Chronic coronary syndromes (CCS) are a widespread phenomenon associated with different clinical entities, mostly characterized by a stable and progressive process of atherosclerotic plaque formation. As symptoms among patients with CCS are often not uniform and atypical, diagnostic tests are needed to confirm the diagnosis and evaluate the risk of events.
- coronary computed tomography
1. Introduction
Over the past years, several noninvasive imaging methods have evolved as the “gatekeeper” for invasive coronary angiography (ICA), like positron emission tomography (PET), stress-echocardiography, single-photon emission CT (SPECT), and stress cardiac magnetic resonance (CMR).
Coronary CT angiography (CCTA) has been validated as a noninvasive anatomical imaging test with an affordable safety profile and diagnostic accuracy in excluding the presence of coronary stenosis [1,2,3]. Rapid developments in scanner technology and recent scientific evidence have raised a great potential for CCTA beyond coronary imaging, as a promising risk stratifier in patients with coronary artery disease (CAD) [4,5]. The possibility of providing detail about coronary anatomy, plaque morphology, disease activity, and hemodynamic effects of coronary lesions in a single examination has made CCTA an appealing noninvasive diagnostic modality for the assessment of CCS.
2. State of the Art: Technology
2.1. Evolution of Cardiac CT Scanners
The first developed electron beam CT scanners, useful for coronary calcification imaging, have been replaced by multidetector CT scanners, which have rapidly developed since the 1980s, for adequate performance of contrast-based cardiovascular imaging such as CCTA. Today, a 64-detector rows technology is required for CCTA studies [6], although calcium scoring systems with 16 detector rows may be sufficient.
In 1998, the “4-detector-row” scanners were introduced in clinical practice for imaging the coronary arteries, although these early models had limited applicability and mainly confined to coronary calcium scoring [7]. Coronary angiography became then feasible with the use of 16-detector-row CT scanners and with retrospective cardiac gated acquisitions [8]. A greater improvement in the diagnostic accuracy was later reached with the introduction of 64 detector rows, with faster gantry rotation times (350–420 ms) and reduced detector size (0.5–0.65 mm). However, the early 64-slice multi-detector CT scanners (MDCT) showed an inherent limited temporal and spatial resolution, with a restricted z-axis coverage. These were rapidly overcome by newer generation MDCTs, which presented improved spatial and temporal resolution, faster scan mode, and whole heart coverage with either wide-detector or dual-source CT. Wide-detector CT scanners with a wide area-coverage (16 cm) can acquire images of the whole heart within one heartbeat, without stair-step artifacts [9]. Table 1 provides an overview on multi-slice CT technology with details on progressive improvement on both spatial and temporal resolution.
Table 1. Chronologic and technical evolution of multi-slice computed tomography (CT) scanners.
| Year | Detector Rows | Detector z-axis Resolution (mm) | Detector z-axis Coverage (mm) |
Temporal Resolution (ms) | Gantry Rotation Time (ms) |
|---|---|---|---|---|---|
| 1998 | 4 | 1–1.25 | 20 | 400 | 500–800 |
| 2001 | 16 | 0.5–0.75 | 24 | 190–250 | 380–500 |
| 2004 | 64 | 0.625 | 40 | 175 | 330–400 |
| 2007–2008 | 256–320 | 0.5–0.625 | 160 | 140–175 | 280–350 |
| 2012 | 640 | 0.5 | 160 | 137 | 275 |
Dual-source CT has high-pitch acquisition platforms that can image the heart in less than 300 ms. Furthermore, the use of thinner detectors with a spatial resolution of 250 microns along the XY planes, faster gantry rotation times (220 ms), noise reduction stretegies with improved detector efficiency, and new electronic circuitry have allowed diagnostic image quality in patients who were considered to be challenging with prior-generation CT. For example, patients with calcium scores >400 HU, coronary artery stent <3 mm, coronary artery bypass grafts, significant heart rate variability and heart rates >65 bpm, and body mass index >30 kg m2 could now be adequately scanned [10,11,12].
2.2. Radiation Exposure and Principle Cardiac CT-Related Risks
Main risks related to CCTA include radiation exposure and contrast agent nephropathy. A significant reduction in total radiation dose as well as contrast material volume has been recently achieved thanks to technological advances.
