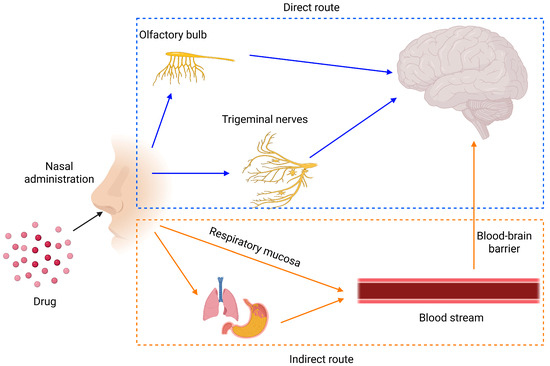
Nose-to-brain drug delivery is an innovative approach that leverages the unique anatomical pathways connecting the nasal cavity to the brain, including the olfactory and trigeminal nerve routes. This method bypasses the blood–brain barrier, enabling direct and efficient transport of therapeutic agents to the central nervous system. It offers significant advantages, such as rapid drug action, reduced systemic side effects, and improved patient compliance through non-invasive administration. This entry summarizes factors affecting the nose-to-brain delivery of drugs and the recent development of nanoparticle-based nose-to-brain delivery.
- nose-to-brain
- blood–brain barrier
- bioavailability
- drug delivery
- nanoparticle
- polymeric nanoparticles
- SLNs
- NLCs
- emulsions
- liposomes
This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia5030091
References
- Prabakaran, A.; Agrawal, M.; Dethe, M.R.; Ahmed, H.; Yadav, A.; Gupta, U.; Alexander, A. Nose-to-brain drug delivery for the treatment of Alzheimer’s disease: Current advancements and challenges. Expert Opin. Drug Deliv. 2022, 19, 87–102.
- Koo, J.; Lim, C.; Oh, K.T. Recent Advances in Intranasal Administration for Brain-Targeting Delivery: A Comprehensive Review of Lipid-Based Nanoparticles and Stimuli-Responsive Gel Formulations. Int. J. Nanomed. 2024, 19, 1767–1807.
- Du, L.; Chen, L.; Liu, F.; Wang, W.; Huang, H. Nose-to-brain drug delivery for the treatment of CNS disease: New development and strategies. Int. Rev. Neurobiol. 2023, 171, 255–297.
- Huang, Q.; Chen, Y.; Zhang, W.; Xia, X.; Li, H.; Qin, M.; Gao, H. Nanotechnology for enhanced nose-to-brain drug delivery in treating neurological diseases. J. Control. Release 2024, 366, 519–534.
- Hong, S.-S.; Oh, K.T.; Choi, H.-G.; Lim, S.-J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540.
- Nguyen, T.-T.-L.; Maeng, H.-J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572.
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood—Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834.
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286.
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177.
- Xu, K.; Duan, S.; Wang, W.; Ouyang, Q.; Qin, F.; Guo, P.; Hou, J.; He, Z.; Wei, W.; Qin, M. Nose-to-brain delivery of nanotherapeutics: Transport mechanisms and applications. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1956.
- Chakraborty, S.; Karmakar, V.; Chatterjee, K.; Chatterjee, A.; Dwivedi, M.; Gorain, B. Chitosan nanoparticle-mediated nose-to-brain delivery of naringenin: Attenuating memory decline in experimental animals via behavioural assessment and modulation of biochemical parameters. Int. J. Biol. Macromol. 2025, 286, 138336.
- Hameed, H.; Faheem, S.; Younas, K.; Jamshaid, M.; Ereej, N.; Hameed, A.; Munir, R.; Khokhar, R. A comprehensive review on lipid-based nanoparticles via nose to brain targeting as a novel approach. J. Microencapsul. 2024, 41, 681–714.
- Liu, Y.; Tan, Y.; Cheng, G.; Ni, Y.; Xie, A.; Zhu, X.; Yin, C.; Zhang, Y.; Chen, T. Customized Intranasal Hydrogel Delivering Methylene Blue Ameliorates Cognitive Dysfunction against Alzheimer’s Disease. Adv. Mater. 2024, 36, e2307081.
- Teng, Z.; Meng, L.-Y.; Yang, J.-K.; He, Z.; Chen, X.-G.; Liu, Y. Bridging nanoplatform and vaccine delivery, a landscape of strategy to enhance nasal immunity. J. Control. Release 2022, 351, 456–475.
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207.
