [21,22,23]. Even at low concentrations, they possess the capacity of perforation of the cell membrane, which is associated with the induction of apoptotic cell death and disruption of extracellular regulated protein kinase (ERK) activity [24,25,26,27]. However, this ability does not exclude the capability of promoting the transport of cations such as K+, Na+, Mg2+, and Ca2+ through the membranes, which leads to the disturbance of cellular ionic homeostasis [28]. This cytotoxic effect on various human cancer cell lines also suggests the potential use of cyclodepsipeptides as anti-cancer drugs [22,29,30,31,32]. All cyclodepsipeptides (BEAs, BEAEs, ENNs) have been shown as compounds exhibiting numerous biological activities, such as antimicrobial, insecticidal, and antibiotic activity, towards Mycobacterium tuberculosis and Plasmodium falciparum (human malaria parasite) because of their potential to inhibit the cholesterol acyltransferase of microbial origin [30,33]. Furthermore, BEA can be used as a co-drug for fungal infections in humans because the combination of BEA and ketoconazole (an anti-fungal drug) enhances its antifungal activities [29,33,34,35]. BEA has been reported as a growth inhibitor of human-pathogenic bacteria, such as Escherichia coli, Enterococcus faecium, Salmonella enterica, Shigella dysenteriae, Listeria monocytogenes, Yersinia enterocolitica, Clostridium perfringens, and Pseudomonas aeruginosa. The chemical properties of cyclodepsipeptides may allow for the emergence of new pharmaceutical products with anti-inflammatory and antibiotic properties [33,36,37]. The studies have shown the divergent impact of cyclodepsipeptides on human health; still, further studies are needed to indicate the potential effects of BEAs, BEAEs, and ENNs on human health. Moreover, it is imperative to study new compounds of the cyclodepsipeptide group, along with their analogues, to better understand the relationships between their structure, diversity, and toxicity.
2. Chemistry
BEAs, ENNs, BEAEs, and allobeauvericins (ALLOBEAs) represent a family of regular cyclodepsipeptides, consisting of three N-methyl amino acids and three hydroxy acid groups [4,38,39,40,41]. Characterization of all cyclodepsipeptides produced by Fusarium fungi, their elemental composition, molecular weights (used for their identification), and chemical structures are presented in Table 1 and Figure 1. Most of the BEAs contain three groups of N-methyl-phenylalanine, except for BEAs J, K, and L, which contain one, two, or three groups of N-methyl-tyrosine, respectively [2,26]. However, BEA D and E have demethylated amino acids-phenylalanine and leucine in their structures [42]. Moreover, BEAs differ in hydroxy acids possession. BEA and BEA D, E, J, K, and L possess D-2-hydroxyisovaleric acid (D-Hiv) (Figure 2a) and BEA A/F, B, and C possess D-2-hydroxy-3-methylpentanoic acid (D-Hmp) (Figure 2b), whereas BEA G1 and G2 possess D-2-hydroxybutyric acid (D-Hbu) (Figure 2c) [2,3,31,33,42]. ALLOBEAs A, B, and C are diastereomeric to BEAs A, B, and C, respectively. These compounds differ in the D-Hmp groups’ configuration [33]. Some of the BEAs, such as BEA B, C, J, K, L, G1, G2, and all ALLOBEAs, were known from previous publications as precursor-directed compounds, detected inside in vitro cultures of fungi belonging to Beauveria, Acremonium, and Paecilomyces genera [26,31,33]. It was proven that phytopathogenic fungi from the Fusarium genus naturally produce all BEAs and ALLOBEAs [2,3,42]. The structures of BEAs have been described in many articles, where they were determined by a variety of chemical methods, including liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR).
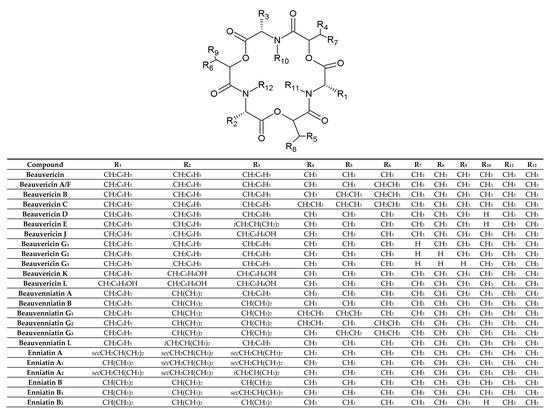
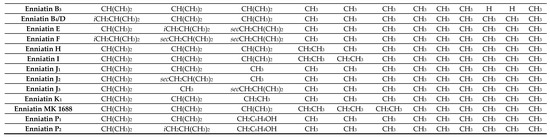
Figure 1. Chemical structures of beauvericin, enniatin, allobeauvericin, and beauvenniatin analogues produced by Fusarium species.
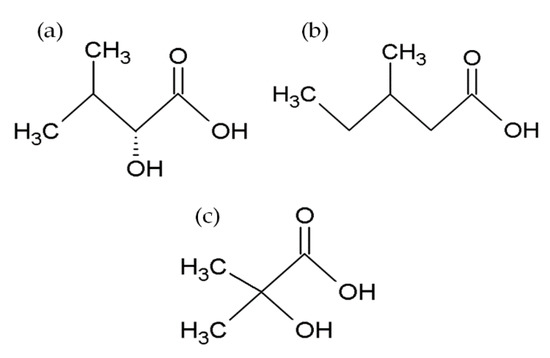
Figure 2. Chemical structures of D-2-hydroxyisovaleric acid (D-Hiv) (a), D-2-hydroxy-3-methylpentanoic acid (D-Hmp) (b), and D-2-hydroxybutyric acid (D-Hbu) (c) groups.
Table 1. Elemental composition and molecular weights of beauvericins, enniatins, and their analogues.
| Compound | MW | MW + NH4+ (18) | MW + Na+ (23) | MW + K+ (39) | Elemental Composition | References |
|---|---|---|---|---|---|---|
| Beauvericin | 783 | 801 | 806 | 822 | C45H57N3O9 | [2,26] |
| Beauvericin A/F/Allobeauvericin A | 797 | 815 | 820 | 836 | C46H59N3O9 | [2,33,42] |
| Beauvericin B/Allobeauvericin B | 811 | 829 | 834 | 850 | C47H61N3O9 | [3,33] |
| Beauvericin C/Allobeauvericin C | 825 | 843 | 848 | 864 | C48H63N3O9 | [2,33] |
| Beauvericin D | 769 | 787 | 792 | 808 | C44H55N3O9 | [2,42] |
| Beauvericin E | 735 | 753 | 758 | 774 | C41H57N3O9 | [3,42] |
| Beauvericin G1 | 769 | 787 | 792 | 808 | C44H55N3O9 | [3,31] |
| Beauvericin G2 | 755 | 773 | 778 | 794 | C43H53N3O9 | [3,31] |
| Beauvericin J | 799 | 817 | 822 | 838 | C45H57N3O10 | [2,26] |
| Beauvericin K | 815 | 833 | 838 | 854 | C45H57N3O11 | [2] |
| Beauvericin L | 831 | 849 | 854 | 870 | C45H57N3O12 | [2] |
| Beauvenniatin A | 735 | 753 | 758 | 774 | C41H57N3O9 | [2,26] |
| Beauvenniatin B | 687 | 705 | 710 | 726 | C37H57N3O9 | [3,26,30] |
| Beauvenniatin G1/G2/G3 | 715 | 733 | 738 | 754 | C39H61N3O9 | [3,30] |
| Beauvenniatin L | 749 | 767 | 772 | 788 | C42H59N3O9 | [2] |
| Enniatin A/F/MK 1688 | 681 | 699 | 704 | 720 | C36H63N3O9 | [25,39,44,45] |
| Enniatin A1/E/I | 667 | 685 | 690 | 706 | C35H61N3O9 | [25,39,44,45] |
| Enniatin A2 | 681 | 699 | 704 | 720 | C35H61N3O9 | [46] |
| Enniatin B | 639 | 657 | 662 | 678 | C33H57N3O9 | [25,39] |
| Enniatin B1/B4/D/H | 653 | 671 | 676 | 692 | C34H59N3O9 | [25,39,44,45,47] |
| Enniatin B2/J2/J3/K1 | 625 | 643 | 648 | 664 | C32H55N3O9 | [25,43] |
| Enniatin B3/J1 | 611 | 629 | 634 | 650 | C31H53N3O9 | [25,43,47] |
| Enniatin P1 | 641 | 659 | 664 | 680 | C33H57N3O10 | [21] |
| Enniatin P2 | 655 | 673 | 678 | 694 | C34H59N3O10 | [21] |
ENNs are typically composed of N-methyl-leucine, N-methyl-isoleucine and/or N-methyl-valine [1,10,41]. However, two of the ENNs: ENN P1 and P2 also possess N-methyl-tyrosine in their structures [21]. ENN J1, J2, and J3 are another group of ENNs that differ from the common ENNs. These cyclodepsipeptides consist of one N-methyl-isoleucine, one N-methyl-valine, and N-methyl-alanine [43]. Most ENNs contain three groups of D-2-hydroxyisovaleric acid (D-Hiv) and only three ENNs: ENN H, I, and MK 1688, containing one, two, or three groups of D-2-hydroxy-3-methylpentanoic acid (D-Hmp), respectively [44]. Some of the reported ENNs are isomers, with the same amino acid composition but in different positions, e.g., ENN J1, J2, J3 or ENN A and F [39,43,45]. On the other hand, even though the ENNs are not isomers, they share the same molecular weight. Therefore, the MS/MS technique with acid hydrolysis or NMR is sometimes necessary during the detection of cyclodepsipeptides for their correct identification.
