The paper aims to advance automated prostate segmentation on T2‑weighted MRI by introducing a hybrid topological atlas‑based method. It leverages a collection of pre‑labeled MRI atlas images to capture anatomical variability and produce accurate prostate segmentations.
Experimental Design
-
Data: The approach was evaluated on 30 T2‑weighted MRI scans.
-
Evaluation metric: Automated contours were compared with manual expert segmentations using the Dice Similarity Coefficient (DSC).
-
Outcome: The method achieved high DSC values, indicating that it closely approximates expert-defined prostate boundaries.
📊 Key Results
-
Demonstrated good overlap between automated and manual segmentations, proving that the hybrid approach is effective.
-
Offers a quantitative performance benchmark: consistently solid DSC scores across the 30-case dataset.
- MRI T2
- image segmentation
- prostate
- image
- segmentation
- atlas
- hybrid method
- medical informatics
1. 📌 Objective & Motivation
The method introduces a hybrid topological atlas-based algorithm for fully automatic prostate segmentation using T2-weighted MRI, designed to address limitations in time and accuracy of manual delineation. It uses a set of pre-labeled atlas images, aiming to deliver robust contours across patients with diverse prostate shapes.
2. 🌐 Atlas-Based & Topological Approach
Atlas Preparation
-
A small but diverse cohort of prostate MRIs are manually segmented to create labeled atlas images.
-
The atlas captures variations in size, shape, and orientation across prostates.
Matching & Registration
-
A new MRI (target) is aligned spatially to each atlas via image registration.
-
The topological similarity (contour shape, directional edges, intensity gradient) between atlases is computed.
-
This determines which atlas (or weighted combination) best matches the target’s topology.
Contour Estimation
-
Leveraging the union of compatible contours from selected atlases, a composite “inferred prostate region” is built.
-
The method then maps these contours precisely to the target image, granting an automated prostate outline.
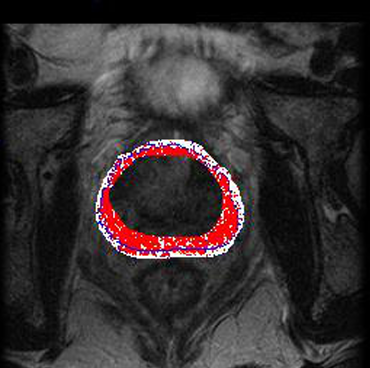
3. ✅ Performance & Evaluation
-
Tested on 30 T2-weighted prostate MRI scans, with segmentations compared to expert manual delineations.
-
Evaluated via Dice Similarity Coefficient (DSC): results indicated high overlap, demonstrating strong segmentation accuracy.
-
The algorithm exhibited fast execution; typically, only a small region of interest is processed, reducing computational load.
4. 🎯 Strengths & Constraints
Strengths
-
Topologically driven matching ensures segmentation respects prostate shape and contour direction.
-
It’s conceptually straightforward and easier to implement than complex deep learning methods.
-
Computationally efficient, as only relevant subregions are processed.
Limitations
-
Relies heavily on the atlas database: new prostate shapes not represented may be missed.
-
If the target’s unique anatomy (e.g., due to severe hypertrophy) lies outside the atlas union, segmentation may fail.
5. 📚 Broader Context & Follow-up Methods
Atlas vs. Deep Learning Today
-
Modern approaches (e.g., U-Net, 3D CNNs, transformers) achieve DSC ≈ 0.9+ for whole gland; zonal segmentation lags slightly behind (DSC ≈ 0.79–0.87).
-
Data robustness is key: domain adaptation and boundary-aware losses help compensate for MRI variance and boundary uncertainty.
Technical Evolution
-
3D multi-planar CNNs (axial + sagittal scans) improved accuracy near prostate apex/base.
-
Transformer-based models now use cross-slice attention to better capture structural continuity.
6. 🧠 Summary Comparison Table
| Aspect | Topological Atlas Method | Modern Deep Learning Models |
|---|---|---|
| Data | 30 manually segmented MRIs | Hundreds to thousands of annotated MRIs |
| Approach | Atlas registration + contour union | CNNs (e.g., U-Net, V-Net), Transformers |
| Performance | Good DSC; efficient contours | Higher DSC (≈0.90 W.G., ≈0.79–0.87 zonal) |
| Speed | Quick due to ROI focus | Varies; commonly fast with GPU acceleration |
| Scalability | Atlas-limited; manual atlas prep | Scalable; needs large annotated datasets |
| Robustness | Sensitive to anatomy not seen in atlas | Generally robust with data augmentation |
7. 🧩 Practical Implications
-
Suite for resource-limited environments: reproducible without massive datasets or specialized hardware.
-
Good fit for institutions with a small atlas archive aiming for quick deployment.
-
Ideal as a baseline or teaching tool to understand topological segmentation before transitioning to neural methods.
8. 🔮 Future Directions
To enhance the topological method, one could:
-
Expand atlas diversity – more cases, including atypical anatomy.
-
Integrate ML-derived shape priors to handle contour variability absent in atlas.
-
Use hybrid pipelines: topological outputs guide or refine deep learning segmenters.
-
Blend active contour techniques to adapt atlas contours dynamically rather than fixed mapping.
9. 📝 In Summary
The topological atlas-based method (2014) offers a fast, interpretable, and implementation-friendly approach for segmenting the prostate on T2 MRI. While deep learning now dominates—with higher accuracy, scalability, and anatomical generalizability—this method remains relevant for small-scale environments and algorithmic education. It underscores the persistent value of topology-driven segmentation, and provides foundational insights for modern hybrid systems that blend atlas priors with data-driven learning.
This entry is adapted from: https://annals-csis.org/proceedings/2014/pliks/204.pdf
References
- Daniela Hristov; Done Stojanov; In silico report on five high-risk Protein C pathogenic variants: G403R, P405S, S421N, C238S, and I243T. Mutat. Res. Mol. Mech. Mutagen. 2025, 831, 111907, .
- Daniela Hristov; Done Stojanov; Exploring Regulatory Properties of Genes Associated with Nonsyndromic Male Infertility. Reprod. Med. 2024, 5, 136-153, .