- Deshmukh, V.; Pathan, N.S.; Haldar, N.; Nalawade, S.; Narwade, M.; Gajbhiye, K.R.; Gajbhiye, V. Exploring intranasal drug delivery via nanocarriers: A promising glioblastoma therapy. Colloids Surf. B Biointerfaces 2024, 245, 114285.
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757.
- Chen, Y.; Zhang, C.; Huang, Y.; Ma, Y.; Song, Q.; Chen, H.; Jiang, G.; Gao, X. Intranasal drug delivery: The interaction between nanoparticles and the nose-to-brain pathway. Adv. Drug Deliv. Rev. 2024, 207, 115196.
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy Disease and Nose-to-Brain Delivery of Polymeric Nanoparticles: An Overview. Pharmaceutics 2019, 11, 118.
- Jin, Z.; Han, Y.; Zhang, D.; Li, Z.; Jing, Y.; Hu, B.; Sun, S. Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics 2022, 14, 2070.
- Semyachkina-Glushkovskaya, O.; Shirokov, A.; Blokhina, I.; Telnova, V.; Vodovozova, E.; Alekseeva, A.; Boldyrev, I.; Fedosov, I.; Dubrovsky, A.; Khorovodov, A.; et al. Intranasal Delivery of Liposomes to Glioblastoma by Photostimulation of the Lymphatic System. Pharmaceutics 2022, 15, 36.
- Yan, M.; Cheng, L.; Zheng, Z.; Lin, Y.; Qin, D.; Chen, H. Advances in the Understanding of ocular and nasal lymphatics. BMC Immunol. 2025, 26, 16.
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200.
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159.
- Singh, A.P.; Saraf, S.K.; Saraf, S.A. SLN approach for nose-to-brain delivery of alprazolam. Drug Deliv. Transl. Res. 2012, 2, 498–507.
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914.
- Nguyen, T.-T.-L.; Duong, V.-A. Advancements in Nanocarrier Systems for Nose-to-Brain Drug Delivery. Pharmaceuticals 2025, 18, 615.
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52.
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601.
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189.
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100.
- Boyuklieva, R.; Pilicheva, B. Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines 2022, 10, 1706.
- Cunha, S.; Almeida, H.; Amaral, M.H.; Lobo, S.J.M.; Silva, A.C. Intranasal Lipid Nanoparticles for the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2017, 23, 6553–6562.
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Gorain, B.; Choudhury, H. Antipsychotic Potential and Safety Profile of TPGS-Based Mucoadhesive Aripiprazole Nanoemulsion: Development and Optimization for Nose-To-Brain Delivery. J. Pharm. Sci. 2021, 110, 1761–1778.
- Uppuluri, C.T.; Ravi, P.R.; Dalvi, A.V. Design, optimization and pharmacokinetic evaluation of Piribedil loaded solid lipid nanoparticles dispersed in nasal in situ gelling system for effective management of Parkinson’s disease. Int. J. Pharm. 2021, 606, 120881.
- Yadav, R.K.; Shah, K.; Dewangan, H.K. Intranasal drug delivery of sumatriptan succinate-loaded polymeric solid lipid nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2022, 48, 21–28.
- Sivadasu, P.; Gowda, D.V.; Subramani, N.K.; Malgur, B.; Vishweshwaraiah, S.S.; Hatna, S. Direct brain targeted nanostructured lipid carriers for sustained release of schizophrenic drug: Formulation, characterization and pharmacokinetic studies. Brain 2020, 9, 12.
- Duan, Z.; Zhou, W.; He, S.; Wang, W.; Huang, H.; Yi, L.; Zhang, R.; Chen, J.; Zan, X.; You, C.; et al. Intranasal Delivery of Curcumin Nanoparticles Improves Neuroinflammation and Neurological Deficits in Mice with Intracerebral Hemorrhage. Small Methods 2024, 8, e2400304.
- Awad, R.; Avital, A.; Sosnik, A. Polymeric nanocarriers for nose-to-brain drug delivery in neurodegenerative diseases and neurodevelopmental disorders. Acta Pharm. Sin. B 2023, 13, 1866–1886.
- Kanoujia, J.; Kishore, A.; Parashar, P. Progress in Polymeric Micelles as Viable Wagons for Brain Targeting. Curr. Pharm. Des. 2023, 29, 116–125.
- Nguyen, T.-T.-L.; Duong, V.-A. Solid Lipid Nanoparticles. Encyclopedia 2022, 2, 952–973.
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781.