BEAEs possess hybrid structures between the aliphatic (enniatin-type) and aromatic (beauvericin-type) cyclodepsipeptides [2,3,26,30]. Moieties of N-methyl-phenylalanine, N-methyl-leucine, and/or N-methyl-valine are the parts of BEAEs’ structures. BEAE A contains one N-methyl-valine, whereas BEAE B, G1, G2, and G3 have two. BEAE L has one N-methyl-leucine in its structure. Apart from the D-2-hydroxyisovaleric acid (D-Hiv) group, three of the BEAE isomers, namely BEAE G1, G2, and G3, contain two D-2-hydroxy-3-methylpentanoic acid (D-Hmp) groups in different combinations. At first, all BEAEs were described as cyclodepsipeptides from Acremonium sp., however further research revealed that Fusarium species are also able to produce these compounds [2,3,26,30].
3. Biosynthesis
Cyclodepsipeptides are biosynthesized by a multi-domain non-ribosomal peptide synthase (NRPS) that is composed of enzymatic modules used to elongate the proteinogenic and non-proteinogenic amino acids, as well as carboxyl and hydroxy acids [48,49]. The modules respond to the order and number of the precursors incorporated into the chain. Separate NRPS modules are required to assemble the product and a minimal module consists of the three core domains: adenylation (A) domain, thiolation or peptidyl-carrier protein (T or PCP) domain, and condensation (C) domain. Moreover, each module and each active site domain is used only once for the recognition and activation of the precursors through adenylation with ATP (A: adenylation domain), covalent thioester tethering (T: thiolation or PCP: peptidyl carrier protein domain), which tethers the activated precursor to a 4′-phosphopantetheine (PP) cofactor through a thioester bond and transport substrates to the active sites of the domains, and condensation (C domain) of the precursors via catalyzing the peptide bond (C-N) formation between the elongated chain and the activated amino acid. The main domains may be supported by additional domains of the NRPS, such as the epimerization (E) domain, which catalyzes the transformation of an L-amino acid into a D-amino acid, or the dual/epimerization (E/C) domains, which catalyze the epimerization and condensation. NRPSs contain an additional reductase (R) domain, which is responsible for reducing the final peptide, the methylation (MT) domain, which catalyzes N-methylation of the amino acid substrate, the cyclization (Cy) domain that catalyzes the formation of oxazoline or thiazoline rings by internal cyclization of cysteine, serine, or threonine residues, and the oxidation (Ox) domain, which catalyzes the formation of an aromatic thiazol through oxidation of a thiazoline ring. The last domains (TE–thioesterase domains), mostly located at the final NRPS module, are responsible for releasing the full-length NRPS product from the enzyme through cyclization or hydrolysis [48,49,50,51,52].
Enniatin biosynthesis is catalyzed by the 347 kDa multienzyme enniatin synthase (ESYN1) purified for the first time from Fusarium oxysporum and further characterized by Zocher and coworkers [53]. Extensive molecular research revealed the basis of cyclic oligopeptide biosynthesis and allowed us to identify esyn1, a gene encoding enniatin synthase, as the essential enzyme of the metabolic pathway [39,54,55,56,57]. The biochemical characterization revealed that the enzyme possesses two substrate activation modules EA and EB, composed of approximately 420 amino acid residues. The EA module activates and participates in binding the α-D-hydroxy acids, while the EB module activates the amino acids. These two modules consist of a conserved 4-phosphopantetheine binding site at the C-terminus, with a highly conserved serine residue. An additional 4-phosphopantetheine group and N-methyltransferase domain M are present in the EB module. Also, a putative condensation (C) domain exists between the EA and EB modules. The M domain is highly conserved among N-methyl peptide synthases of prokaryotic and eukaryotic origin, thus it represents only local sequence similarities to the structural elements of other AdoMet-dependent methyltransferases. A dipeptidol unit is formed due to the interaction between the EA and EB modules and later, it is transferred and condensed into a thiol group. Three such successive condensations of the enzyme-bound dipeptidols are followed by the ring′s closure into the enniatin (ENN) molecule [4,58,59,60,61] (Figure 3A,B).
Figure 3. Mechanism of enniatin B formation according to Hornbogen et al. [4]. (A) Scheme of partial reactions leading to the formation of ENN B, P1, P2, P3 = 4′-phosphopantetheine. (B) Model of the arrangement of catalytic sites in enniatin synthase; Cy: cyclization cavity; EA: D-Hiv-activation module; EB: L-valine-activation module; M: N-methyltransferase domain.
The primary precursors of the ENNs are valine, leucine or isoleucine, D-2-hydroxyisovaleric acid, and S-adenosylmethionine and their synthesis is entirely dependent on the cyclization reaction of linear hexadepsipeptide. The amino acid specificity of ESYN1 contributes to the chemical diversity of ENNs and this is why different types of ENNs are produced by Fusarium scirpi, F. lateritium, and F. sambucinum. The Esyn domains activating L-valine in F. scirpi and preferably activating L-isoleucine in F. sambucinum are nearly identical, with an exception of the three regions showing significant differences in their structures. This difference in the activation can be accredited to the mutations that eventually occurred in the amino acid recognition sites of various enniatin synthases. In spite of the variability in amino acid units, certain ENNs can only be isolated from specific Fusarium strains, in which the enniatin synthase prefers some amino acids over others during biosynthesis [4,53,62,63,64,65].
BEAs are also formed as cyclic trimers assembled from three D-Hiv-N-methyl-L-amino acid dipeptidol monomers (Figure 4A) [50,51]. Similarly, they are also produced by a thiol template mechanism and synthesized by beauvericin synthase (BEAS) enzyme, which consists of a single polypeptide chain of about 351 kD [41,50]. For the first time, the 250 kDa BEAS enzyme was characterized by Peeters et al. [66] from the entomopathogenic fungus Beauveria bassiana, although Xu et al. [50], who conducted a more in-depth analysis, described a 33,475 bp beauvericin gene cluster including a 9570 bp bbBeas gene. Five years later, Zhang and coworkers [51] cloned and characterized 9413 bp beauvericin synthase gene (fpBeas) from Fusarium proliferatum.
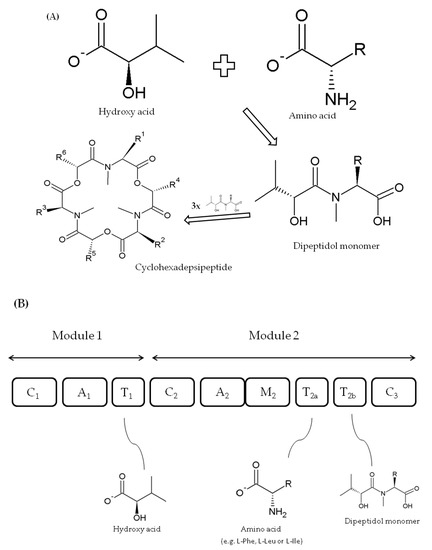
Figure 4. Biosynthesis of fungal cyclodepsipeptides (A) and model of beauvericins (BEAS) synthase structure with domain roles (domains not to scale) (B) according to Xu et al. [50,52].
The C1, A1, and T1 domains within the first module of FpBEAS and ESYN (EA module) synthases have the same role in cyclodepsipeptide formation [51]. Nevertheless, the two depsipeptide synthases differ in A2 domain substrate specificity within module 2 (ESYN EB module), i.e., apart from that of enniatin synthase, beauvericin synthase preferably accepts N-methyl-L-phenylalanine and some other aliphatic hydrophobic amino acids (e.g., leucine or isoleucine) [50]. Furthermore, their incorporation efficiency reduces with the length of side chains, where ortho-, meta-, and para-fluoro-substituted phenylalanine derivatives and N-methyl-L-leucine, N-methyl-L-norleucine, and N-methyl-L-isoleucine residues could replace N-methyl-L-phenylalanine. Domains C2, T2a;b, M2, and C3 within module 2 of BEAS and ESYN play the same role in both synthases (Figure 4B) [50,66].
The depsipeptides, including BEAs, have a common 2-hydroxycarboxylic acid ingredient–D-2-hydroxyisovalerate (D-Hiv) that is formed from 2-ketoisovalerate (2-Kiv) by a highly specific chiral reduction reaction catalyzed by 2-ketoisovalerate reductase (KIVR) enzyme [50,52,67,68,69,70]. KIVR has a significant role in the biosynthesis of BEAs as was clearly understood when BEA production was inhibited in a KIVR knock-out B. bassiana mutant [67]. Kiv is formed from pyruvate during the biosynthesis of valine and it is the key intermediate in several metabolic pathways, including pantothenate biosynthesis in fungi, bacteria, and plants. It is also involved in producing phosphopantetheinyl prosthetic groups of acyl or peptidyl carrier proteins and co-enzyme A (Figure 5) [50,52,67,69,70].
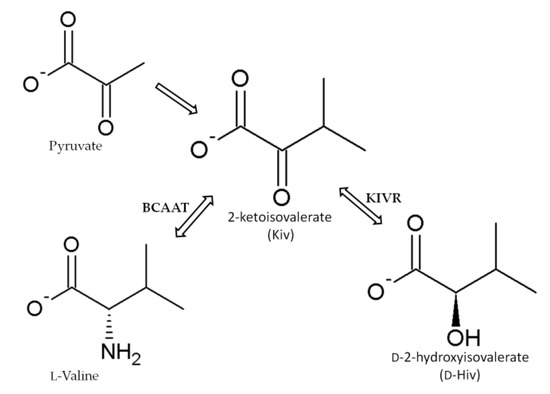
Figure 5. Synthesis of 2-ketoisovalerate (Kiv), a substrate used in the formation of D-2-hydroxyisovaleric acid (D-Hiv) moiety by 2-ketoisovalerate reductase (KIVR) according to Xu et al. [67]. BCAAT: branched-chain amino acid aminotransferase.
Significant sequence homologies were identified for certain Fusarium enzymes, which shows a common genetic background for the synthesis of both depsipeptide compounds. Zhang et al. [51] revealed in their analysis that FpBEAS (GenBank acc. no. JF826561.1) has 64% identity to ESYN (GenBank acc. no. CAA79245) as it was proven that some Fusarium species, like F. poae, F. proliferatum, or F. oxysporum were found to produce ENNs and BEA simultaneously. This is justified by the fact that both toxins share a metabolic pathway [1,44,71,72]. Reports suggest that there is a high probability that the single PCR based esyn1- and/or BEAS- specific marker can detect potential BEAs and ENNs-producing fungi from contaminated soil and plant material [39,55,73].
