There are limited proven therapies for COVID-19. Vitamin C’s antioxidant, anti-inflammatory and immunomodulating effects make it a potential therapeutic candidate, both for the prevention and amelioration of COVID-19 infection, and as an adjunctive therapy in the critical care of COVID-19. This literature review focuses on vitamin C deficiency in respiratory infections, including COVID-19, and the mechanisms of action in infectious disease, including support of the stress response, its role in preventing and treating colds and pneumonia, and its role in treating sepsis and COVID-19. The evidence to date indicates that oral vitamin C (2–8 g/day) may reduce the incidence and duration of respiratory infections and intravenous vitamin C (6–24 g/day) has been shown to reduce mortality, intensive care unit (ICU) and hospital stays, and time on mechanical ventilation for severe respiratory infections. Further trials are urgently warranted. Given the favourable safety profile and low cost of vitamin C, and the frequency of vitamin C deficiency in respiratory infections, it may be worthwhile testing patients’ vitamin C status and treating them accordingly with intravenous administration within ICUs and oral administration in hospitalised persons with COVID-19.
- COVID-19
- SARS-CoV-2
- coronavirus
- vitamin C
- ascorbate
- colds
- pneumonia
- sepsis
- immunonutrition
- ascorbic acid
1. Introduction
Vitamin C, ascorbic acid, is an essential water-soluble nutrient. It is synthesised in plants from fructose and in almost all animals from glucose. It is not synthesised by primates, most bats, guinea pigs, and a small number of birds and fish since the final enzyme, gulonolactone oxidase (GULO), required for ascorbic acid synthesis is missing due to gene mutations that occurred prior to the evolution of Homo sapiens [1]. All these species are therefore dependent on vitamin C in their food. Primates are dependent on an adequate supply provided by fruits and vegetation intake ranging from 4.5 g/day for gorillas [2] to 600 mg/day for smaller monkeys (7.5 kg—a tenth of human size) [3].
The EU Average Requirement of 90 mg/day for men and 80 mg/day for women is to maintain a normal plasma level of 50 µmol/L [4], which is the mean plasma level in UK adults [5]. This is sufficient to prevent scurvy but may be inadequate when a person is under viral exposure and physiological stress. An expert panel in cooperation with the Swiss Society of Nutrition recommended that everyone supplement with 200 mg “to fill the nutrient gap for the general population and especially for the adults age 65 and older. This supplement is targeted to strengthen the immune system” [6]. The Linus Pauling Institute recommends 400 mg for older adults (>50 years old) [7].
Pharmacokinetic studies in healthy volunteers support a 200 mg daily dose to produce a plasma level of circa 70 to 90 µmol/L [8,9]. Complete plasma saturation occurs between 1 g daily and 3 g every four hours, being the highest tolerated oral dose, giving a predicted peak plasma concentration of circa 220 µmol/L [10]. The same dose given intravenously raises plasma vitamin C levels approximately ten-fold. Higher intakes of vitamin C are likely to be needed during viral infections with 2–3 g/day required to maintain normal plasma levels between 60 and 80 µmol/L [11,12].
2. Vitamin C Deficiency in Pneumonia, Sepsis and COVID-19
Human plasma vitamin C levels decline rapidly under conditions of physiological stress including infection, trauma, and surgery, not uncommonly resulting in overt vitamin C deficiency in hospitalised patients, defined as a plasma level of vitamin C ≤ 11 µmol/L [13,14,15,16,17,18]. Two studies in hospitals in Paris reported that 17 to 44% of patients had vitamin C plasma levels less than ≤ 11 µmol/L [14,15]. In a Canadian university hospital, it was found that 19% of patients had vitamin C plasma levels ≤ 11 µmol/L [16]. In a study of surgical patients in Australia, it was found that 21% had vitamin C plasma levels ≤ 11 µmol/L [17]. A survey of elderly Scottish patients hospitalised as a consequence of acute respiratory infections reported that 35% of patients had vitamin C plasma levels ≤ 11 µmol/L [18]. The UK’s National Diet and Nutrition Survey, based on a cross section of the UK population, reports that 4% of 65+ year olds and 40% of those institutionalised in care homes have vitamin C levels ≤ 11 µmol/L [5,19], indicating the way in which older people with low vitamin C status may be especially susceptible to critical infection.