Patients at higher risk for contrast-induced nephropathy are those with reduced renal function (eGFR <45 mL/min/1.73 m2) and with diabetes, especially when both present [13]. Therefore, since a significant proportion of patients with CCS are at risk for contrast-induced nephropathy, optimization of the contrast material dose is essential, though allowing a proper magnification of the vessel lumen [14]. The latest CT scanners can allow a very short acquisition time, therefore requiring a markedly reduced contrast material volume for coronary opacification. For example, 75–100 mL are now sufficient for 64-detector-row CT systems, while 100–140 mL were originally needed with 16-detector rows. [15]. Even less than 60 mL may be appropriate for newer wide-detector CT scanners [16,17]. Iterative reconstruction techniques and low tube potential (70–100 kV) have also favored a reduction in contrast dose. However, in order to allow an accurate delineation of coronary disease, attenuation values between 250 and 350 HU should be guaranteed [18,19].
Dose reduction strategies are currently needed to limit patient exposure, especially with the introduction of multi-detector CT [20]. In regard to dose reduction strategies, axial volumetric acquisition of CCTA may reduce the overall radiation dose without additional radiation exposure derived from helical oversampling or sequential axial scanning [21]. For example, an effective dose of 8.3 ± 3.4 mSv was reported for 320-detector-row CT scanners, due to tube current and X-ray emission modification based on the patient’s body size [22]. Several years later, an even lower radiation dose, till less than 1 mSV, was achieved with faster gantry rotation times and other technical improvements, though providing an excellent image quality over a wide range of body sizes and heart rates at lower radiation dose [23,24]. Table 2 underlines the different radiation exposure ranges for different CT modalities. Of note, FFR-CT does not add further risk to standard CCTA.
Table 2. Radiation exposure and presumed risk of cardiac computed tomography (CT) techniques.
| CT Modality | Effective Dose (mSv) | Additional Risks |
|---|---|---|
| CACS | 1.0–1.5 | - |
| CCTA | <1.0–13.5 | Contrast-related, Beta-blockers/Nitroglycerine |
| FFR-CT | <1.0–13.5 | Contrast-related, Beta-blockers/Nitroglycerine |
| Stress-CTP | 2.5–21.6 | Contrast-related, Beta-blockers/Nitroglycerine, Adenosine |
CT: computed tomography; CCTA: coronary computed tomography angiography; FFR-CT: fractional flow reserve derived from computed tomography; CTP: computed tomography perfusion.
Other risks in CT examinations may concern additional contrast agent-related effects, allergic reactions, and medications side effects. Severe allergic reactions are rare (0.04%) [25]. Contrast medium extravasation is relatively rare (frequency <1%) and generally results in minimal damage at superficial skin level, although it may eventually lead to severe stages of necrosis and ulceration which may also favor topic infection [26]. Beta-blockers should be used with caution in patients with asthma or bronchospastic disease, atrio-ventricular conduction defects, and should not be administered if systolic blood pressure <90 mmHg. Care should regard administration of nitroglycerine in aortic stenosis patients, and it should be avoided with phosphodiesterase type 5 inhibitors and hypertrophic cardiomyopathy [27].
2.3. Future Technical Perspectives
Some future perspectives in the field of cardiac CT hardware technology include the use of true cardiac-capable photon counting detectors, which enable nearly 100% geometric dose efficiency, permitting relevant radiation dose reduction [28,29] with far superior spatial resolution, giving way to improved coronary lumen visualization through better edge delineation [30]. These advances may also offer the ability to perform advanced tissue analysis with distinction of lipid, fibrous, and calcified elements [31].
Apart from advancements in hardware technologies, the integration of validated advanced analytic tools and engineering solutions, such as machine learning and deep learning, is promisingly leading to an innovative and impressive pathway in study analysis and reporting. Early experience has outlined the emerging role of these analytical tools in CT datasets to unmask their new potential compared with standard visual evaluation [32]. These techniques could significantly overcome limitations related to human interpretation, with its inherent limitations, with a rapid and objective image dataset evaluation. Development of these complex algorithms requires highly advanced mathematics, engineering, and computer programming levels, which are still under construction and evolving. These tools may represent an increasing part of routine clinical practice in the future, but further large randomized trials are necessary to validate these innovative approaches.
3. Screening of Patients: The Calcium Score
Coronary artery calcification shows a significant association with advanced coronary disease burden [33], and represents a final step of progression for coronary plaque [34]. Various techniques are used to detect the amount of calcium deposits, including the Agatston calcium score. This score was first developed in the 1990s, being the most broadly used method to detect coronar artery calcification, as it shows a discrete reproducibility and accuracy.
Coronary artery calcium score (CACS) is commonly achieved in the initial noncontrast low-radiation phase of CCTA, by assigning a weighted density score to the location of calcium with the highest attenuation (measured in Hounsfield units) and then multiplying by the area of calcification. It is defined as >130 HU and >1 mm2 in size. In detail, grading of coronary calcium burden is defined as a 0, 1 to 10, 11 to 100, 101 to 400, and greater than 400 CACS, which correspond to no, minimal, mild, moderate, and severe CAD [35].