4. Fusarium Species and Cyclodepsipeptide Mycotoxins in Food and Feed
Plant crops are critical mainly in terms of yield and diverse use for foods and feeds. They suffer from a range of fungal diseases and Fusarium species are among the most damaging pathogens, producing toxic secondary metabolites, such as cyclodepsipeptides. Cyclodepsipeptides biosynthesis has been observed for 44 Fusarium species (Table 2) and F. acuminatum, F. concentricum, F. proliferatum, F. verticillioides, F. oxysporum, and F. tricinctum produce a broad spectrum of ENN, BEA, and BEAE analogues. The remaining Fusarium species formed only individual mycotoxin groups, such as BEA, ENNs, or a mixture of these. However, in a few research papers, it was not specified which Fusarium species produced ENNs and the presence of mycotoxins was described as a “mix of ENNs” (Table 2).
Table 2. Cyclodepsipeptides mycotoxins produced by various Fusarium species.
| Fusarium Species | Compound | References |
|---|---|---|
| F. acuminatum | BEA, ENN A, ENN A1, ENN B, ENN B1, ENN B2, ENN B3, ENN B4, ENN P1, ENN P2, BEA C, BEA D, BEA G1, ALLOBEA C | [2,3,5,21,39,47,78] |
| F. acutatum | BEA, mix of ENNs | [79] |
| F. ananatum | BEA, ENN A, ENN B, ENN B1 | [39] |
| F. anthophilum | BEA, ENN A, ENN B, ENN B1 | [39,78] |
| F. arthrosporioides | mix of ENNs | [15] |
| F. avenaceum | BEA, ENN A, ENN A1, ENN B, ENN B1, ENN B2, ENN B3, ENN B4 | [25,39,78,80,81] |
| F. beomiforme | BEA | [78] |
| F. bulbicola | BEA | [79] |
| F. circinatum | BEA | [79,82] |
| F. concentricum | BEA, ENN A, ENN A1, ENN B, ENN B1, BEA A/F, BEA B, BEA C, BEA D, BEA E, BEA G1, BEA G2, BEA J, BEA K, BEA L, BEAE A, BEAE B, BEAE G1/G2/G3, BEAE L, ALLOBEA A, ALLOBEA B, ALLOBEA C | [2,3,39,79,82] |
| F. compactum | ENN A, ENN A1, ENN B, ENN B1, ENN B2 | [47] |
| F. culmorum | mix of ENNs, ENN B | [83] |
| F. denticulatum | BEA | [79] |
| F. dlamini | BEA, ENN A, ENN A1, ENN B1 | [39,78,79] |
| F. equiseti | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,78] |
| F. fujikuoi | BEA | [79] |
| F. globosum | BEA | [84] |
| F. guttiforme | BEA | [79,82] |
| F. graminearum | ENN A, ENN A1, ENN B, ENN B1 | [85] |
| F. konzum | BEA | [86] |
| F. kyushuense | ENN B, ENN B1 | [87] |
| F. lactis | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,79] |
| F. langsethiae | BEA, ENN A1, ENN B, ENN B1 | [87] |
| F. lateritium | mix of ENNs | [15] |
| F. longipes | BEA | [78] |
| F. merismoides | mix of ENNs | [15] |
| F. nygamai | BEA, ENN A, ENN A1, ENN B | [39,78,79] |
| F. oxysporum | BEA, BEA A/F, BEA B, BEA C, BEA D, BEA E, BEA G1, BEA G2, BEA J, BEAE A, BEAE B, BEAE L, ALLOBEA A, ALLOBEA B, ALLOBEA C, ENN A1, ENN B, ENN B1, ENN H, ENN I, ENN MK1688 | [2,3,39,44,78] |
| F. poae | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,71,78,87] |
| F. phyllophilum | BEA | [79] |
| F. proliferatum | BEA, ENN A1, ENN B, ENN B1, BEA A/F, BEA B, BEA C, BEA D, BEA E, BEA G1, BEA G2, BEA J, BEA K, BEAE A, BEAE B, BEAE L, ALLOBEA A, ALLOBEA B, ALLOBEA C | [2,3,39,84] |
| F. pseudoanthophilum | BEA | [82] |
| F. pseudocircinatum | BEA | [79] |
| F. redolens | BEA | [37] |
| F. sacchari | BEA | [79] |
| F. sambucinum | BEA, mix of ENNs | [15,78] |
| F. scirpi | mix of ENNs | [15] |
| F. semitectum | BEA | [88] |
| F. sporotrichioides | BEA, ENN A, ENN B, ENN B1, ENN A1 | [39,71,87] |
| F. subglutinans | BEA, ENN A, ENN B, ENN B1 | [39,88,89,90] |
| F. succisae | BEA | [79] |
| F. temperatum | BEA, ENN A, ENN A1, ENN B, ENN B1 | [39,90] |
| F. torulosum | ENN B | [91,92] |
| F. tricinctum | BEA, ENN A, ENN A1, ENN B, ENN B1, ENN B4, ENN J1 | [5,36,39,93] |
| F. verticillioides | BEA, ENN B, ENN B1, BEA C, BEA D, BEA G1, BEA K, BEAE A, ALLOBEA C | [2,3,39,94] |
Fusarium species can cause many plant diseases and one of them is Fusarium head blight (FHB), which is devastating for cereal species, particularly as it is a major problem regarding wheat production in many countries. Usually, one or more Fusarium species (F. graminearum, F. culmorum, F. avenaceum, F. poae, and F. sporotrichioides) are involved as causal agents [74]. The occurrence of many Fusarium species may increase the accumulation of mycotoxins in grains or plants and introduce them into the food chain [71,75,76]. Humidity and temperature determine the disease severity, but geographical conditions, plant genotype, and local pathogen populations also play essential roles [54,77].
Available literature data relate both to identifying Fusarium fungi isolated from various hosts and analyzing their mycotoxin biosynthesis capacity (Table 3). Efforts are also being made to assess contamination levels with these toxins of raw plant materials and food and feed products (Table 4). Mainly, the content of BEA and four ENNs (ENN A, ENN A1, ENN B, ENN B1) has been investigated [8,25]. BEA and ENNs are common contaminants and were detected in plant crops and grains throughout the world. The occurrence of BEA, ENN A, ENN A1, ENN B, and ENN B1 in naturally contaminated crops has been studied much more extensively than the occurrence of other cyclodepsipeptides [1,39]. Table 3 summarizes the most effective producers of depsipeptides among Fusarium fungi isolated from different crops and geographical areas. F. avenaceum, F. equiseti, F. proliferatum, and F. sporotrichioides were the most common species isolated from plants. The best producer of BEA was F. proliferatum (FPG61_CM), isolated from garlic in Spain, with the concentration reaching 671.80 μg/g [6]. The highest yielding producers of ENNs were F. avenaceum (KF1330), isolated from wheat in Poland, and F. tricinctum (3405), isolated from wheat in Finland [5,39]. Both strains produced in the highest amounts ENN B (895.46 μg/g, 690 μg/g) and ENN B1 (452.46 μg/g, 1200 μg/g) [5,39].
Table 3. The strains of Fusarium species from different origins and hosts, producing the highest amounts of cyclodepsipeptides [μg/g].