The vitamin C-deficiency disease scurvy has long been associated with pneumonia which led to the view that vitamin C may influence susceptibility to respiratory infections [20]. In other words, people deficient in vitamin C may be more susceptible to severe respiratory infections such as pneumonia. A prospective study of 19,357 men and women followed over 20 years found that people in the top quartiles of baseline plasma vitamin C concentrations had a 30% lower risk of pneumonia [21]. Furthermore, meta-analysis has indicated a reduction in the risk of pneumonia with oral vitamin C supplementation, particularly in individuals with low dietary intakes [22].
Post-mortem investigations of severe COVID-19 have demonstrated a secondary organising pneumonia phenomenon [23]; therefore, studies investigating vitamin C in relation to pneumonia may be relevant [18,24,25,26,27] (Table 1). The most recent study, from New Zealand, reported that patients with pneumonia had depleted vitamin C levels compared with healthy controls (23 µmol/L vs. 56 µmol/L, p < 0.001). The pneumonia cohort comprised 62% with hypovitaminosis C and 22% with vitamin C ≤ 11 µmol/L, compared with 8% hypovitaminosis C and no cases with ≤11µmol/L in the healthy controls [24]. The more severely ill patients in the ICU had mean vitamin C levels of 11 µmol/L. Similar findings have been reported in other studies of critically ill septic patients [28,29,30,31,32,33] (Table 1). A New Zealand study of patients with sepsis found that 40% had vitamin C ≤ 11 µmol/L and the majority of the patients had hypovitaminosis C (serum level < 23 µmol/L), despite receiving recommended enteral and parenteral intakes of the vitamin [29].
Table 1. Vitamin C status of patients with pneumonia, sepsis and severe COVID-19.
| Study Type | Cohort | Vitamin C (µmol/L) (% Deficient, % Hypovitaminosis C) |
Refs. |
|---|---|---|---|
| Pneumonia | |||
| Case control | Healthy volunteers (n = 50) | 56 ± 2 a (0% b, 8% c) | [24] |
| Community-acquired pneumonia (n = 50) | 23 ± 3 (22%, 62%) | ||
| Case control | Healthy volunteers (n = 20) | 66 ± 3 | [25] |
| Pneumonia cases (n = 11) | 31 ± 9 | ||
| Case control | Healthy participants (n = 28) | 49 ± 1 | [26] |
| Lobular pneumonia (n = 35): | |||
| Acute—did not survive (n = 7) | 17 ± 1 | ||
| Acute—survived (n = 15) | 24 ± 1 | ||
| Convalescent cases (n = 13) | 34 ± 1 | ||
| Intervention (placebo group) | Pneumonia/bronchitis (n = 29): | [18] | |
| Week 0 | 24 ± 5 (40%) b | ||
| Week 2 | 19 ± 3 (37%) | ||
| Week 4 | 24 ± 6 (25%) | ||
| Intervention (control group) | Pneumonia cases (n = 70): | [27] | |
| Day 0 | 41 | ||
| Day 5–10 | 23–24 | ||
| Day 15–20 | 32–35 | ||
| Day 30 | 39 | ||
| Sepsis | |||
| Intervention (baseline) | Sepsis with ARDS (n = 83): | [28] | |
| Day 0 | 22 (11–37) d | ||
| Day 2 | 23 (9–37) | ||
| Day 4 | 26 (9–41) | ||
| Day 7 | 29 (12–39) | ||
| Observational | Septic shock patients (n = 24) | 15 ± 2 (38% b, 88% c) | [29] |
| Intervention (baseline) | Severe sepsis patients (n = 24) | 18 ± 2 | [30] |
| Case control | Healthy controls (n = 6) | 48 ± 6 | [31] |
| Severe sepsis (n = 19) | 14 ± 3 | ||
| Septic shock (n = 37) | 14 ± 3 | ||
| Case control | Healthy controls (n = 14) | 76 ± 6 | [32] |
| Septic encephalopathy (n = 11) | 19 ± 11 | ||
| Case control | Healthy controls (n = 34) | 62 (55–72) d | [33] |
| ICU (injury, surgery, sepsis) (n = 62) | 11 (8–22) | ||
| Severe COVID-19 | |||
| Observational | Critically ill COVID-19 (n = 21) | 22 ± 4 (45%b, 70% c) e | [34] |
| Survivors (n = 11) | 29 ± 7 (40%, 50%) | ||
| Non-survivors (n = 10) | 15 ± 2 (50%, 90%) | ||
| Observational | COVID-associated ARDS (n = 18) | 17 with <9 µmol/L | [35] |
| 1 with 14 µmol/L |
a—Data represent mean and SEM; d—median (and interquartile range); b—Percentage of patients with vitamin C deficiency (<11 µmol/L); c—Percentage of patients with hypovitaminosis C (<23 µmol/L); e—Personal communication (Cristian Arvinte, North Suburban Medical Center, Thornton, CO, USA). COVID—coronavirus disease; ICU—intensive care unit; ARDS—acute respiratory distress syndrome. A part of this table has been reproduced from [36].