Numerous studies have outlined the prognostic value of CACS over conventional risk factors in asymptomatic patients with a pre-test intermediate risk of cardiovascular disease (CVD). Approximately 20% of patients in the intermediate risk group will have an improvement in risk prediction when CACS is considered (6–20% risk for events in the next 10 years according to the Framingham risk score). The lowest risk of adverse events is in the 1–100 group (HR 3.61), with the highest in the >400 group (HR 9.67) [36]. A low prevalence of obstructive disease is related to an Agatston = 0, with a <1% annual risk for nonfatal myocardial infarction, providing a “warranty” against cardiovascular events for 10–15 years [37,38].
The recent 2019 European Society of Cardiology (ESC) guidelines for CCS give CACS a Class IIb recommendation as a screening tool for CAD in asymptomatic patients, while the current 2016 European guidelines on preventive strategies give CACS a Class IIa recommendation for intermediate risk patients [39]. CACS, along with several CVD risk factors, namely age, sex, ethnicity, diabetes, tobacco use, cholesterol level, blood pressure, and use of cholesterol or hypertensive medications can provide an adequate predictive model of 10-year-risk for CVD events [40,41]. Recent studies have therefore supported the integration of CACS into CVD risk prediction models above traditional risk factors. For example, the Astronaut Cardiovascular and Health Modification (Astro-CHARM) calculator demonstrated the improvement of adding CACS to the Framingham Risk Score [42]. The Multi-Ethnic Study of Atherosclerosis demonstrated that CACS predicted CVD events beyond traditional risk factors with adequate strength in all ethnic groups represented in the study [43]. Calcium scoring is also considered a decisive factor in the decision to begin statin therapy as recently incorporated into the 2018 US guidelines for the management of blood cholesterol [44]. For example, a CACS of 0 generally supports deferral of statin therapy unless a patient has diabetes, is a cigarette smoker, or a family history of premature coronary disease. Otherwise, a CACS of 1–99 favors statin therapy [45].
On the other hand, CACS seems to be less useful in low-risk patients. In addition, high-risk or symptomatic patients do not benefit from this examination [46]. One of the major benefits of CACS is in the reclassification of intermediate risk patients, who are not symptomatic, into a higher or lower risk group, thus identifying those who may benefit more from an aggressive primary preventive treatment, based on the very favorable prognosis of a CACS of zero [45,46,47]. CACS may therefore be useful to adjust the pharmacologic therapy and adapt lifestyle modifications in order to provide targeted risk factors modifications, in particular treatment of hypertension, dyslipidemia, and diabetes. CACS can be considered for CAD population screening due to its little radiation exposure and need for patient preparation. Of note, seriated CACS performance would easily help to monitor vessel disease progression and/or regression, though with limited information than CCTA.
However, certain aspects should be taken in consideration. For instance, traditional CACS cannot provide the number and size of calcifications, thus limiting comprehensive assessment of total plaque burden, which is considered an important feature of adverse event risk. In addition, CACS alone is not able to identify noncalcified coronary artery plaques, which represents a large portion of total plaque content, thus significantly underestimating and missing a part of atherosclerotic pathology [48,49]. If histological data suggest that plaques with high calcium amount have smaller lipid cores and less positive remodeling, which are defined features of vulnerable plaques, CACS actually targets a stable type of plaque, which is less susceptible to rupture or cause adverse events.
Although current recommendations for CACS performance in routine clinical practice remains, future and rapidly evolving advances in plaque imaging as well as newer improvements in CT technology will likely overcome the role of CACS as a screening tool for CAD in the future.
4. Clinical Indications of CCTA in the Context of Chronic Coronary Syndromes
The 2019 European Society of Cardiology (ESC) guidelines on the diagnosis and management of CCS [50] introduced the term CCS, which refers to a defined spectrum of clinical scenarios of CAD, without including acute coronary events.
Patients suspected of CCS should be managed through a stepwise approach, to select the most appropriate noninvasive functional or anatomical modality from clinical patient’s characteristics (gender, age, and symptoms) so as to assess the risk of obstructive CAD and possibly intervene with revascularization. A pre-test probability (PTP) <5% does not suggest further testing, while in patients with PTP >15% or PTP between 5–15% and a strong clinical suspect of obstructive CAD, a subsequent noninvasive diagnostic test should be performed [51]. Only if the assessed risk of obstructive CAD results is very high, ICA should be recommended.
