This review illuminates established knowledge of root–arbuscular mycorrhizal fungi (AMF)–plant mutualism to study the uptake of phosphorus (P) as a critical element for plant nutrition. We focus on P cycling, underscoring the role of AMF in enhancing P acquisition and plant resilience in the rhizosphere. The role(s) of plant roots, root exudates, and biomolecules in relevant soil processes is emphasized in this manuscript. Enhancing P uptake efficiency through AMF interaction presents a promising avenue for sustainable agriculture, with future research opportunities focusing on understanding underlying mechanisms and developing innovative technologies as a need to transition from the use of AMF as a biofertilizer or as an inoculation alternative for seeds to being an inspiration for the development of technology adapted to different crops. This is important to promote responsible agricultural practices and improve crop yields. We provide definitions of key terms and concepts for one of the best-known natural sustainable phosphorus systems. This manuscript illuminates and aims to inspire technology development to overcome the challenge of plant nutrition under P scarcity conditions.
- arbuscular mycorrhizal
- fungi
- phosphorus
- technology
- sensors
1.1. Arbuscular mycorrhizal Fungi
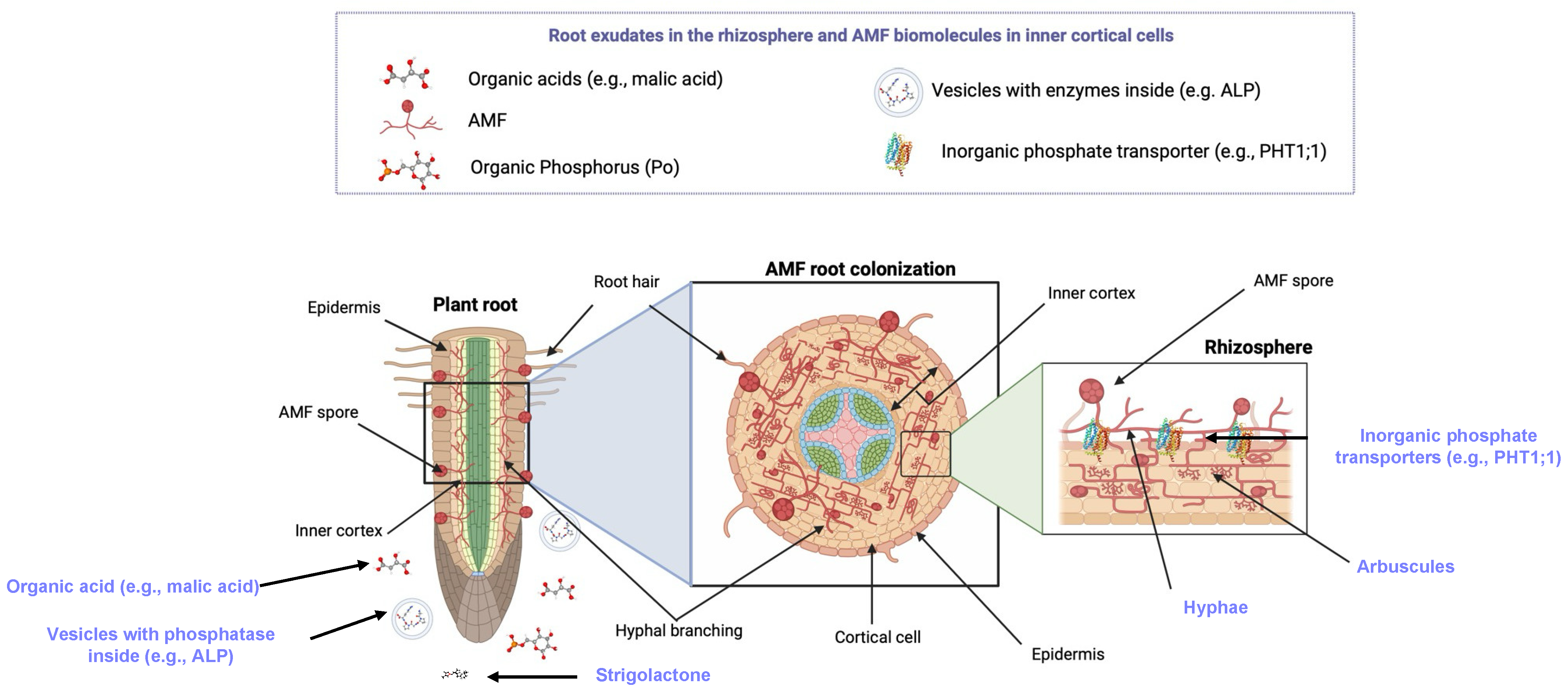 Figure 1. AMF root colonization is a mutualistic relationship for phosphate uptake by plants. The ectomycorrhizae shown on the left side of the diagram depict fungal hyphae [7] extending into the plant root during AMF colonization. The exploded view (middle, right) shows endomycorrhizae, where AMF hyphae extend into the root cortex. The diagrams expand on the work by [5] and highlight seven key structures in purple color. These key structures include: (i) AMF, (ii) root exudates (PubChem ID: 15102684; [8]), (iii) D-glucose-6-phosphate (as an example of organic phosphorus) (PubChem ID: 5958, [9]), (iv) inorganic phosphorus transporters (PiPT; PBD Entry: 8FVZ, [10]), and (v) hyphae. Malic acid was used as an example of an organic acid in soil (PubChem ID: 525). Figure created in Biorender.com.