As yet, there have been few studies reporting the vitamin C status of patients with COVID-19 (Table 1). A study of 21 critically ill COVID-19 patients admitted to ICU in the US found a mean level of 22 µmol/L, thus a majority had hypovitaminosis C. The mean level for 11 survivors was 29 µmol/L compared to 15 µmol/L for the 10 non-survivors; of these five (50%) had ≤11 µmol/L [34]. A study in an ICU in Barcelona of 18 COVID-19 patients meeting acute respiratory distress syndrome (ARDS) criteria found that 17 had undetectable levels of vitamin C (i.e., <9 µmol/L) and one patient had a low vitamin C (14 µmol/L) [35]. Thus, low vitamin C levels are common in critically ill hospitalised patients with respiratory infections, pneumonia, sepsis and COVID-19, the most likely explanation being increased metabolic consumption [37].
3. Clinical Evidence for the Role of Vitamin C in COVID-19
Given the potential benefit of vitamin C, in oral and intravenous doses of 2–8 g/day, to reduce duration and severity of the common cold, pneumonia, sepsis and ARDS, this warrants investigation in relation to whether early oral supplementation could be beneficial in preventing conversion from mild infection to more critical COVID-19 infection and, if given intravenously to those with critical COVID-19 symptoms, in reducing mortality and ICU stay, thus speeding up recovery.
Interestingly, many of the risk factors for COVID-19 overlap with those for vitamin C deficiency [94]. Certain sub-groups (male, African American, older, those suffering with co-morbidities of diabetes, hypertension, COPD), all at higher risk of severe COVID-19, have also been shown to have lower serum vitamin C levels [95]. Average plasma vitamin C levels are generally lower in men than women, even with comparative intakes of vitamin C, which has been attributed to their higher body weight [94]. A hypothesis of altered sodium-dependent vitamin C transporter (SVCT1 and 2) expression in these sub-groups has also been proposed [95]. In old versus young rat hepatocytes, the vitamin C level declines by 66%, which is largely attributed to reduced absorption due to a 45% decline in SVCT1 with age [96]. It is noteworthy that inflammatory cytokines, also present in co-morbidities, downregulate SVCT2, resulting in the depletion of intracellular vitamin C [97,98].
There are currently 45 trials registered on Clinicaltrials.gov investigating vitamin C with or without other treatments for COVID-19. In the first RCT to test the value of vitamin C in critically ill COVID-19 patients, 54 ventilated patients in Wuhan, China, were treated with a placebo (sterile water) or intravenous vitamin C at a dose of 24 g/day for 7 days [82] (Table 2). After 7 days of treatment, the ratio of PaO2/FiO2 in the vitamin C group was 229 mmHg versus 151 mmHg in the control group (p = 0.01), and this also improved over time in the vitamin C group, but fell in the control group. On day 7, the IL-6 level was lower in the vitamin C group than in the placebo group: 19 pg/mL versus 158 pg/mL (p = 0.04). The more severely ill patients with SOFA scores ≥ 3 in the vitamin C group exhibited a reduction in 28-day mortality: 18% versus 50% (p = 0.05) in univariate survival analysis (Figure 2). No study-related adverse events were reported. The effects of treatment on the ratio PaO2/FiO2 and on IL-6 are clinically important, but further studies are needed to determine if the trend in lower mortality can be confirmed. The trial was originally designed for 140 subjects and was thus underpowered, with only 54 patients due to a lack of new admissions.