The choice for noninvasive diagnostic testing in patients with intermediate PTP of CAD may depend on local availability, expertise, and patient’s characteristics. However, current 2019 ESC guidelines on CCS recommend CCTA as the first-line test (class IB) in suitable patients with low to intermediate clinical likelihood of CCS, due to its highest rule-out capability compared to other noninvasive tests. This gives a growing role to CCTA, representing a dramatic change in respect to the previous version of the guideline. Otherwise, functional imaging tests such as stress-echocardiography, PET, SPECT, or CMR perfusion imaging may be preferred in certain healthcare settings mainly due to local availability or as an alternative of CCTA when doubtful or not diagnostic, as well as in patients with known CAD or who have undergone previous percutaneous coronary intervention (PCI) [50].
The SCOT-HEART (Scottish Computed Tomography of the Heart) trial randomized 8000 patients with suspected obstructive CAD to either CCTA or standard care (predominantly stress ECG), thus revealing a significantly lower rate of the primary endpoint of cardiovascular death or nonfatal MI (2.3% vs. 3.9% at 5-year follow-up) in patients who underwent CCTA [52,53]. Rates of ICA and revascularization did not differ significantly between the two strategies, CCTA led to the beginning of more preventive therapies [53].
The PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial randomized 10,003 patients with symptoms suggestive for CAD randomized to either CCTA or functional tests, without showing any relevant difference in the primary outcome (3.3% vs. 3.0%, at 25-month follow-up) [54].
In the EVINCI (Evaluation of Integrated Cardiac Imaging in Ischemic Heart Disease) study, CCTA was compared with several diagnostic modalities (stress CMR, PET, SPECT, stress echocardiography) in patients with suspected CAD and likelihood of intermediate disease. In this study, the CT scan was found to be the method with the best diagnostic performance (sensitivity, specificity, and diagnostic accuracy of 91%, 92%, 91%, respectively) [55]. Moreover, other randomized trials documented that CCTA performs exclusion of CAD in a rapid, safe method, with a very low rate of complications related to contrast medium (<1/1000 patients), and with similar or superior cardiac results compared to noninvasive functional testing [52,54,55,56].
CCTA shows very high sensitivity in detecting both coronary artery stenosis defined as obstructive by ICA and non-obstructive calcified or non-calcified lesions [56]. Meta-analysis assessing the diagnostic performance of CCTA in respect to ICA (for >50% coronary stenosis) demonstrated an overall sensitivity and specificity of 96.6% and 81.5%, respectively [57]. Although CCTA may be associated with an increasing number of total ICAs, it allows a reduced percentage of negative ICA studies performed, as well as myocardial infarctions rate, with more appropriate revascularizations in respect to functional tests [58]. The favorable long-term outcome may be favored by the reliable ability in the identification of CAD and the subsequent begin of preventive therapies [59]. Figure 1 is an example of CCTA acquisition and subsequent ICA in the same patient.
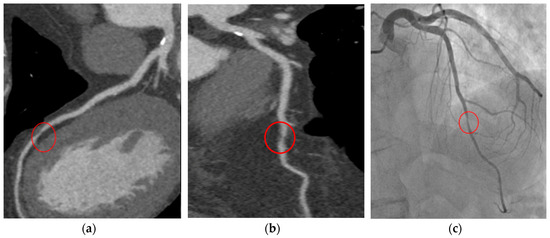
Figure 1. T.F. 67 years-old, male; dyslipidaemia, smoker; typical exertional chest pain; exercise test negative; CCTA (a,b): subocclusive stenosis of mid-LAD; ICA (c): significant stenosis was confirmed and elective PCI was performed. CCTA: coronary computed tomography angiography; LAD: left anterior descending artery; ICA: invasive coronary angiography; PCI: percutaneous coronary intervention.
CCTA can also provide important prognostic information [60] and may allow risk stratification and guide future therapy decisions in CAD patients [37,59]. The culmination of recent advances in the field of CCTA has favored a change in the latest National Institute for Health and Care Excellence (NICE) guidelines for recent onset chest pain, which highly recommend CCTA for patients with typical or even atypical nonanginal chest pain with ECG changes [61]. This gives CCTA a more relevant role in the diagnosis of CAD in respect to calculations of pre-test probability. An updated version of the American guidelines in the context of stable CAD is expected in the near future, which will probably further define the cardiac CT role among CCS patients. According to existing guidelines, CCTA is not recommended in the presence of adverse features that could compromise good image quality, such as diffuse coronary calcifications, irregular heart rates, obesity, and difficulty in breath-holding [50]. However, growing technologies already permit acquisition of good image quality in patients historically considered not suitable for this examination. Thus, this recommendation could potentially be overcome in the next few years, further amplifying the already wide field of application for CCTA.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9123925
