- CAR T cells,immunotherapy,pediatric neuroblastom
1. Use in Cancer
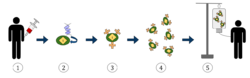
1. T-cells (represented by objects labeled as ’t’) are removed from the patient's blood.
2. Then in a lab setting the gene that encodes for the specific antigen receptors are incorporated into the T-cells.
3. Thus producing the CAR receptors (labeled as c) on the surface of the cells.
4. The newly modified T-cells are then further harvested and grown in the lab.
5. After a certain time period, the engineered T-cells are infused back into the patient.
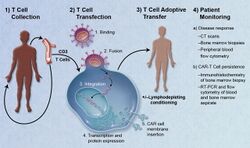
Adoptive transfer of T cells expressing chimeric antigen receptors is a promising anti-cancer therapeutic as CAR-modified T cells can be engineered to target virtually any tumor associated antigen. There is great potential for this approach to improve patient-specific cancer therapy in a profound way. Following collection of a patient's T cells, the cells are genetically engineered to express CARs specifically directed toward antigens on the patient's tumor cells, then infused back into the patient.[6]
Preparation
The first step in the introduction of CAR-T cells into the body of a patient is the removal of activated leukocytes from the blood in a process known as leukocyte apheresis. The leukocytes are removed using a blood cell separator. The patient’s autologous peripheral blood mononuclear cells (PBMC) are then separated and collected from the buffy coat that forms.[7] The products of leukocyte apheresis are then transferred into a cell processing center. In the cell processing centre, specific T-cells are activated in a certain environment in which they can actively proliferate. The cells are activated using a type of cytokine called an interleukin, specifically Inter-Leukin 2 (IL-2) as well as anti-CD3 antibodies.[8]
The T-cells are then transfected with CD19 CAR genes by either an integrating gammaretrovirus (RV) or by lentivirus (LV) vectors. These vectors are very safe in modern times due to a partial deletion of the U3 region.[9] The patient undergoes lymphodepletion chemotherapy prior to the introduction of the engineered CD CAR-T cells.[10] The depletion of the number of circulating leukocytes in the patient upregulates the number of cytokines that are produced which help to promote the expansion of the engineered CAR-T cell.[11]
Safety concerns
CAR-T cells are undoubtedly a major breakthrough in cancer treatment. However, there are still expected and unexpected toxicities that result from CAR-T cells being introduced into the body. These toxicities include cytokine release syndrome, neurological toxicity, on-target/off-tumor recognition, insertional mutagenesis, and anaphylaxis.[10]
Cytokine release syndrome (CRS) is a condition in which the immune system is activated and releases an increased number of inflammatory cytokines. The clinical manifestations of this syndrome include high fever, fatigue, myalgia, nausea, tachycardia, capillary leakages, cardiac dysfunction, hepatic failure, and renal impairment.[12]
The neurological toxicity associated with CAR-T cells have clinical manifestations that include delirium, the partial loss of the ability to speak a coherent language while still having the ability to interpret language (expressive aphasia), lowered alertness (obtundation), and seizures.[13] During some clinical trials deaths caused by neurotoxicity have occurred. The main cause of death from neurotoxicity is cerebral edema. In a study carried out by Juno Therapeutics, Inc., five patients enrolled in the trial died as a result of cerebral edema. Two of the patients were treated with cyclophosphamide alone and the remaining three were treated with a combination of cyclophosphamide and fludarabine.[14] In another clinical trial sponsored by the Fred Hutchinson Cancer Research Center, there was one reported case of irreversible and fatal neurological toxicity 122 days after the administration of CAR-T cells.[15]
On-target/off-tumor recognition occurs when the CAR-T cell recognizes the correct antigen, but the antigen is expressed on healthy, non-pathogenic tissue. This results in the CAR-T cells attacking non-tumor tissue, such as healthy B cells that express CD19. The severity of this adverse effect can vary from B-cell aplasia to extreme toxicity leading to death.[8]
Anaphylaxis is an expected side effect, as the CAR is made with a foreign monoclonal antibody and as a result, invokes an immune response.
There is also a potential for insertional mutagenesis, which can occur when using a virus to insert the CAR vector DNA into a host T cell. Lentiviral (LV) vectors carry a lower risk than retroviral (RV) vectors. However, both have the potential to be oncogenic.
Because it is a relatively new treatment, there is little data about the long-term effects of CAR-T cell therapy. There are still concerns about long-term survival as well as pregnancy complications in female patients treated with CAR-T cells.[13]
2. Structure
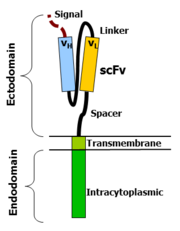
CAR-T cells create a link between an extracellular ligand recognition domain to an intracellular signalling molecule which in turn activates T cells. The extracellular ligand recognition domain is usually a single-chain variable fragment (scFv). CARs are composed of three regions: the ectodomain, the transmembrane domain and the endodomain.