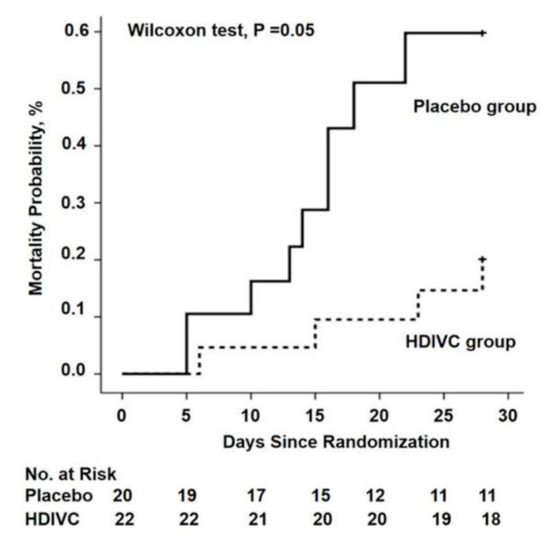
Figure 2. The 28-day mortality from randomization (day 1) to day 28 in a trial of high-dose intravenous vitamin C (HDIVC) in patients with COVID-19. Kaplan–Meier analysis was used to estimate the 28-day mortality and survival curves were compared with the Wilcoxon test (p = 0.05) among severe COVID-19 patients (baseline SOFA score ≥ 3). Cox regression was used as multiple comparisons (HR, 0.32 (95%CI, 0.10–1.06); p = 0.06). HDIVC—high-dose intravenous vitamin C. Reproduced with permission from Zhang J. et al. [82].
The largest registered trial is the Lessening Organ Dysfunction with Vitamin C-COVID (LOVIT-COVID) trial in Canada, which is recruiting 800 patients who are randomly assigned to vitamin C (intravenous, 50 mg/kg every 6 h) or a placebo for 96 h, i.e., equivalent to 15 g/day for a 75 kg person (NCT04401150). This protocol has also been added as a vitamin C arm in the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP; NCT02735707). The study design provides further rationale for the use of vitamin C in COVID-19 patients [99]. There is also a high-dose (10 g/day) vitamin C intervention study in 500 adults is in progress in Palermo, Italy (NCT04323514).
There is concern, however, that these study designs limit the use of vitamin C to a maximum of four days, which may be inadvisable in acutely ill patients due to the potential return of symptoms if the inflammation is not resolved. This issue was illustrated by the CITRIS-ALI trial, which showed a maximum reduction in mortality compared to placebo on day 4, the final day of vitamin C administration, but a decreased difference between the groups after 28 days [87,89].
In the UK, the Chelsea and Westminster hospital ICU, where adult ICU patients were administered 1 g of intravenous vitamin C every 12 h together with anticoagulants [100], has reported 29% mortality [101], compared to the average 41% reported by the Intensive Care National Audit and Research Centre (ICNARC) for all UK ICUs [102]. While the authors have stated that the addition of an antioxidant in the form of vitamin C could have contributed to the lower mortality rate, it should be noted that other clinical factors and procedures could also account for the improved mortality and that the Chelsea and Westminster ICU serves a more affluent sector of the population with less deprivation on the basis of the Index of Multiple Deprivation (IMD). Deprivation, while a risk factor for COVID-19 mortality, is also a predictor of low vitamin C status. In the UK, an estimated 25% of men and 16% of women in the low-income/materially deprived population are deficient in vitamin C > 11 µmol/L [103].
The Frontline COVID-19 Critical Care Expert Group (FLCCC), a group of emergency medicine experts, have reported that, with the combined use of 6 g/day intravenous vitamin C (1.5 g every 6 h), plus steroids and anticoagulants, mortality was 5% in two ICUs in the US (United Memorial Hospital in Houston, Texas, and Norfolk General Hospital in Norfolk, Virginia), the lowest mortality rates in their respective counties [88].
A case report of 17 COVID-19 patients who were given 1 g of intravenous vitamin C every 8 h for 3 days reported a mortality rate of 12% with 18% rates of intubation and mechanical ventilation and a significant decrease in inflammatory markers, including ferritin and D-dimer, and a trend towards decreasing FiO2 requirements [104]. Another case of unexpected recovery following high-dose intravenous vitamin C has also been reported [105]. While these case reports are subject to confounding and are not prima facie evidence of effects, they do illustrate the feasibility of using vitamin C for COVID-19 with no adverse effects reported.