| Species | ID Strain | Host | Origin | ENN A | ENN A1 | ENN B | ENN B1 | ENN B2 | ENN B3 | BEA | Analytical Method | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F. acuminatum | KF 3713 | Pea | Poland | 19.62 | 26.92 | 90.89 | 31.49 | NA | NA | 5.31 | HPLC | [39] |
| F. ananatum | KF 3557 | Pineapple | Costa Rica | 6.94 | ND | 8.81 | 27.60 | NA | NA | 27.68 | HPLC | [39] |
| F. avenaceum | KF 3803 | Asparagus | Poland | ND | ≤0.01 | 0.03 | ND | NA | NA | ND | HPLC | [39] |
| 11B14 | Barley | Italy | 10.9 | 193 | 45 | 172 | 55 | 1.58 | NA | LC-MS/MS | [104] | |
| KF 3717 | Pea | Poland | 6.09 | 5.65 | 6.71 | 11.46 | NA | NA | ND | HPLC | [39] | |
| Fa40 | Wheat | Italy | 165.8 | 109.2 | 35.5 | 60.2 | NA | NA | ND | LC-DAD | [71] | |
| KF 1337 | Wheat | Poland | 34.55 | 71.90 | 895.46 | 452.46 | NA | NA | ND | HPLC | [39] | |
| 44 | Wheat | Italy | 7.24 | 34.3 | 6.6 | 17.8 | 0.67 | ≤0.01 | ≤0.01 | LC-MS/MS | [105] | |
| Fa34 | Wheat | Italy | 332.8 | 181.7 | 64.9 | 101.9 | NA | NA | ND | LC-DAD | [71] | |
| KF 3390 | Maize | Poland | 29.12 | 32.40 | 255.08 | 138.15 | NA | NA | ND | HPLC | [39] | |
| F. concentricum | KF 3755 | Pineapple | Costa Rica | 11.40 | 8.69 | 17.33 | 18.17 | NA | NA | 312.2 | HPLC | [39] |
| F. culmorum | KF 3798 | Asparagus | Poland | ND | ND | 0.06 | ND | NA | NA | ND | HPLC | [39] |
| F. equiseti | KF 3563 | Asparagus | Poland | 43.47 | 36.81 | 29.18 | 30.39 | NA | NA | ND | HPLC | [39] |
| KF 3749 | Tomato | Poland | 39.27 | 38.18 | ND | 29.22 | NA | NA | ND | HPLC | [39] | |
| KF 3430 | Banana | Ecuador | 31.17 | 32.15 | 32.98 | 41.22 | NA | NA | ND | HPLC | [39] | |
| Feq16 | Wheat | Italy | ND | ≤0.01 | ≤0.01 | ≤0.01 | NA | NA | ≤0.01 | LC-DAD | [71] | |
| Feq136 | Wheat | Italy | ≤0.01 | 0.02 | ≤0.01 | 0.02 | NA | NA | ND | LC-DAD | [71] | |
| F. fujikuroi | KF 3631 | Rice | Thailand | ND | ND | ND | ND | NA | NA | 428.09 | HPLC | [39] |
| F. globosum | 6646 | Maize | South Africa | NA | NA | NA | NA | NA | NA | 110 | LC-MS | [84] |
| F. lactis | KF 3641 | Pepper | Poland | 30.97 | 26.94 | ND | ND | NA | NA | ND | HPLC | [39] |
| F. nygamai | KF 337 | Pigeon Pea | India | 10.45 | ND | 9.50 | ND | NA | NA | 22.86 | HPLC | [39] |
| F. oxysporum | KF 3567 | Garlic | Poland | ND | 6.42 | 8.25 | 7.28 | NA | NA | 80.03 | HPLC | [39] |
| KF 3805 | Asparagus | Poland | ND | ND | ND | ND | NA | NA | 0.53 | HPLC | [39] | |
| F. poae | Fp26 | Wheat | Italy | ≤0.01 | 0.07 | 0.03 | 0.05 | NA | NA | 3.5 | LC-DAD | [71] |
| 156 | Wheat | Italy | ≤0.01 | 0.03 | 0.03 | ND | ND | ND | 10.5 | LC-MS/MS | [105] | |
| Fp49 | Wheat | Italy | ≤0.01 | 0.1 | 0.05 | 0.04 | NA | NA | 9.4 | LC-DAD | [71] | |
| KF 2576 | Maize | Poland | 34.31 | 26.89 | 28.71 | ND | NA | NA | 37.53 | HPLC | [39] | |
| F. proliferatum | KF 3382 | Pineapple | Hawaii | ND | ND | ND | ND | NA | NA | 3.39 | HPLC | [39] |
| FPG61_CM | Garlic | Spain | NA | NA | NA | NA | NA | NA | 671.80 | HPLC | [6] | |
| KF 3363 | Garlic | Poland | ND | ND | ND | ND | NA | NA | 45.13 | HPLC | [39] | |
| KF 3792 | Asparagus | Poland | ND | 0.39 | 0.13 | 0.06 | NA | NA | 0.41 | HPLC | [39] | |
| KF 3584 | Rice | Thailand | ND | 6.39 | 12.92 | 19.64 | NA | NA | 291.87 | HPLC | [39] | |
| KF 3560 | Rhubarb | Poland | ND | ND | ND | ND | NA | NA | 149.67 | HPLC | [39] | |
| KF 496 | Maize | Italy | ND | 5.48 | 9.61 | 12.89 | NA | NA | ND | HPLC | [39] | |
| F. sambucinum | 179 | Wheat | Italy | ND | ND | ND | ND | ND | ND | 10.1 | LC-MS/MS | [105] |
| F. subglutinans | 1084 | Maize | South Africa | NA | NA | NA | NA | NA | NA | 700 | LC-MS | [84] |
| F. sporotrichioides | KF 3815 | Asparagus | Poland | ND | 0.09 | ND | ND | NA | NA | 0.21 | HPLC | [39] |
| KF 3728 | Pea | Poland | 12.67 | ND | 5.99 | 18.15 | NA | NA | 5.13 | HPLC | [39] | |
| Fsp50 | Wheat | Italy | ND | ≤0.01 | ≤0.01 | 0.02 | NA | NA | 13.7 | LC-DAD | [71] | |
| 194 | Wheat | Italy | ND | ND | ND | ND | ND | ND | 6.89 | LC-MS/MS | [105] | |
| F. temperatum | KF 3321 | Pineapple | Costa Rica | 27.79 | 34.39 | 39.20 | 29.21 | NA | NA | 290.97 | HPLC | [39] |
| RCFT 934 | Maize | Argentina | NA | NA | NA | NA | NA | NA | 1151 | HPLC | [106] | |
| KF 506 | Maize | Poland | ND | ND | 15.17 | 9.88 | NA | NA | 17.47 | HPLC | [39] | |
| F. tricinctum | KF 3795 | Asparagus | Poland | 0.1 | 0.17 | 0.28 | 0.38 | NA | NA | 0.55 | HPLC | [39] |
| 27B14 | Malting barley | Italy | 8.45 | 118 | 39 | 124 | 27 | 0.13 | NA | LC-MS/MS | [104] | |
| 3405 | Wheat | Finland | NA | 94 | 690 | 1200 | NA | NA | 33 | HPLC | [5] | |
| F. verticillioides | KF 393 | Maize | USA | ND | ND | 8.75 | 12.43 | NA | NA | 2.34 | HPLC | [39] |
“ND”—not detected; “NA”—not analyzed.
Table 4. Maximum levels [μg/g] of naturally occurring depsipeptides in foods and feeds from different countries.
| Sample | Origin | ENN A | ENN A1 | ENN B | ENN B1 | ENN B4 | BEA | Reference |
|---|---|---|---|---|---|---|---|---|
| Asparagus | Poland | ND | 0.05 | 0.06 | ND | NA | 0.1 | [8] |
| Barley | Italy | ND | ND | ND | ≤0.01 | 0.02 | ≤0.01 | [100] |
| Italy | 0.02 | 0.06 | 0.07 | 0.07 | NA | ≤0.01 | [104] | |
| Finland | 0.95 | 2 | 9.76 | 5.72 | NA | 0.02 | [1] | |
| Morocco | ND | 220 | 49 | 32 | NA | 5 | [107] | |
| Norway | ≤0.01 | 0.04 | 0.49 | 0.17 | NA | ≤0.01 | [108] | |
| Spain | ND | 361.57 | 21.37 | 45.94 | NA | 6.94 | [97] | |
| Tunisia | 33.6 | 149 | 29.2 | 31 | NA | NA | [96] | |
| Maize | Brazil | ≤0.01 | 0.31 | ≤0.01 | ≤0.01 | NA | 0.16 | [109] |
| Croatia | NA | NA | NA | NA | NA | 1.84 | [110] | |
| Denmark | ≤0.01 | ≤0.01 | 0.58 | 0.09 | NA | 0.09 | [111] | |
| Japan | NA | NA | NA | NA | NA | 0.03 | [112] | |
| Morocco | ND | 445 | 100 | 8 | NA | 59 | [107] | |
| Poland | NA | NA | NA | NA | NA | 1.73 | [95] | |
| Serbia | 0.02 | 0.03 | ≤0.01 | 0.02 | NA | 0.14 | [7] | |
| Slovakia | NA | NA | NA | NA | NA | 3 | [113] | |
| Spain | ND | 813.01 | 6.31 | 4.34 | NA | 9.31 | [97] | |
| Tunisia | ND | 29.6 | ND | 17 | NA | NA | [96] | |
| USA | NA | NA | NA | NA | NA | 0.5 | [114] | |
| Oats | Finland | ≤0.01 | ≤0.01 | 0.02 | ≤0.01 | NA | 0.02 | [1] |
| Italy | ND | ≤0.01 | ≤0.01 | ND | 0.05 | ≤0.01 | [100] | |
| Norway | ≤0.01 | ≤0.01 | 0.05 | 0.02 | NA | 0.02 | [108] | |
| Rice | Iran | ND | ≤0.01 | ND | ND | ND | ≤0.01 | [115] |
| Spain | ND | 814.42 | 7.95 | ND | NA | 11.78 | [97] | |
| Rye | Finland | ND | ≤0.01 | 0.05 | ≤0.01 | NA | ND | [1] |
| Italy | ≤0.01 | ND | ≤0.01 | ND | ≤0.01 | ≤0.01 | [100] | |
| Sorghum | Tunisia | 95.6 | 480 | ND | 120.1 | NA | NA | [96] |
| Spelt wheat | Italy | ≤0.01 | ND | ND | ND | ND | ND | [100] |
| Wheat | Finland | 0.49 | 0.94 | 18.3 | 5.1 | NA | ≤0.01 | [1] |
| Italy | ≤0.01 | ≤0.01 | 0.02 | ≤0.01 | 0.04 | ≤0.01 | [100] | |
| Morocco | 0.08 | 0.13 | 2.57 | 0.35 | NA | 0.02 | [116] | |
| Morocco | 34 | 209 | 11 | 19 | NA | 4 | [107] | |
| Norway | ≤0.01 | 0.02 | 0.79 | 0.18 | NA | ≤0.01 | [108] | |
| Poland | 0.27 | 3.6 | 28.52 | 11.8 | NA | 0.02 | [57] | |
| Romania | 0.14 | 0.36 | 0.41 | 0.51 | NA | NA | [117] | |
| Spain | ND | 634.85 | ND | ND | NA | 3.5 | [97] | |
| Tunisia | 75.1 | 177.7 | 180.6 | 58.5 | NA | NA | [96] | |
| UK | 0.04 | 0.17 | 0.13 | 0.30 | NA | NA | [85] | |
| Breakfast cereals | Morocco | 29.7 | 688 | 81.1 | 795 | NA | 5.3 | [99] |
| Spain | ND | 268.54 | ND | ND | NA | 3.12 | [97] | |
| Tunisia | 121.3 | 480 | 295 | 120.1 | NA | NA | [96] | |
| Infant cereals | Morocco | ND | 52 | 5.7 | 14.5 | NA | 10.6 | [99] |
| Pasta | Italy | ≤0.01 | ≤0.01 | 0.11 | ≤0.01 | ≤0.01 | ND | [100] |
| Oat flour | Spain | ND | 388.38 | ND | ND | NA | 4.18 | [97] |
| Wheat flour | Japan | ≤0.01 | 0.03 | 0.63 | 0.09 | NA | ≤0.01 | [112] |
| Corn grits | Japan | ND | ND | ND | ND | NA | 0.03 | [112] |
| Bovine feed | Spain | ND | ≤0.01 | 0.04 | 0.02 | NA | 0.05 | [98] |
| Ovine feed | Spain | ND | ≤0.01 | 0.09 | 0.03 | NA | 0.13 | [98] |
| Caprine feed | Spain | ND | ≤0.01 | 0.02 | ≤0.01 | NA | 0.02 | [98] |
| Horses feed | Spain | ND | ≤0.01 | 0.04 | ≤0.01 | NA | 0.03 | [98] |
| Porcine feed | Finland | 0.31 | 0.55 | 1.51 | 1.85 | NA | 0.41 | [102] |
| Spain | ND | ≤0.01 | 0.06 | 0.02 | NA | ≤0.01 | [98] | |
| Poultry feed | Brazil | ND | ≤0.01 | ≤0.01 | ≤0.01 | NA | 0.02 | [109] |
| Spain | ND | ≤0.01 | 0.05 | 0.02 | NA | 0.02 | [98] | |
| UK | 0.04 | 0.03 | 2.19 | 0.40 | NA | 0.48 | [101] | |
| Rabbits feed | Spain | ND | ≤0.01 | 0.05 | 0.02 | NA | ≤0.01 | [98] |
| Dogs feed | Spain | ND | ≤0.01 | 0.02 | ≤0.01 | NA | 0.04 | [98] |
| Cats feed | Spain | ND | ND | ≤0.01 | ≤0.01 | NA | ND | [98] |
| Fish feed | Scotland/Norway/ Spain | ≤0.01 | ≤0.01 | 0.03 | ≤0.01 | NA | 0.08 | [103] |
“ND”—not detected; “NA”—not analyzed.