Figure 1. AMF root colonization is a mutualistic relationship for phosphate uptake by plants. The ectomycorrhizae shown on the left side of the diagram depict fungal hyphae [7] extending into the plant root during AMF colonization. The exploded view (middle, right) shows endomycorrhizae, where AMF hyphae extend into the root cortex. The diagrams expand on the work by [5] and highlight seven key structures in purple color. These key structures include: (i) AMF, (ii) root exudates (PubChem ID: 15102684; [8]), (iii) D-glucose-6-phosphate (as an example of organic phosphorus) (PubChem ID: 5958, [9]), (iv) inorganic phosphorus transporters (PiPT; PBD Entry: 8FVZ, [10]), and (v) hyphae. Malic acid was used as an example of an organic acid in soil (PubChem ID: 525). Figure created in Biorender.com.1.2. Phosphorus Is a Key Element
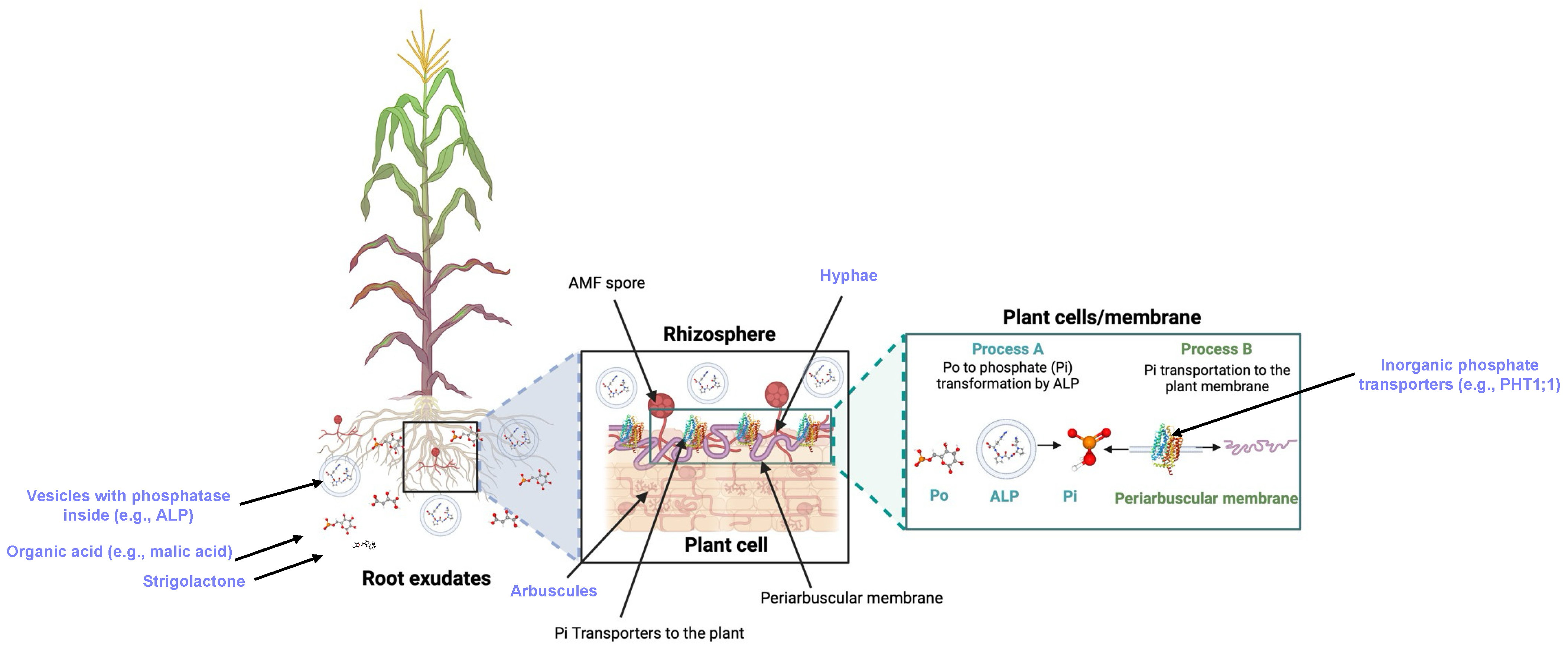 Figure 2. The root–AMF network involves AMF–exudate interactions for P uptake and transport to the plant. The diagram expands on the work by [16][17] and highlights seven key structures in purple color: (i) root exudates (PubChem ID: 15102684; [8]), (ii) D-glucose-6-phosphate as representative Po (PubChem ID: 5958, [9]), (iii) inorganic phosphorus (Pi) (PubChem ID: 1003, [18]), (iv) inorganic phosphorus transporters to the plant membrane (PiPT (PBD Entry: 8FVZ, [10]), (v) Enzymes (PubChem ID: 18985873, [19]) transformation of Po to Pi in the plant cells, (vi) arbuscules, (vii) hyphae, and (viii) vesicles. Figure created in Biorender.com.