Reference (we'll rearrange the references after you submitted it)
- Drouin, G.; Godin, J.R.; Page, B. The genetics of vitamin C loss in vertebrates. Curr. Genomics 2011, 12, 371–378.
- Milton, K. Micronutrient intakes of wild primates: Are humans different? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 47–59.
- Milton, K. Nutritional characteristics of wild primate foods: Do the diets of our closest living relatives have lessons for us? Nutrition 1999, 15, 488–498.
- European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on dietary reference values for vitamin C. EFSA J. 2013, 11, 3418.
- Bates, B.; Collins, D.; Cox, L.; Nicholson, S.; Page, P.; Roberts, C.; Steer, T.; Swan, G. National Diet and Nutrition Survey Years 1 to 9 of the Rolling Programme (2008/2009–2016/2017): Time Trend and Income Analyses; Public Health England: London, UK, 2019.
- Berger, M.M.; Bischoff-Ferrari, H.A.; Zimmermann, M.; Herter, I.; Spieldenner, J.; Eggersdorfer, M. White Paper on Nutritional Status in Supporting a Well-Functioning Immune System for Optimal Health with a Recommendation for Switzerland; SGE: Bern, Switzerland, 2020.
- Linus Pauling Institute; Micronutrient Information Center. Micronutrients for Older Adults Oregon State University. 2020. Available online: https://lpi.oregonstate.edu/mic/life-stages/older-adults (accessed on 20 October 2020).
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709.
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846.
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537.
- De Grooth, H.J.; Manubulu-Choo, W.P.; Zandvliet, A.S.; Spoelstra-de Man, A.M.E.; Girbes, A.R.; Swart, E.L.; Oudemans-van Straaten, H.M. Vitamin-C pharmacokinetics in critically ill patients: A randomized trial of four intravenous regimens. Chest 2018, 153, 1368–1377.
- Hume, R.; Weyers, E. Changes in leucocyte ascorbic acid during the common cold. Scott. Med. J. 1973, 18, 3–7.
- Evans-Olders, R.; Eintracht, S.; Hoffer, L.J. Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 2010, 26, 1070–1074.
- Teixeira, A.; Carrie, A.S.; Genereau, T.; Herson, S.; Cherin, P. Vitamin C deficiency in elderly hospitalized patients. Am. J. Med. 2001, 111, 502.
- Fain, O.; Paries, J.; Jacquart, B.; Le Moel, G.; Kettaneh, A.; Stirnemann, J.; Heron, C.; Sitbon, M.; Taleb, C.; Letellier, E.; et al. Hypovitaminosis C in hospitalized patients. Eur. J. Intern. Med. 2003, 14, 419–425.
- Gan, R.; Eintracht, S.; Hoffer, L.J. Vitamin C deficiency in a university teaching hospital. J. Am. Coll. Nutr. 2008, 27, 428–433.
- Ravindran, P.; Wiltshire, S.; Das, K.; Wilson, R.B. Vitamin C deficiency in an Australian cohort of metropolitan surgical patients. Pathology 2018, 50, 654–658.
- Hunt, C.; Chakravorty, N.K.; Annan, G.; Habibzadeh, N.; Schorah, C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam. Nutr. Res. 1994, 64, 212–219.
- Bates, C.J.; Prentice, A.; Cole, T.J.; van der Pols, J.C.; Doyle, W.; Finch, S.; Smithers, G.; Clarke, P.C. Micronutrients: Highlights and research challenges from the 1994-5 National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 1999, 82, 7–15.
- Hemilä, H.; Louhiala, P. Vitamin C may affect lung infections. J. R. Soc. Med. 2007, 100, 495–498.
- Myint, P.K.; Wilson, A.M.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.T. Plasma vitamin C concentrations and risk of incident respiratory diseases and mortality in the European Prospective Investigation into Cancer-Norfolk population-based cohort study. Eur. J. Clin. Nutr. 2019, 73, 1492–1500.
- Hemilä, H.; Louhiala, P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013.