Table 4 presents the maximum amounts of BEA and ENNs in naturally contaminated plant crops described in the literature. The highest contamination level of BEA was found to be 1731.55 μg/g in Polish maize [95]. When compared to other cyclodepsipeptides, it was also the highest concentration of mycotoxin in crops. In Tunisian sorghum, maximum concentrations of ENN A (95.6 μg/g) and ENN B1 (120.1 μg/g) were detected [96]. The highest amount of ENN A1 was 813.01 μg/g and 814.42 μg/g in Spanish maize and rice, respectively [97]. ENN B was found with a maximum level of 180.6 μg/g in Tunisian wheat [96]. The data show very high variability of investigated cyclodepsipeptides and it can be concluded that each strain of Fusarium species possesses a unique ability to biosynthesize these compounds. In addition to crops, cyclodepsipeptides are also found in food and feed [98,99,100,101,102,103]. Cyclodepsipeptides were identified mainly in cereal food, with very high levels of ENN A1 and B1 in breakfast cereals from Morocco (668 and 795 μg/g, respectively) [99]. In feed samples, ENNs and BEA levels were very low and did not exceed 0.48 μg/g for BEA (poultry feed) and 2.19 μg/g for ENNs (poultry feed) [101].
References
- Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K. Presence and Concentrations of the Fusarium-Related Mycotoxins Beauvericin, Enniatins and Moniliformin in Finnish Grain Samples. Food Addit. Contam. 2004, 21, 794–802, doi:10.1080/02652030410001713906.
- Urbaniak, M.; Stepien, L.; Uhlig, S. Evidence for Naturally Produced Beauvericins Containing N-Methyl-Tyrosine in Hypocreales Toxins 2019, 11, 182, doi:10.3390/toxins11030182.
- Urbaniak, M.; Waskiewicz, A.; Trzebny, A.; Koczyk, G.; Stepien, L. Cyclodepsipeptide Biosynthesis in Hypocreales Fungi and Sequence Divergence of the Non-Ribosomal Peptide Synthase Genes. Pathogens 2020, 9, 552, doi:10.3390/pathogens9070552.
- Hornbogen, T.; Glinski, M.; Zocher, R. Biosynthesis of Depsipeptide Mycotoxins in Fusarium. J. Plant Pathol. 2002, 108, 713–718, doi:10.1023/A:1020687231810.
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of Beauvericin and Enniatins in Wheat Affected by Fusarium avenaceum Head Blight. Environ. Microbiol. 2002, 68, 82–85, doi:10.1128/AEM.68.1.82-85.2002.
- Galvez, L.; Urbaniak, M.; Waskiewicz, A.; Stepien, L.; Palmero, D. Fusarium proliferatum—Causal Agent of Garlic Bulb Rot in Spain: Genetic Variability and Mycotoxin Production. Food Microbiol. 2017, 67, 41–48, doi:10.1016/j.fm.2017.05.006.
- Jajic, I.; Dudas, T.; Krstovic, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savic, Z.; Guljas, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357, doi:10.3390/toxins11060357.
- Stepien, L.; Waskiewicz, A.; Urbaniak, M. Wildly Growing Asparagus (Asparagus officinalis) Hosts Pathogenic Fusarium Species and Accumulates Their Mycotoxins. Microb. Ecol. 2016, 71, 927–937, doi:10.1007/s00248-015-0717-1.
- Tomczyk, L.; Stepien, L.; Urbaniak, M.; Szablewski, T.; Cegielska-Radziejewska, R.; Stuper-Szablewska, K. Characterisation of the Mycobiota on the Shell Surface of Table Eggs Acquired from Different Egg-Laying Hen Breeding Systems. Toxins 2018, 10, 293, doi:10.3390/toxins10070293.
- Jestoi, M. Emerging Fusarium-Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin: A Review. Rev. Food Sci. Nutr. 2008, 48, 21–49, doi:10.1080/10408390601062021.
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium Molds and Mycotoxins: Potential Species-Specific Effects. Toxins 2018, 10, 244, doi:10.3390/toxins10060244.
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins 2017, 9, 228, doi:10.3390/toxins9070228.
- Belen Serrano, A.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Ventura, S.; Lagana, A. Development of a Rapid LC-MS/MS Method for the Determination of Emerging Fusarium Mycotoxins Enniatins and Beauvericin in Human Biological Fluids. Toxins 2015, 7, 3554–3571, doi:10.3390/toxins7093554.
- Perincherry, L.; Lalak-Kanczugowska, J.; Stepien, L. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664, doi:10.3390/toxins11110664.
- Herrmann, M.; Zocher, R.; Haese, A. Enniatin Production by Fusarium Strains and Its Effect on Potato Tuber Tissue. Environ. Microbiol. 1996, 62, 393–398, doi:10.1128/AEM.62.2.393-398.1996.
- Huang, C.H.; Wang, F.T.; Chan, W.H. Enniatin B Induces Dosage-Related Apoptosis or Necrosis in Mouse Blastocysts Leading to Deleterious Effects on Embryo Development. Drug Chem. Toxicol. 2020, 1–12, doi:10.1080/01480545.2020.1838537.
- Zuzek, M.C.; Grandic, M.; Jakovac Strajn, B.; Frangez, R. Beauvericin Inhibits Neuromuscular Transmission and Skeletal Muscle Contractility in Mouse Hemidiaphragm Preparation. Sci. 2016, 150, 283–291, doi:10.1093/toxsci/kfv326.
- Schoevers, E.J.; Santos, R.R.; Fink-Gremmels, J.; Roelen, B.A. Toxicity of Beauvericin on Porcine Oocyte Maturation and Preimplantation Embryo Development. Toxicol. 2016, 65, 159–169, doi:10.1016/j.reprotox.2016.07.017.
- Albonico, M.; Schutz, L.F.; Caloni, F.; Cortinovis, C.; Spicer, L.J. In Vitro Effects of the Fusarium Mycotoxins Fumonisin B1 and Beauvericin on Bovine Granulosa Cell Proliferation and Steroid Production. Toxicon 2017, 128, 38–45, doi:10.1016/j.toxicon.2017.01.019.
- Kalayou, S.; Ndossi, D.; Frizzell, C.; Groseth, P.K.; Connolly, L.; Sorlie, M.; Verhaegen, S.; Ropstad, E. An Investigation of the Endocrine Disrupting Potential of Enniatin B Using in Vitro Bioassays. Lett. 2015, 233, 84–94, doi:10.1016/j.toxlet.2015.01.014.
- Uhlig, S.; Ivanova, L.; Petersen, D.; Kristensen, R. Structural Studies on Minor Enniatins from Fusarium VI 03441: Novel N-Methyl-Threonine Containing Enniatins. Toxicon 2009, 53, 734–742, doi:10.1016/j.toxicon.2009.02.014.
- Jow, G.M.; Chou, C.J.; Chen, B.F.; Tsai, J.H. Beauvericin Induces Cytotoxic Effects in Human Acute Lymphoblastic Leukemia Cells through Cytochrome C Release, Caspase 3 Activation: The Causative Role of Calcium. Cancer Lett. 2004, 216, 165–173, doi:10.1016/j.canlet.2004.06.005.
- Chen, B.F.; Tsai, M.C.; Jow, G.M. Induction of Calcium Influx from Extracellular Fluid by Beauvericin in Human Leukemia Cells. Biophys Res. Commun. 2006, 340, 134–139, doi:10.1016/j.bbrc.2005.11.166.
- Watjen, W.; Debbab, A.; Hohlfeld, A.; Chovolou, Y.; Kampkotter, A.; Edrada, R.A.; Ebel, R.; Hakiki, A.; Mosaddak, M.; Totzke, F.; et al. Enniatins A1, B and B1 from an Endophytic Strain of Fusarium tricinctum Induce Apoptotic Cell Death in H4IIE Hepatoma Cells Accompanied by Inhibition of ERK Phosphorylation. Nutr. Food Res. 2009, 53, 431–440, doi:10.1002/mnfr.200700428.
- Ivanova, L.; Skjerve, E.; Eriksen, G.S.; Uhlig, S. Cytotoxicity of Enniatins A, A1, B, B1, B2 and B3 from Fusarium avenaceum. Toxicon 2006, 47, 868–876, doi:10.1016/j.toxicon.2006.02.012.
- Isaka, M.; Yangchum, A.; Sappan, M.; Suvannakad, R.; Srikitikulchai, P. Cyclohexadepsipeptides from Acremonium Sp Bcc 28424. Tetrahedron 2011, 67, 7929–7935, doi:10.1016/j.tet.2011.08.041.