Ectodomain
The ectodomain is the region of the receptor that is exposed to the extracellular fluid and consists of 3 components: a signalling peptide, an antigen recognition region and a spacer.
A signal peptide directs the nascent protein into the endoplasmic reticulum. The signal protein in CAR is called a single-chain variable fragment (scFv),[16] a type of protein known as a fusion protein or chimeric protein. A fusion protein is a protein that is formed by merging two or more genes that code originally for different proteins but when they are translated in the cell, the translation produces one or more polypeptides with functional properties derived for each of the original genes.[17]
A scFv is a chimeric protein made up of the light and heavy chains of immunoglobins connected with a short linker peptide. The linker consists of hydrophilic residues with stretches of glycine and serine in it for flexibility as well as stretches of glutamate and lysine for added solubility.[18]
Transmembrane domain
The transmembrane domain is a hydrophobic alpha helix that spans the membrane. The transmembrane domain is essential for the stability of the receptor as a whole. At present, the CD28 transmembrane domain is the most stable of the domains.
Generally, the transmembrane domain from the most membrane proximal component of the endodomain is used. Using the CD3-zeta transmembrane domain may result in incorporation of the artificial TCR into the native TCR, a factor that is dependent on the presence of the native CD3-zeta transmembrane charged aspartic acid residue.[19] Different transmembrane domains result in different receptor stability. The CD28 transmembrane domain results in a highly expressed, stable receptor.
Endodomain
This is the functional end of the receptor. After antigen recognition, receptors cluster and a signal is transmitted to the cell.[16] The most commonly used endodomain component is CD3-zeta which contains 3 ITAMs. This transmits an activation signal to the T cell after the antigen is bound. CD3-zeta may not provide a fully competent activation signal and co-stimulatory signaling is needed. For example, chimeric CD28 and OX40 can be used with CD3-Zeta to transmit a proliferative/survival signal or all three can be used together.
3. History
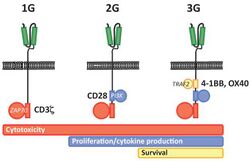
First generation CARs were developed in 1989 by Gideon Gross and Zelig Eshhar[21][22] at Weizmann Institute, Israel.[23] The first generation of CARs are composed of an extracellular binding domain, a hinge region, a transmembrane domain, and one or more intracellular signaling domains.[4] Extracellular binding domain contains single‐chain variable fragments (scFvs) derived from tumor antigen‐reactive antibodies and usually have high specificity to tumor antigen.[4] All CARs harbor the CD3ζ chain domain as the intracellular signaling domain, which is the primary transmitter of signals. Second generation CARs also contain co‐stimulatory domains, like CD28 and/or 4‐1BB. The involvement of these intracellular signaling domains improve T cell proliferation, cytokine secretion, resistance to apoptosis, and in vivo persistence.[4] Besides co-stimulatory domains, the third‐generation CARs combine multiple signaling domains, such as CD3z-CD28-41BB or CD3z-CD28-OX40, to augment T cell activity. Preclinical data shows the third-generation CARs exhibit improved effector functions and in vivo persistence as compared to second‐generation CARs.[4] Recently, the fourth‐generation CARs (also known as TRUCKs or armored CARs), combine the expression of a second‐generation CAR with factors that enhance anti‐tumoral activity (e.g., cytokines, co‐stimulatory ligands).[24]
The evolution of CAR therapy is an excellent example of the application of basic research to the clinic. The PI3K binding site used was identified in co-receptor CD28,[25] while the ITAM motifs were identified as a target of the CD4- and CD8-p56lck complexes.[26]
The introduction of Strep-tag II sequence (an eight-residue minimal peptide sequence (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys) that exhibits intrinsic affinity toward streptavidin[27]) into specific sites in synthetic CARs or natural T-cell receptors provides engineered T cells with an identification marker for rapid purification, a method for tailoring spacer length of chimeric receptors for optimal function and a functional element for selective antibody-coated, microbead-driven, large-scale expansion.[28][29] Strep-tag can be used to stimulate the engineered cells, causing them to grow rapidly. Using an antibody that binds the Strep-tag, the engineered cells can be expanded by 200-fold. Unlike existing methods this technology stimulates only cancer-specific T cells.