Figure 2. The root–AMF network involves AMF–exudate interactions for P uptake and transport to the plant. The diagram expands on the work by [16][17] and highlights seven key structures in purple color: (i) root exudates (PubChem ID: 15102684; [8]), (ii) D-glucose-6-phosphate as representative Po (PubChem ID: 5958, [9]), (iii) inorganic phosphorus (Pi) (PubChem ID: 1003, [18]), (iv) inorganic phosphorus transporters to the plant membrane (PiPT (PBD Entry: 8FVZ, [10]), (v) Enzymes (PubChem ID: 18985873, [19]) transformation of Po to Pi in the plant cells, (vi) arbuscules, (vii) hyphae, and (viii) vesicles. Figure created in Biorender.com.This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia4030077
References
- Bagyaraj, D.J.; Sharma, M.P.; Maiti, D. Phosphorus Nutrition of Crops through Arbuscular Mycorrhizal Fungi. Curr. Sci. 2015, 108, 1288–1293.
- Bhattacharya, A. Chapter 5—Changing Environmental Condition and Phosphorus-Use Efficiency in Plants. In Changing Climate and Resource Use Efficiency in Plants; Bhattacharya, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 241–305. ISBN 978-0-12-816209-5.
- Lee, E.-H.; Eo, J.-K.; Ka, K.-H.; Eom, A.-H. Diversity of Arbuscular Mycorrhizal Fungi and Their Roles in Ecosystems. Mycobiology 2013, 41, 121.
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J. A Phosphate Transporter Gene from the Extra-Radical Mycelium of an Arbuscular Mycorrhizal Fungus Glomus Intraradices Is Regulated in Response to Phosphate in the Environment. Mol. Plant-Microbe Interact. 2001, 14, 1140–1148.
- Doydora, S.; Gatiboni, L.; Grieger, K.; Hesterberg, D.; Jones, J.L.; McLamore, E.S.; Peters, R.; Sozzani, R.; Van den Broeck, L.; Duckworth, O.W. Accessing Legacy Phosphorus in Soils. Soil Syst. 2020, 4, 74.
- Qin, Z.; Peng, Y.; Yang, G.; Feng, G.; Christie, P.; Zhou, J.; Zhang, J.; Li, X.; Gai, J. Relationship between Phosphorus Uptake via Indigenous Arbuscular Mycorrhizal Fungi and Crop Response: A 32P-Labeling Study. Appl. Soil Ecol. 2022, 180, 104624.
- Ahammed, G.J.; Hajiboland, R. Introduction to Arbuscular Mycorrhizal Fungi and Higher Plant Symbiosis: Characteristic Features, Functions, and Applications. In Arbuscular Mycorrhizal Fungi and Higher Plants: Fundamentals and Applications; Ahammed, G.J., Hajiboland, R., Eds.; Springer Nature: Singapore, 2024; pp. 1–17. ISBN 978-981-9982-20-2.
- National Center for Biotechnology Information PubChem Compound Summary for CID 15102684, 5-Deoxystrigol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/15102684 (accessed on 25 May 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 5958, Glucose 6-Phosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5958 (accessed on 25 May 2024).
- Gupta, M.; Finer-Moore, J.; Stroud, R.M. PDB Entry - 8FV- PiPT Y150A. Available online: https://www.wwpdb.org/pdb?id=pdb_00008fvz (accessed on 1 June 2024).
- Aggarwal, A.; Kadian, N.; Tanwar, A.; Yadav, A.; Gupta, K.K. Role of Arbuscular Mycorrhizal Fungi (AMF) in Global Sustainable Development. J. Appl. Nat. Sci. 2011, 3, 340–351.
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005.
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate–Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618.
- Yang, S.-Y.; Huang, T.-K.; Kuo, H.-F.; Chiou, T.-J. Role of Vacuoles in Phosphorus Storage and Remobilization. J. Exp. Bot. 2017, 68, 3045–3055.
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D.; Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular Mycorrhizal Fungi Contribute to Phosphorous Uptake and Allocation Strategies of Solidago Canadensis in a Phosphorous-Deficient Environment. Front. Plant Sci. 2022, 13, 831654.
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95.
- Chiu, C.H.; Paszkowski, U. Mechanisms and Impact of Symbiotic Phosphate Acquisition. Cold Spring Harb. Perspect. Biol. 2019, 11, a034603.
- National Center for Biotechnology Information PubChem Compound Summary for CID 1003, Dihydrogenphosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1003 (accessed on 25 May 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 18985873, Alkaline Phosphatase. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/18985873 (accessed on 25 May 2024).