- Hyun, U.; Lee, D.-H.; Lee, C.; Shin, C.-G. Apoptosis induced by enniatins H and MK1688 isolated from Fusarium oxysporum Toxicon 2009, 53, 723–728, doi:10.1016/j.toxicon.2009.02.01.
- Kamyar, M.; Rawnduzi, P.; Studenik, C.R.; Kouri, K.; Lemmens-Gruber, R. Investigation of the Electrophysiological Properties of Enniatins. Biochem. Biophys 2004, 429, 215–223, doi:10.1016/j.abb.2004.06.013.
- Zhang, L.X.; Yan, K.Z.; Zhang, Y.; Huang, R.; Bian, J.; Zheng, C.S.; Sun, H.X.; Chen, Z.H.; Sun, N.; An, R.; et al. High-Throughput Synergy Screening Identifies Microbial Metabolites as Combination Agents for the Treatment of Fungal Infections. Natl. Acad. Sci. USA 2007, 104, 4606–4611, doi:10.1073/pnas.0609370104.
- Bunyapaiboonsri, T.; Vongvilai, P.; Auncharoen, P.; Isaka, M. Cyclohexadepsipeptides from the Filamentous Fungus Acremonium Bcc 2629. Helv. Chim. Acta. 2012, 95, 963–972, doi:10.1002/hlca.201100482.
- Xu, Y.; Zhan, J.; Wijeratne, E.M.K.; Burns, A.M.; Gunatilaka, A.A.L.; Molnar, I. Cytotoxic and Antihaptotactic Beauvericin Analogues from Precursor-Directed Biosynthesis with the Insect Pathogen Beauveria bassiana ATCC 7159. Nat. Prod. 2007, 70, 1467–1471, doi:10.1021/np070262f.
- Dornetshuber, R.; Heffeter, P.; Kamyar, M.R.; Peterbauer, T.; Berger, W.; Lemmens-Gruber, R. Enniatin Exerts P53-Dependent Cytostatic and P53-Independent Cytotoxic Activities against Human Cancer Cells. Res. Toxicol. 2007, 20, 465–473, doi:10.1021/tx600259t.
- Nilanonta, C.; Isaka, M.; Kittakoop, P.; Trakulnaleamsai, S.; Tanticharoen, M.; Thebtaranonth, Y. Precursor-Directed Biosynthesis of Beauvericin Analogs by the Insect Pathogenic Fungus Paecilomyces tenuipes BCC 1614. Tetrahedron 2002, 58, 3355–3360, doi:10.1016/S0040-4020(02)00294-6.
- Fukuda, T.; Arai, M.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Omura, S. New Beauvericins, Potentiators of Antifungal Miconazole Activity, Produced by Beauveria FKI-1366—I. Taxonomy, Fermentation, Isolation and Biological Properties. J. Antibiot. 2004, 57, 110–116, doi:10.7164/antibiotics.57.110.
- Supothina, S.; Isaka, M.; Kirtikara, K.; Tanticharoen, M.; Thebtaranonth, Y. Enniatin Production by the Entomopathogenic Fungus Verticillium hemipterigenum BCC 1449. Antibiot. 2004, 57, 732–738, doi:10.7164/antibiotics.57.732.
- Meca, G.; Sospedra, I.; Soriano, J.M.; Ritieni, A.; Moretti, A.; Manes, J. Antibacterial Effect of the Bioactive Compound Beauvericin Produced by Fusarium proliferatum on Solid Medium of Wheat. Toxicon 2010, 56, 349–354, doi:10.1016/j.toxicon.2010.03.022.
- Xu, L.; Wang, J.; Zhao, J.; Li, P.; Shan, T.; Wang, J.; Li, X.; Zhou, L. Beauvericin from the Endophytic Fungus, Fusarium redolens, Isolated from Dioscorea zingiberensis and Its Antibacterial Activity. Prod. Commun. 2010, 5, 811–814, doi:10.1177/1934578X1000500527.
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. The Structure of Beauvericin, a New Depsipeptide Antibiotic Toxic to Artemia salina. Tetrahedron Lett. 1969, 10, 4255–4258, doi:10.1016/S0040-4039(01)88668-8.
- Stepien, L.; Waskiewicz, A. Sequence Divergence of the Enniatin Synthase Gene in Relation to Production of Beauvericin and Enniatins in Fusarium Toxins 2013, 5, 537–555, doi:10.3390/toxins5030537.
- Wang, Q.; Xu, L. Beauvericin, a Bioactive Compound Produced by Fungi: A Short Review. Molecules 2012, 17, 2367–2377, doi:10.3390/molecules17032367.
- Sivanathan, S.; Scherkenbeck, J. Cyclodepsipeptides: A Rich Source of Biologically Active Compounds for Drug Research. Molecules 2014, 19, 12368–12420, doi:10.3390/molecules190812368.
- Fukuda, T.; Arai, M.; Tomoda, H.; Omura, S. New Beauvericins, Potentiators of Antifungal Miconazole Activity, Produced by Beauveria FKI-1366—II. Structure Elucidation. J. Antibiot. 2004, 57, 117–124, doi:10.7164/antibiotics.57.117.
- Pohanka, A.; Capieau, K.; Broberg, A.; Stenlid, J.; Stenstrom, E.; Kenne, L. Enniatins of Fusarium Strain F31 and Their Inhibition of Botrytis cinerea Spore Germination. J. Nat. Prod. 2004, 67, 851–857, doi:10.1021/np0340448.
- Song, H.H.; Lee, H.S.; Jeong, J.H.; Park, H.S.; Lee, C. Diversity in Beauvericin and Enniatins H, I, and Mk1688 by Fusarium oxysporum Isolated from Potato. J. Food Microbiol. 2008, 122, 296–301, doi:10.1016/j.ijfoodmicro.2008.01.009.
- Tomoda, H.; Nishida, H.; Huang, X.H.; Masuma, R.; Kim, Y.K.; Omura, S. New Cyclodepsipeptides, Enniatins D, E and F Produced by Fusarium FO-1305. J. Antibiot. 1992, 45, 1207–1215, doi:10.7164/antibiotics.45.1207.
- Blais, L.A.; ApSimon, J.W. Isolation and Characterization of Enniatins from Fusarium avenaceum DAOM 196490. J. Chem. 1992, 70, 1281–1287, doi:10.1139/v92-165.
- Visconti, A.; Blais, L.A.; ApSimon, J.W.; Greenhalgh, R.; Miller, J.D. Production of Enniatins by Fusarium acuminatum and Fusarium compactum in Liquid Culture: Isolation and Characterization of Three New Enniatins, B2, B3, and B4. Agric. Food Chem. 1992, 40, 1076–1082, doi:10.1021/jf00018a034.
- Bushley, K.E.; Turgeon, B.G. Phylogenomics Reveals Subfamilies of Fungal Nonribosomal Peptide Synthetases and Their Evolutionary Relationships. BMC Evol. Biol. 2010, 10, 26, doi:10.1186/1471-2148-10-26.
- Gallo, A.; Ferrara, M.; Perrone, G. Phylogenetic Study of Polyketide Synthases and Nonribosomal Peptide Synthetases Involved in the Biosynthesis of Mycotoxins. Toxins 2013, 5, 717–742, doi:10.3390/toxins5040717.
- Xu, Y.; Orozco, R.; Wijeratne, E.M.; Gunatilaka, A.A.; Stock, S.P.; Molnar, I. Biosynthesis of the Cyclooligomer Depsipeptide Beauvericin, a Virulence Factor of the Entomopathogenic Fungus Beauveria bassiana. Biol. 2008, 15, 898–907, doi:10.1016/j.chembiol.2008.07.011.
- Zhang, T.; Zhuo, Y.; Jia, X.; Liu, J.; Gao, H.; Song, F.; Liu, M.; Zhang, L. Cloning and Characterization of the Gene Cluster Required for Beauvericin Biosynthesis in Fusarium proliferatum. China Life Sci. 2013, 56, 628–637, doi:10.1007/s11427-013-4505-1.
- Xu, Y.; Orozco, R.; Kithsiri Wijeratne, E.M.; Espinosa-Artiles, P.; Leslie Gunatilaka, A.A.; Patricia Stock, S.; Molnar, I. Biosynthesis of the Cyclooligomer Depsipeptide Bassianolide, an Insecticidal Virulence Factor of Beauveria bassiana. Fungal Genet. Biol. 2009, 46, 353–364, doi:10.1016/j.fgb.2009.03.001.
- Zocher, R.; Keller, U.; Kleinkauf, H. Enniatin Synthetase, a Novel Type of Multifunctional Enzyme Catalyzing Depsipeptide Synthesis in Fusarium oxysporum. Biochemistry 1982, 21, 43–48, doi:10.1021/bi00530a008.
- Liuzzi, V.C.; Mirabelli, V.; Cimmarusti, M.T.; Haidukowski, M.; Leslie, J.F.; Logrieco, A.F.; Caliandro, R.; Fanelli, F.; Mule, G. Enniatin and Beauvericin Biosynthesis in Fusarium Species: Production Profiles and Structural Determinant Prediction. Toxins 2017, 9, 45, doi:10.3390/toxins9020045.
- Kulik, T.; Pszczolkowska, A.; Fordonski, G.; Olszewski, J. Pcr Approach Based on the Esyn1 Gene for the Detection of Potential Enniatin-Producing Fusarium Int. J. Food Microbiol. 2007, 116, 319–324, doi:10.1016/j.ijfoodmicro.2007.02.003.
- Nicholson, P.; Simpson, D.; Wilson, A.; Chandler, E.; Thomsett, M. Detection and Differentiation of Trichothecene and Enniatin-Producing Fusarium Species on Small-Grain Cereals. J. Plant Pathol. 2004, 110, 503–514, doi:10.1023/B:EJPP.0000032390.65641.a7.