Smart T cell
Combined with exogenous molecules, some synthetic control devices have been implemented on CAR-T cells and alter the cell activity. Smart T cell is engineered with suicide gene or other synthetic control panels to precisely control therapeutic function over the timing and dosage, there by alleviating cytotoxicity.[30] Several strategies to improve safety and efficacy of CAR-T cells are:
Suicide gene engineering: engineered T cells are incorporated with suicide genes, which can be activated by extracellular molecule and then induce T cell apoptosis. Herpes simplex virus thymidine kinase (HSV-TK) and inducible caspase 9 (iCas9) are two types suicide genes have been integrated into CAR-T cells.[30][31][32] In iCas9 system, the suicide gene is composed of the sequence of the mutated FK506-binding protein with high specificity to a small-molecule, AP1903 and a gene encoding human caspase 9 switch. When the release of cytokines by CAR-T cells becomes more pronounced than basic levels, the iCas9 can be dimerized and lead to rapid apoptosis of T cells. Although both suicide genes demonstrate a noticeable function of as a safety switch in clinical trials for cellular therapies, some hinder defects limit the application of this strategy. HSV-TK is derived from virus and may be immunogenic to humans.[30][33] The suicide gene strategies may not act quickly enough to eliminate off-tumor cytotoxicity as well.
Dual-antigen receptor: T cells are engineered to express two tumor-associated antigen receptors at the same time. The dual-antigen receptor of engineered T cell module has been reported to have less intense side effects.[34] The activation of CAR-T cell via TCR-CD3ζ signal transduction pathway is transient and a complementary signal pathway provided by co-stimulatory molecules on antigen presenting cells promotes survival of modified-T cell can ability in controlling tumor.[35] An in vivo study in mice shows the dual-receptor T cells effectively eradicated prostate cancer and achieved complete long-term survival.[36]
ON-switch: ON-switch CAR-T cell split synthetic receptors into two parts: the first part mainly contains an antigen binding domain towards and the other part features two different downstream signaling elements (e.g. CD3ζ and 4-1BB). Upon the presence of an exogenous molecule (rapamycin analogs for example), two physically separated signaling elements fuse together and CAR-T cells exert therapeutic functions.[37] In this mechanism, the engineered T cell shows therapeutic function only in the presence of both tumor antigen and a benign exogenous molecule.
Bifunctional molecules as switches: The bispecific antibodies are developed as an efficacious bridge to target cytotoxic T cells to cancer cells and causes localized T cell activation. In this strategy, the bispecific antibody targets CD3 molecule of T cell and tumor-associated antigen presented on cancer cell surface.[38] The anti-CD20/CD3 bispecific molecule shows high specificity to both malignant B cells and cancer cells in mice.[39] FITC is another bifunctional molecule used in this strategy. FITC can redirect and regulate the activity of the FITC-specific CAR-T cells toward tumor cells with folate receptors.[40]
SMDC adaptor technology
SMDCs (small molecule drug conjugates) platform in immuno-oncology is a novel (currently experimental) approach that makes possible the engineering of a single universal CAR T cell, which binds with extraordinarily high affinity to a benign molecule designated as FITC. These cells are then used to treat various cancer types when co-administered with bispecific SMDC adaptor molecules. These unique bispecific adaptors are constructed with a FITC molecule and a tumor-homing molecule to precisely bridge the universal CAR T cell with the cancer cells, which causes localized T cell activation. Anti-tumor activity in mice is induced only when both the universal CAR T cells plus the correct antigen-specific adaptor molecules are present. Anti-tumor activity and toxicity can be controlled by adjusting the administered adaptor molecule dosing. Treatment of antigenically heterogeneous tumors can be achieved by administration of a mixture of the desired antigen-specific adaptors. Thus, several challenges of current CAR T cell therapies, such as:
- the inability to control the rate of cytokine release and tumor lysis
- the absence of an “off switch” that can terminate cytotoxic activity when tumor eradication is complete
- a requirement to generate a different CAR T cell for each unique tumor antigen
may be solved or mitigated using this approach.[41][42][43]
4. Clinical Studies
As of August 2017, there were around 200 clinical trials happening globally involving CAR-T cells.[10] Around 65% of those trials targeted blood cancers, and 80% of them involved CD19 CAR-T cells targeting B-cell cancers.[10] In 2016, studies began to explore the viability of other antigens, such as CD20.[44]
FDA approvals
The first two FDA approved CAR-T therapies both target the CD19 antigen, which is found on many types of B-cell cancers.[45] Tisagenlecleucel (Kymriah) is approved to treat relapsed/refractory B-cell precursor acute lymphoblastic leukemia (ALL), while axicabtagene ciloleucel (Yescarta) is approved to treat relapsed/refractory diffuse large B-cell lymphoma (DLBCL).[45]
Kymriah's clinical trial showed an 83% remission rate of all types of B-cell ALL after three months post treatment.[46] However, 49% of patients also suffered cytokine release syndrome (CRS), a serious side effect that has been responsible for several deaths in clinical trials run by Novartis’ competitors.[46]
This entry is adapted from the peer-reviewed paper 10.3390/vaccines8040753