- Kory, P.; Kanne, J.P. SARS-CoV-2 organising pneumonia: ‘Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?’. BMJ Open Respir. Res. 2020, 7, e000724.
- Carr, A.C.; Spencer, E.; Dixon, L.; Chambers, S.T. Patients with community acquired pneumonia exhibit depleted vitamin C status and elevated oxidative stress. Nutrients 2020, 12, 1318.
- Bakaev, V.V.; Duntau, A.P. Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int. J. Tuberc. Lung. Dis. 2004, 8, 263–266.
- Chakrabarti, B.; Banerjee, S. Dehydroascorbic acid level in blood of patients suffering from various infectious diseases. Proc. Soc. Exp. Biol. Med. 1955, 88, 581–583.
- Mochalkin, N.I. Ascorbic acid in the complex therapy of acute pneumonia. Voen. Med. Zhurnal 1970, 9, 17–21.
- Fowler, A.A., III; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., II; Natarajan, R.; Brophy, D.F.; et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA 2019, 322, 1261–1270.
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300.
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32.
- Doise, J.M.; Aho, L.S.; Quenot, J.P.; Guilland, J.C.; Zeller, M.; Vergely, C.; Aube, H.; Blettery, B.; Rochette, L. Plasma antioxidant status in septic critically ill patients: A decrease over time. Fundam. Clin. Pharmacol. 2008, 22, 203–209.
- Voigt, K.; Kontush, A.; Stuerenburg, H.-J.; Muench-Harrach, D.; Hansen, H.C.; Kunze, K. Decreased plasma and cerebrospinal fluid ascorbate levels in patients with septic encephalopathy. Free Radic. Res. 2002, 36, 735–739.
- Schorah, C.J.; Downing, C.; Piripitsi, A.; Gallivan, L.; Al-Hazaa, A.H.; Sanderson, M.J.; Bodenham, A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996, 63, 760–765.
- Arvinte, C.; Singh, M.; Marik, P.E. Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a north American community hospital intensive care unit in May 2020: A pilot study. Med. Drug Discov. 2020.
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 522.
- Carr, A.C. Vitamin C in pneumonia and sepsis. In Vitamin C: New Biochemical and Functional Insights; Chen, Q., Vissers, M., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2020; pp. 115–135.
- Marik, P.E.; Hooper, M.H. Doctor-your septic patients have scurvy! Crit. Care 2018, 22, 23.
- Marik, P.E. Vitamin C: An essential “stress hormone” during sepsis. J. Thorac. Dis. 2020, 12, S84–S88.
- Marik, P.E. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol. Ther. 2018, 189, 63–70.
- Colunga Biancatelli, R.M.L.; Berrill, M.; Marik, P.E. The antiviral properties of vitamin C. Expert Rev. Anti Infect. Ther. 2020, 18, 99–101.
- Thomas, W.R.; Holt, P.G. Vitamin C and immunity: An assessment of the evidence. Clin. Exp. Immunol. 1978, 32, 370–379.
- Dahl, H.; Degre, M. The effect of ascorbic acid on production of human interferon and the antiviral activity in vitro. Acta Pathol. Microbiol. Scand. B 1976, 84, 280–284.
- Webb, A.L.; Villamor, E. Update: Effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr. Rev. 2007, 65, 181–217.
- Hemila, H. Vitamin C and infectious diseases. In Vitamin C; Paoletti, R., Sies, H., Bug, J., Grossi, E., Poli, A., Eds.; Springer: Milan, Italy, 1998; pp. 73–85.
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211.
- Wang, Y.; Russo, T.A.; Kwon, O.; Chanock, S.; Rumsey, S.C.; Levine, M. Ascorbate recycling in human neutrophils: Induction by bacteria. Proc. Natl. Acad. Sci. USA 1997, 94, 13816–13819.
- Nualart, F.J.; Rivas, C.I.; Montecinos, V.P.; Godoy, A.S.; Guaiquil, V.H.; Golde, D.W.; Vera, J.C. Recycling of vitamin C by a bystander effect. J. Biol. Chem. 2003, 278, 10128–10133.
- May, J.M.; Qu, Z.C. Ascorbic acid prevents oxidant-induced increases in endothelial permeability. Biofactors 2011, 37, 46–50.
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal 2013, 19, 2068–2083.