- Stepien, L.; Jestoi, M.; Chelkowski, J. Cyclic Hexadepsipeptides in Wheat Field Samples and Esyn1 Gene Divergence among Enniatin Producing Fusarium avenaceum World Mycotoxin J. 2013, 6, 399–409, doi:10.3920/WMJ2012.1464.
- Hornbogen, T.; Riechers, S.P.; Prinz, B.; Schultchen, J.; Lang, C.; Schmidt, S.; Mugge, C.; Turkanovic, S.; Sussmuth, R.D.; Tauberger, E.; et al. Functional Characterization of the Recombinant N-Methyltransferase Domain from the Multienzyme Enniatin Synthetase. Chembiochem 2007, 8, 1048–1054, doi:10.1002/cbic.200700076.
- Zocher, R.; Keller, U.; Kleinkauf, H. Mechanism of Depsipeptide Formation Catalyzed by Enniatin Synthetase. Biophys. Res. Commun. 1983, 110, 292–299, doi:10.1016/0006-291x(83)91294-9.
- Zocher, R.; Keller, U. Thiol Template Peptide Synthesis Systems in Bacteria and Fungi. Microb. Physiol. 1997, 38, 85–131, doi:10.1016/s0065-2911(08)60156-3.
- Feifel, S.C.; Schmiederer, T.; Hornbogen, T.; Berg, H.; Sussmuth, R.D.; Zocher, R. In Vitro Synthesis of New Enniatins: Probing the Alpha-D-Hydroxy Carboxylic Acid Binding Pocket of the Multienzyme Enniatin Synthetase. Chembiochem 2007, 8, 1767–1770, doi:10.1002/cbic.200700377.
- Pieper, R.; Kleinkauf, H.; Zocher, R. Enniatin Synthetases from Different Fusaria Exhibiting Distinct Amino Acid Specificities. Antibiot. 1992, 45, 1273–1277, doi:10.7164/antibiotics.45.1273.
- Krause, M.; Lindemann, A.; Glinski, M.; Hornbogen, T.; Bonse, G.; Jeschke, P.; Thielking, G.; Gau, W.; Kleinkauf, H.; Zocher, R. Directed Biosynthesis of New Enniatins. Antibiot. 2001, 54, 797–804, doi:10.7164/antibiotics.54.797.
- Billich, A.; Zocher, R. Constitutive Expression of Enniatin Synthetase During Fermentative Growth of Fusarium scirpi. Environ. Microbiol. 1988, 54, 2504–2509, doi:10.1128/AEM.54.10.2504-2509.1988.
- Haese, A.; Schubert, M.; Herrmann, M.; Zocher, R. Molecular Characterization of the Enniatin Synthetase Gene Encoding a Multifunctional Enzyme Catalysing N-Methyldepsipeptide Formation in Fusarium scirpi. Microbiol. 1993, 7, 905–914, doi:10.1111/j.1365-2958.1993.tb01181.x.
- Peeters, H.; Zocher, R.; Kleinkauf, H. Synthesis of Beauvericin by a Multifunctional Enzyme. Antibiot. 1988, 41, 352–359, doi:10.7164/antibiotics.41.352.
- Xu, Y.; Wijeratne, E.M.; Espinosa-Artiles, P.; Gunatilaka, A.A.; Molnar, I. Combinatorial Mutasynthesis of Scrambled Beauvericins, Cyclooligomer Depsipeptide Cell Migration Inhibitors from Beauveria bassiana. Chembiochem 2009, 10, 345–354, doi:10.1002/cbic.200800570.
- Heider, J.; Mai, X.; Adams, M.W. Characterization of 2-Ketoisovalerate Ferredoxin Oxidoreductase, a New and Reversible Coenzyme a-Dependent Enzyme Involved in Peptide Fermentation by Hyperthermophilic Archaea. Bacteriol. 1996, 178, 780–787, doi:10.1128/jb.178.3.780-787.1996.
- Kim, J.; Yoon, D.H.; Oh, J.; Hyun, M.W.; Han, J.G.; Sung, G.H. Calmodulin-Mediated Suppression of 2-Ketoisovalerate Reductase in Beauveria bassiana Beauvericin Biosynthetic Pathway. Microbiol. 2016, 18, 4136–4143, doi:10.1111/1462-2920.13461.
- Zhang, T.; Jia, X.; Zhuo, Y.; Liu, M.; Gao, H.; Liu, J.; Zhang, L. Cloning and Characterization of a Novel 2-Ketoisovalerate Reductase from the Beauvericin Producer Fusarium proliferatum BMC Biotechnol. 2012, 12, 55, doi:10.1186/1472-6750-12-55.
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Meca, G.; Juan, C.; Manes, J. Biosynthesis of Beauvericin and Enniatins in Vitro by Wheat Fusarium Species and Natural Grain Contamination in an Area of Central Italy. Food Microbiol. 2015, 46, 618–626, doi:10.1016/j.fm.2014.09.009.
- Chełkowski, J.; Ritieni, A.; Wiśniewska, H.; Mulè, G.; Logrieco, A. Occurrence of Toxic Hexadepsipeptides in Preharvest Maize Ear Rot Infected by Fusarium poae in Poland. Phytopathol. 2006, 155, 8–12, doi:10.1111/j.1439-0434.2006.01173.x.
- Kulik, T.; Pszczolkowska, A.; Lojko, M. Multilocus Phylogenetics Show High Intraspecific Variability within Fusarium avenaceum. J. Mol. Sci. 2011, 12, 5626–5640, doi:10.3390/ijms12095626.
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waskiewicz, A.; Stepien, L. Fusarium Head Blight Incidence and Mycotoxin Accumulation in Three Durum Wheat Cultivars in Relation to Sowing Date and Density. Sci Nat-Heidelberg.2018, 105, doi:10.1007/s00114-017-1528-7.
- Arie, T. Fusarium Diseases of Cultivated Plants, Control, Diagnosis, and Molecular and Genetic Studies. Pestic. Sci. 2019, 44, 275–281, doi:10.1584/jpestics.J19-03.
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Manes, J. Presence of Enniatins and Beauvericin in Romanian Wheat Samples: From Raw Material to Products for Direct Human Consumption. Toxins 2017, 9, 189, doi:10.3390/toxins9060189.
- Decleer, M.; Landschoot, S.; De Saeger, S.; Rajkovic, A.; Audenaert, K. Impact of Fungicides and Weather on Cyclodepsipeptide-Producing Fusarium And Beauvericin and Enniatin Levels in Wheat Grains. J. Sci. Food Agric. 2019, 99, 253–262, doi:10.1002/jsfa.9167.
- Logrieco, A.; Moretti, A.; Castella, G.; Kostecki, M.; Golinski, P.; Ritieni, A.; Chelkowski, J. Beauvericin Production by Fusarium Appl. Environ. Microb. 1998, 64, 3084–3088, doi:10.1128/AEM.64.8.3084-3088.1998.
- Moretti, A.; Mule, G.; Ritieni, A.; Logrieco, A. Further Data on the Production of Beauvericin, Enniatins and Fusaproliferin and Toxicity to Artemia salina by Fusarium Species of Gibberella fujikuroi Species Complex. J. Food Microbiol. 2007, 118, 158–163, doi:10.1016/j.ijfoodmicro.2007.07.004.
- Morrison, E.; Kosiak, B.; Ritieni, A.; Aastveit, A.H.; Uhlig, S.; Bernhoft, A. Mycotoxin Production by Fusarium avenaceum Strains Isolated from Norwegian Grain and the Cytotoxicity of Rice Culture Extracts to Porcine Kidney Epithelial Cells. Agric. Food Chem. 2002, 50, 3070–3075, doi:10.1021/jf011532h.
- Fanelli, F.; Ferracane, R.; Ritieni, A.; Logrieco, A.F.; Mule, G. Transcriptional Regulation of Enniatins Production by Fusarium avenaceum. Appl. Microbiol. 2014, 116, 390–399, doi:10.1111/jam.12371.
- Fotso, J.; Leslie, J.F.; Smith, J.S. Production of Beauvericin, Moniliformin, Fusaproliferin, and Fumonisins B(1), B(2), and B(3) by Fifteen Ex-Type Strains of Fusarium Appl. Environ. Microbiol. 2002, 68, 5195–5197, doi:10.1128/aem.68.10.5195-5197.2002.
- Bosch, U.; Mirocha, C.J.; Abbas, H.K.; di Menna, M. Toxicity and Toxin Production by Fusarium Isolates from New Zealand. Mycopathologia 1989, 108, 73–79, doi:10.1007/BF00436056.
- Shephard, G.S.; Sewram, V.; Nieuwoudt, T.W.; Marasas, W.F.; Ritieni, A. Production of the Mycotoxins Fusaproliferin and Beauvericin by South African Isolates in the Fusarium Section Liseola. Agric. Food Chem. 1999, 47, 5111–5115, doi:10.1021/jf9903713.
- Garcia-Cela, E.; Kiaitsi, E.; Medina, A.; Sulyok, M.; Krska, R.; Magan, N. Interacting Environmental Stress Factors Affects Targeted Metabolomic Profiles in Stored Natural Wheat and That Inoculated with F. graminearum. Toxins 2018, 10, 56, doi:10.3390/toxins10020056.
- Leslie, J.F.; Zeller, K.A.; Logrieco, A.; Mule, G.; Moretti, A.; Ritieni, A. Species Diversity of and Toxin Production by Gibberella fujikuroi Species Complex Strains Isolated from Native Prairie Grasses in Kansas. Environ. Microbiol. 2004, 70, 2254–2262, doi:10.1128/aem.70.4.2254-2262.2004.
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in Metabolite Production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. J. Food Microbiol. 2004, 95, 257–266, doi:10.1016/j.ijfoodmicro.2003.12.005.
- Gupta, S.; Krasnoff, S.B.; Underwood, N.L.; Renwick, J.A.; Roberts, D.W. Isolation of Beauvericin as an Insect Toxin from Fusarium semitectum and Fusarium moniliforme subglutinans. Mycopathologia 1991, 115, 185–189, doi:10.1007/BF00462223.
- Moretti, A.; Mule, G.; Ritieni, A.; Laday, M.; Stubnya, V.; Hornok, L.; Logrieco, A. Cryptic Subspecies and Beauvericin Production by Fusarium subglutinans from Europe. J. Food Microbiol. 2008, 127, 312–315, doi:10.1016/j.ijfoodmicro.2008.08.003.
- Fumero, M.V.; Villani, A.; Susca, A.; Haidukowski, M.; Cimmarusti, M.T.; Toomajian, C.; Leslie, J.F.; Chulze, S.N.; Moretti, A. Fumonisin and Beauvericin Chemotypes and Genotypes of the Sister Species Fusarium subglutinans and Fusarium temperatum. Environ. Microbiol. 2020, 86, doi:10.1128/AEM.00133-20.
- Langseth, W.; Bernhoft, A.; Rundberget, T.; Kosiak, B.; Gareis, M. Mycotoxin Production and Cytotoxicity of Fusarium Strains Isolated from Norwegian Cereals. Mycopathologia 1998, 144, 103–113, doi:10.1023/a:1007016820879.
- Altomare, C.; Logrieco, A.; Bottalico, A.; Mule, G.; Moretti, A.; Evidente, A. Production of Type a Trichothecenes and Enniatin B by Fusarium sambucinum Fuckel Sensu Lato. Mycopathologia 1995, 129, 177–181, doi:10.1007/BF01103344.
- Cuomo, V.; Randazzo, A.; Meca, G.; Moretti, A.; Cascone, A.; Eriksson, O.; Novellino, E.; Ritieni, A. Production of Enniatins A, A1, B, B1, B4, J1 by Fusarium tricinctum in Solid Corn Culture: Structural Analysis and Effects on Mitochondrial Respiration. Food Chem. 2013, 140, 784–793, doi:10.1016/j.foodchem.2012.10.136.
- Bottalico, A.; Logrieco, A.; Ritieni, A.; Moretti, A.; Randazzo, G.; Corda, P. Beauvericin and Fumonisin B1 in Preharvest Fusarium moniliforme Maize Ear Rot in Sardinia. Food Addit. Contam. 1995, 12, 599–607, doi:10.1080/02652039509374348.
- Gromadzka, K.; Blaszczyk, L.; Chelkowski, J.; Waskiewicz, A. Occurrence of Mycotoxigenic Fusarium Species and Competitive Fungi on Preharvest Maize Ear Rot in Poland. Toxins 2019, 11, 224, doi:10.3390/toxins11040224.
- Oueslati, S.; Meca, G.; Mliki, A.; Ghorbel, A.; Mañes, J. Determination of Fusarium Mycotoxins Enniatins, Beauvericin and Fusaproliferin in Cereals and Derived Products from Tunisia. Food Control. 2011, 22, 1373–1377, doi:10.1016/j.foodcont.2011.02.015.
- Meca, G.; Zinedine, A.; Blesa, J.; Font, G.; Manes, J. Further Data on the Presence of Fusarium Emerging Mycotoxins Enniatins, Fusaproliferin and Beauvericin in Cereals Available on the Spanish Markets. Food Chem. Toxicol. 2010, 48, 1412–1416, doi:10.1016/j.fct.2010.03.010.
- Tolosa, J.; Rodriguez-Carrasco, Y.; Ferrer, E.; Manes, J. Identification and Quantification of Enniatins and Beauvericin in Animal Feeds and Their Ingredients by LC-QTRAP/MS/MS. Metabolites 2019, 9, 33, doi:10.3390/metabo9020033.
- Mahnine, N.; Meca, G.; Elabidi, A.; Fekhaoui, M.; Saoiabi, A.; Font, G.; Mañes, J.; Zinedine, A. Further Data on the Levels of Emerging Fusarium Mycotoxins Enniatins (A, A1, B, B1), Beauvericin and Fusaproliferin in Breakfast and Infant Cereals from Morocco. Food Chem. 2010, 124, 481–485, doi:10.1016/j.foodchem.2010.06.058.
- Juan, C.; Ritieni, A.; Manes, J. Occurrence of Fusarium Mycotoxins in Italian Cereal and Cereal Products from Organic Farming. Food Chem. 2013, 141, 1747–1755, doi:10.1016/j.foodchem.2013.04.061.
- Kolawole, O.; Graham, A.; Donaldson, C.; Owens, B.; Abia, W.A.; Meneely, J.; Alcorn, M.J.; Connolly, L.; Elliott, C.T. Low Doses of Mycotoxin Mixtures Below Eu Regulatory Limits Can Negatively Affect the Performance of Broiler Chickens: A Longitudinal Study. Toxins 2020, 12, 433, doi:10.3390/toxins12070433.
- Novak, B.; Rainer, V.; Sulyok, M.; Haltrich, D.; Schatzmayr, G.; Mayer, E. Twenty-Eight Fungal Secondary Metabolites Detected in Pig Feed Samples: Their Occurrence, Relevance and Cytotoxic Effects in Vitro. Toxins 2019, 11, 537, doi:10.3390/toxins11090537.
- Nacher-Mestre, J.; Beltran, E.; Strachan, F.; Dick, J.R.; Perez-Sanchez, J.; Berntssen, M.H.G.; Tocher, D.R. No Transfer of the Non-Regulated Mycotoxins, Beauvericin and Enniatins, from Feeds to Farmed Fish Reared on Plant-Based Diets. Food Chem. 2020, 323, 126773, doi:10.1016/j.foodchem.2020.126773.
- Beccari, G.; Senatore, M.T.; Tini, F.; Sulyok, M.; Covarelli, L. Fungal Community, Fusarium Head Blight Complex and Secondary Metabolites Associated with Malting Barley Grains Harvested in Umbria, Central Italy. J. Food Microbiol. 2018, 273, 33–42, doi:10.1016/j.ijfoodmicro.2018.03.005.
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal Agents of Fusarium Head Blight of Durum Wheat (Triticum durum ) in Central Italy and Their in Vitro Biosynthesis of Secondary Metabolites. Food Microbiol. 2018, 70, 17–27, doi:10.1016/j.fm.2017.08.016.
- Fumero, M.V.; Reynoso, M.M.; Chulze, S. Fusarium temperatum and Fusarium subglutinans Isolated from Maize in Argentina. J. Food Microbiol. 2015, 199, 86–92, doi:10.1016/j.ijfoodmicro.2015.01.011.
- Zinedine, A.; Meca, G.; Mañes, J.; Font, G. Further Data on the Occurrence of Fusarium Emerging Mycotoxins Enniatins (A, A1, B, B1), Fusaproliferin and Beauvericin in Raw Cereals Commercialized in Morocco. Food Control. 2011, 22, 1–5, doi:10.1016/j.foodcont.2010.05.002.
- Uhlig, S.; Torp, M.; Heier, B.T. Beauvericin and Enniatins A, A1, B and B1 in Norwegian Grain: A Survey. Food Chem. 2006, 94, 193–201, doi:10.1016/j.foodchem.2004.11.004.
- De Lourdes Mendes de Souza, M.; Sulyok, M.; Freitas-Silva, O.; Costa, S.S.; Brabet, C.; Machinski Junior, M.; Sekiyama, B.L.; Vargas, E.A.; Krska, R.; Schuhmacher, R. Cooccurrence of Mycotoxins in Maize and Poultry Feeds from Brazil by Liquid Chromatography/Tandem Mass Spectrometry. World J. 2013, 2013, 427369, doi:10.1155/2013/427369.
- Jurjevic, Z.; Solfrizzo, M.; Cvjetkovic, B.; De Girolamo, A.; Visconti, A. Occurrence of Beauvericin in Corn from Croatia. Food Technol. Biotechnol. 2002, 40, 91–94.
- Sorensen, J.L.; Nielsen, K.F.; Rasmussen, P.H.; Thrane, U. Development of a LC-MS/MS Method for the Analysis of Enniatins and Beauvericin in Whole Fresh and Ensiled Maize. Agric. Food Chem. 2008, 56, 10439–10443, doi:10.1021/jf802038b.
- Yoshinari, T.; Suzuki, Y.; Sugita-Konishi, Y.; Ohnishi, T.; Terajima, J. Occurrence of Beauvericin and Enniatins in Wheat Flour and Corn Grits on the Japanese Market, and Their Co-Contamination with Type B Trichothecene Mycotoxins. Food Addit. Contam. Part A 2016, 33, 1620–1626, doi:10.1080/19440049.2016.1228126.
- Srobarova, A.; Moretti, A.; Ferracane, R.; Ritieni, A.; Logrieco, A. Toxigenic Fusarium Species of Liseola Section in Pre-Harvest Maize Ear Rot, and Associated Mycotoxins in Slovakia. J. Plant Pathol. 2002, 108, 299–306, doi:10.1023/A:1015645813231.
- Munkvold, G.; Stahr, H.M.; Logrieco, A.; Moretti, A.; Ritieni, A. Occurrence of Fusaproliferin and Beauvericin in Fusarium-Contaminated Livestock Feed in Iowa. Environ. Microbiol. 1998, 64, 3923–3926, doi:10.1128/AEM.64.10.3923-3926.1998.
- Nazari, F.; Sulyok, M.; Kobarfard, F.; Yazdanpanah, H.; Krska, R. Evaluation of Emerging Fusarium Mycotoxins Beauvericin, Enniatins, Fusaproliferin and Moniliformin in Domestic Rice in Iran. J. Pharm. Res. 2015, 14, 505–512.
- Blesa, J.; Moltó, J.-C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous Determination of Fusarium Mycotoxins in Wheat Grain from Morocco by Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry. Food Control 2014, 46, 1–5, doi:10.1016/j.foodcont.2014.04.019.
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Manes, J. Occurrence and Co-Occurrence of Fusarium Mycotoxins in Wheat Grains and Wheat Flour from Romania. Food Control 2017, 73, 147–155, doi:10.1016/j.foodcont.2016.07.042.
This entry is adapted from the peer-reviewed paper 10.3390/toxins12120765