- Sen, C.K.; Packer, L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996, 10, 709–720.
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014, 2014, 426740.
- Erol, N.; Saglam, L.; Saglam, Y.S.; Erol, H.S.; Altun, S.; Aktas, M.S.; Halici, M.B. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: A rat trial. Inflammation 2019, 42, 1585–1594.
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-a/b at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013, 13, 70–74.
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339.
- Atherton, J.G.; Kratzing, C.C.; Fisher, A. The effect of ascorbic acid on infection chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978, 56, 195–199.
- Davelaar, F.G.; Bos, J. Ascorbic acid and infectious bronchitis infections in broilers. Avian Pathol. 1992, 21, 581–589.
- Gan, R.; Rosoman, N.P.; Henshaw, D.J.E.; Noble, E.P.; Georgius, P.; Sommerfeld, N. COVID-19 as a viral functional ACE2 deficiency disorder with ACE2 related multi-organ disease. Med. Hypotheses 2020, 144, 110024.
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24.
- Ma, S.; Sun, S.; Li, J.; Fan, Y.; Qu, J.; Sun, L.; Wang, S.; Zhang, Y.; Yang, S.; Liu, Z.; et al. Single-cell transcriptomic atlas of primate cardiopulmonary aging. Cell Res. 2020.
- Kumar, V.; Jena, M. In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against COVID-19. Res. Square 2020.
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136.
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307.
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535.
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A., III; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151.
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A., III; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L20–L32.
- Hornig, D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975, 258, 103–118.
- Padayatty, S.J.; Doppman, J.L.; Chang, R.; Wang, Y.; Gill, J.; Papanicolaou, D.A.; Levine, M. Human adrenal glands secrete vitamin C in response to adrenocorticotrophic hormone. Am. J. Clin. Nutr. 2007, 86, 145–149.
- Kodama, M.; Kodama, T.; Murakami, M.; Kodama, M. Vitamin C infusion treatment enhances cortisol production of the adrenal via the pituitary ACTH route. In Vivo 1994, 8, 1079–1085.
- Barabutis, N.; Khangoora, V.; Marik, P.E.; Catravas, J.D. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest 2017, 152, 954–962.
- Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with Covid-19-preliminary report. N. Engl. J. Med. 2020.
- Pauling, L. The significance of the evidence about ascorbic acid and the common cold. Proc. Natl. Acad. Sci. USA 1971, 68, 2678–2681.
- Pauling, L. Vitamin C the Common Cold and Flu; Freeman: San Francisco, CA, USA, 1970.
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013.
- Van Straten, M.; Josling, P. Preventing the common cold with a vitamin C supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2002, 19, 151–159.
- Klenner, F.R. Massive doses of vitamin C and the virus diseases. South Med. Surg. 1951, 113, 101–107.
- Pitt, H.A.; Costrini, A.M. Vitamin C prophylaxis in marine recruits. JAMA 1979, 241, 908–911.
- Kimbarowski, J.A.; Mokrow, N.J. Colored precipitation reaction of the urine according to Kimbarowski (FARK) as an index of the effect of ascorbic acid during treatment of viral influenza. Dtsch Gesundheitsw. 1967, 22, 2413–2418.
- Glazebrook, A.J.; Thomson, S. The administration of vitamin C in a large institution and its effect on general health and resistance to infection. J. Hyg. 1942, 42, 1–19.
- Nabil Habib, T.; Ahmed, I. Early adjuvant intravenous vitamin C treatment in septic shock may resolve the vasopressor dependence. Int. J. Microbiol. Adv. Immunol. 2017, 5, 77–81.
- Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose ascorbic acid on vasopressor’s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100.
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. High-dose vitamin C infusion for the treatment of critically ill COVID-19. Res. Square 2020.
- Zhang, M.; Jativa, D.F. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018, 6.
- Hemila, H.; Chalker, E. Vitamin C can shorten the length of stay in the ICU: A meta-analysis. Nutrients 2019, 11, 708.
- Hemila, H.; Chalker, E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: A meta-regression analysis. J. Intensive Care 2020, 8, 15.
- Long, C.L.; Maull, K.I.; Krishnan, R.S.; Laws, H.L.; Geiger, J.W.; Borghesi, L.; Franks, W.; Lawson, T.C.; Sauberlich, H.E. Ascorbic acid dynamics in the seriously ill and injured. J. Surg. Res. 2003, 109, 144–148.
- Kashiouris, M.G.; L’Heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The emerging role of vitamin C as a treatment for sepsis. Nutrients 2020, 12, 292.
- Marik, P.E.; Kory, P.; Varon, J.; Iglesias, J.; Meduri, G.U. MATH+ protocol for the treatment of SARS-CoV-2 infection: The scientific rationale. Expert Rev. Anti Infect. Ther. 2020, 1–7.
- Hemilä, H.; Chalker, E. Reanalysis of the effect of vitamin C on mortality in the CITRIS-ALI trial: Important findings dismissed in the trial report. Front. Med. 2020.
- Fowler, A.A., III; Fisher, B.J.; Kashiouris, M.G. Vitamin C for sepsis and acute respiratory failure—Reply. JAMA 2020, 323, 792–793.
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients with Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020, 323, 423–431.
- Long, M.T.; Kory, P.; Marik, P. Vitamin C, hydrocortisone, and thiamine for septic shock. JAMA 2020, 323, 2203–2204.
- Carr, A.C. Is the VITAMINS RCT indicating potential redundancy between corticosteroids and vitamin C? Crit. Care 2020, 24, 129.
- Carr, A.C.; Rowe, S. Factors affecting vitamin C status and prevalence of deficiency: A global health perspective. Nutrients 2020, 12, 1963.
- Patterson, G.; Isales, C.M.; Fulzele, S. Low level of vitamin C and dysregulation of vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2020, 12.
- Michels, A.J.; Joisher, N.; Hagen, T.M. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch. Biochem. Biophys. 2003, 410, 112–120.
- Subramanian, V.S.; Sabui, S.; Subramenium, G.A.; Marchant, J.S.; Said, H.M. Tumor Necrosis Factor alpha (TNF-alpha) reduces intestinal vitamin C uptake: A role for NF-kB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G241–G248.
- Subramanian, V.S.; Sabui, S.; Moradi, H.; Marchant, J.S.; Said, H.M. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta Biomembr. 2018, 1860, 556–565.
- Domain-Specific Appendix: VITAMIN, C. REMAP-CAP: Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia 2020. Available online: https://static1.squarespace.com/static/5cde3c7d9a69340001d79ffe/t/5f1bba732cda7f10310643fe/1595652735252/REMAP-CAP+Vitamin+C+Domain+Specific+Appendix+V2+-+08+June+2020_WM.pdf (accessed on 26 September 2020).
- Vizcaychipi, M.P.; Shovlin, C.L.; McCarthy, A.; Howard, A.; Brown, A.; Hayes, M.; Singh, S.; Christie, L.; Sisson, A.; Davies, R.; et al. Development and implementation of a COVID-19 near real-time traffic light system in an acute hospital setting. Emerg. Med. J. 2020, 37, 630–636.
- ICNARC Report on COVID-19 in Critical Care: Chelsea and Westminster Hospital Intensive Care Unit. London. 2020. Available online: https://www.patrickholford.com/uploads/2020/chelwesticnarcreportjune.pdf (accessed on 12 June 2020).
- ICNARC Report on COVID-19 in Critical Care. London. 2020. Available online: https://www.patrickholford.com/uploads/2020/nationwideicnarcreportjune.pdf (accessed on 26 June 2020).
- Mosdol, A.; Erens, B.; Brunner, E.J. Estimated prevalence and predictors of vitamin C deficiency within UK’s low-income population. J. Public Health 2008, 30, 456–460.
- Hiedra, R.; Lo, K.B.; Elbashabsheh, M.; Gul, F.; Wright, R.M.; Albano, J.; Azmaiparashvili, Z.; Patarroyo Aponte, G. The use of IV vitamin C for patients with COVID-19: A case series. Expert Rev. Anti Infect. Ther. 2020, 18, 1259–1261.
- Waqas Khan, H.M.; Parikh, N.; Megala, S.M.; Predeteanu, G.S. Unusual early recovery of a critical COVID-19 patient after administration of intravenous vitamin C. Am. J. Case Rep. 2020, 21, e925521.
This entry is adapted from the peer-reviewed paper 10.3390/nu12123760
